Abstract
A promising and increasingly exploited property of hematopoietic stem cells is their ability to efflux the fluorescent dye Hoechst 33342. The Hoechst-negative cells are isolated by fluorescence-activated cell sorting as a so-called side “population” (SP) of bone marrow. This SP from bone marrow, as well as other tissues, is reported to contain immature stem cells with considerable plasticity. Some cell lines also efflux Hoechst and generate SP profiles. Reverse transcription–polymerase chain reaction (RT-PCR) and efflux inhibition studies with the lung carcinoma cell line, A549, implicated the ABCG2 transporter as a Hoechst efflux pump. Furthermore, it is shown that transient expression of ABCG2 generates a robust SP phenotype in human embryonic kidney (HEK293) cells. The results allow the conclusion thatABCG2 is a potent Hoechst efflux pump. Semiquantitative RT-PCR was used to characterize the developmental pattern of expression of ABCG2 in hematopoiesis. It is expressed at relatively high levels in putative hematopoietic stem cells (isolated as SP, 34+/38− or 34+/KDR+populations) and drops sharply in committed progenitors (34+/38+, 34+/33+, or 34+/10+). Expression remains low in most maturing populations, but rises again in natural killer cells and erythroblasts. Comparison of messenger RNA (mRNA) levels for the 3 major multidrug-resistant efflux pumps, MDR1,MRP1, and ABCG2, in bone marrow SP cells reveals that ABCG2 is the predominant form in these cells. These data suggest that ABCG2 contributes significantly to the generation of the SP phenotype in hematopoietic stem cells. Furthermore, the sharp down-regulation of ABCG2 at the stage of lineage commitment suggests that this gene may play an important role in the unique physiology of the pluripotent stem cell.
Introduction
The ability of early hematopoietic progenitors to efflux certain fluorescent dyes such as rhodamine 123 and Hoechst 33342 has long been appreciated and exploited for the isolation of these cells by fluorescence-activated cell sorting (FACS).1-3More recently, one method has proven to be especially useful for the isolation of the most primitive bone marrow cells from a number of different species.4 This method relies on incubating the target cell population with the fluorescent dye Hoechst 33342 and subsequent FACS analysis of dual-wavelength Hoechst fluorescence with gating on a specific side population displaying low red and low blue fluorescence. Hence, the isolated cells are termed side-population (SP) cells. This low-staining SP is lost after treatment with verapamil, which has led to the assumption that the MDR1-encoded adenosine triphosphate–binding cassette (ABC) transporter, P-glycoprotein (P-gp), is responsible for Hoechst dye efflux in these cells.4
Recently, excitement has been generated by the finding that putative stem cells from solid tissues may also share this SP phenotype.5-7 Moreover, the degree of efflux activity seems to correlate with the maturation state, such that cells exhibiting the highest efflux activity are the most primitive.8 Aside from its value in stem cell purification, the dye efflux phenomenon raises 2 important questions: (1) what are the biochemical mechanisms by which it is produced in SP cells and (2) what physiologic roles do such export activities play in stem cells?
The multidrug-resistance gene, MDR1, is the best-studied member of the ABC transporter super-family of genes. The products of these genes are transmembrane proteins involved in energy-dependent transport of a wide spectrum of substrates across membranes.9,10 Currently, more than 30 members have been identified in humans.11 To date, most drug efflux phenomena have been ascribed to expression ofMDR1.12 Due to known Hoechst transport activity of MDR1,13 and to the expression ofMDR1 in early hematopoietic progenitor cells,14,15 the export activity in stem cells has largely been attributed to MDR1.4,14,16 17
MDR1 is only one of many candidate transporters for Hoechst. Members of the B, C, and G families of the ABC transport super-family are all associated with multidrug resistance and exhibit overlapping substrate specificities. Of particular importance here, they possess the ability to efflux broad and overlapping ranges of lipophilic molecules, a category that includes Hoechst.18 A careful review of the literature revealed that the efflux activity of rhodamine in murine hematopoietic stem cells is only partially blocked by theMDR1 inhibitor, verapamil. In fact, the murine long-term repopulating cells, considered to be the most primitive stem cells, are isolated on the basis of rhodamine efflux (Rho−) in the presence of verapamil.16 Thus, it seems probable that another transporter may contribute to the SP phenotype associated with stem cells.
Given that the transporter activity causing the efflux phenotype is important for the isolation of stem cells and hypothetically may play an important physiologic role in stem cell function, we have addressed the genetic and biochemical identity of this activity. In this study, we demonstrate that a strong SP phenotype is exhibited in the A549 cell line and that the inhibitor profile of this activity correlates better with the G family member, ABCG2 (ABCP/BCRP/MXR) thanMDR1 activity. Transfection experiments establish thatABCG2 is necessary and sufficient to generate the SP phenotype in human embryonic kidney (HEK293) cells. Furthermore, we show that the expression of ABCG2 is restricted to the most immature hematopoietic progenitors in human bone marrow and is sharply down-regulated at the committed progenitor level, consistent with an in vivo role for this transporter in immature cells. Direct comparison of messenger RNA (mRNA) levels for ABCG2, MDR1, andMRP1 reveals that ABCG2 is the predominant form in bone marrow SP cells, indicating that it probably plays a major role in the characteristic Hoechst efflux capacity of these cells.
Materials and methods
Cell culture
The 293 cells (American Type Culture Collection [ATCC] no. CRL-1573), kindly provided by J. Vierra (FHCRC), were grown in Dulbecco modified Eagle medium (DMEM) containing 10% fetal calf serum (FCS). A549 human lung carcinoma cells (ATCC no. CCL-185) were kindly provided by M. Cornwell (FHCRC) and grown in 50% Ham F12 medium and 50% DMEM containing 10% FCS. The following human leukemia cell lines were cultured according to ATCC recommendations: HL-60 (promyelocytic leukemia, ATCC no. CCL-240), K-562 (chronic myelogenous leukemia, ATCC no. CCL-243), KG-1 and KG-1a (acute myelogenous leukemia, ATCC no. CCL-246 and CCL-246.1) and HEL-DR+ and HEL-DR−erythroleukemia lines.19 Cells were grown at 37°C in a 5% CO2 atmosphere and maintained in log phase. Cell numbers were determined by hemocytometer and cell viability was assessed by trypan blue exclusion.
Hoechst efflux studies
The A549 or 293 cells from log-phase cultures were trypsinized and resuspended at 0.5 to 1.0 × 106 cells/mL in complete RPMI. Hoechst was added to a final concentration of 5 μg/mL and cells were incubated for 45 minutes in a 37°C water bath. Cells were gently agitated every 15 minutes. After this staining period, the cells were washed in cold Hanks balanced saline solution/1 mM HEPES/2% FCS (HBSS+) once and resuspended in warm (37°C) media and incubated for a 45-minute efflux period under the indicated conditions. This poststaining efflux period served to clear cells of membrane-associated fluorescence and lower background. When efflux inhibitors were used, they were maintained at the designated concentration throughout the staining and efflux periods. ATP depletion was performed by preincubating the cells for 20 minutes at 37°C with complete medium containing 15 mM sodium azide and 50 mM deoxyglucose, and maintaining the same inhibitor levels throughout the staining and efflux periods. Probenecid (Sigma, St Louis, MO) was used at 5 mM.20 Verapamil (Sigma) was used at 25 or 75 μg/mL as indicated. Fumitremorgin C (the kind gift of S. Bates, National Institutes of Health) was used at 10 μM. Cells were then washed in ice-cold HBSS+ and resuspended in cold HBSS+containing 2 μg/mL propidium iodide (PI) to exclude nonviable cells for flow cytometric analysis. A Vantage II flow cytometer equipped with 360 nm UV and 488 nm argon lasers was used to read the fluorescence of Hoechst and PI, respectively. The 424/44 BP and 675 LP filters, in combination with a 640 long pass dichroic mirror, were the appropriate filters for detection of Hoechst blue and red, respectively. Concomitant PI fluorescence was read using a 630/22 bond pass filter and a 610 short pass dichroic mirror. At least 100 000 events were collected.
Fluorescent microscopy
The 293 cells were transfected with the bicistronic vectors using FUGENE (Roche Molecular Biochemicals, Indianapolis, IN). Forty-eight hours after transfection, cells were trypsinized and plated on fibronectin-coated chamber slides (Nunc, Naperville, IL) and allowed to adhere overnight. Sixty-four hours after transfection, cells were stained with Hoechst under the same conditions as described above and examined using a Deltavision Microscope Zeiss Axiovert 100 (Applied Precision, Issaquah, WA). Images were captured using 360/40 and 457/20 filters (Hoechst), and 490/20 and 529/38 (green fluorescent protein [GFP]), for excitation and emission, respectively. The software used for capturing and viewing images was SoftWoRx 2.50 (Applied Precision). Images were subsequently deconvolved mathematically.21 Data are presented as representative optical sections.
Flow sorting of hematopoietic cells
Aliquots of normal bone marrow were diverted for research purposes from aspirated marrow collected from 15 healthy adult marrow transplant donors. Informed consent for this research use was obtained as determined by the Institutional Review Board of the FHCRC. After collection, Ficoll density centrifugation was performed to isolate mononuclear cells. For isolation of SP cells, mononuclear cells were counted and resuspended at 106 cells/mL in Iscoves/1 mM HEPES/2% heat-inactivated calf serum warmed to 37°C. Hoechst was added to 5 μg/mL and cells were incubated for 90 minutes in a 37°C water bath with gentle agitation every 20 minutes. After staining, the cells were centrifuged at 300g for 5 minutes, washed once in ice-cold HBSS+, and resuspended in cold HBSS+containing 2 μg/mL PI. Cells were kept on ice until sorting.
For isolation of all other cell populations, mononuclear cells were pre-enriched for CD34+ cells using the Miltenyi (Auburn, CA) Auto-MACS system and subsequently stained with monoclonal antibodies against human surface markers. The antibodies included CD34 (HPCA-2), CD38 (HB-7), CD33 (P67.6), and CD10, all from Becton Dickinson (San Jose, CA). The monoclonal antibody against human vascular endothelial growth factor receptor 2 (KDR) was kindly provided by Dr Chengchao Shou and Dr Donghai Chen (Beijing Institute for Cancer Research and Beijing Cancer Hospital, People's Republic of China). All staining was performed for 30 minutes on ice. Hoechst efflux was measured on a Vantage II SE, essentially as described by Goodell et al,4 using 424/44 BP and 675 LP filters, for detection of Hoechst blue and red, respectively. Dead cells were excluded based on PI uptake. Ten thousand cells of each defined cell population were then sorted directly into lysis buffer (Rneasy kit, Qiagen, Valencia, CA).
Analysis of gene expression
Total RNA was isolated from cell lines by extraction with Trizol (Gibco BRL, Grand Island, NY), 2 rounds of precipitation with ethanol, and resuspension in RNAase-free distilled water. Total RNA from FACS-sorted cells (usually 10 000 cells) was isolated with the Rneasy Mini Kit (Qiagen), precipitated in ethanol with glycogen carrier (Gibco BRL), and resuspended in 5 μL RNAase-free distilled water. First strand complementary DNA (cDNA) was synthesized from either 40 ng total RNA from cell lines or total RNA from 10 000 sorted cells, using 200 U Superscript II reverse transcriptase (Gibco BRL), and a combination of 5 μM OligodT18 and 10 μM random hexamer primers. Completed reverse transcription (RT) reactions were diluted to 400 μL (about 25 cells/μL) with DNAase-free distilled water and stored at −20°C. Specific cDNAs were amplified from dilutions of the RT reactions with Advantage 2 polymerase mix (Clontech Laboratories, Palo Alto, CA) using 30 to 40 cycles of a 2-step program (94°C for 20 seconds, 68°C for 2 minutes). Gene-specific primers for human cDNAs were as follows: GAPDH: 5′- GGAAGGACTCATGACCACAGTCC;GAPDH: 3′- TCGCTGTTGAAGTCAGAGGAGACC; MDR1: 5′- CAGAAACAACGCATTGCCATAGCTC. MDR1: 3′- TGATGATGTCTCTCACTCTGTTCC. MRP1: 5′- GGGGATGCTGAAGAACAAGACGC.MRP1: 3′- GCTGAGGAAGGAGATGAAGAGTCC. ABCG2: 5′-GGGTTCTCTTCTTCCTGACGACC. ABCG2: 3′- TGGTTGTGAGATTGACCAACAGACC. Amplified products (400-500 base pairs) were separated by electrophoresis on 1.5% agarose gels, stained with ethidium bromide, detected by fluorescence-based laser scanning with a Typhoon 8600 Imager (Amersham Pharmacia, Piscataway, NJ) and quantitated with ImageQuant software (Amersham Pharmacia). Prior to analysis of transporter gene expression, all RT reactions were normalized to GAPDH by first amplifying over a range of cycle numbers to generate a cycle/signal curve, and then adjusting the RT dilutions to obtain equivalent GAPDH signals for a given cycle number.
Semiquantitative reverse transcription–polymerase chain reaction (RT-PCR) of ABCG2, MDR1, and MRP1transcripts in sorted cell populations was performed by amplification of the GAPDH-normalized RT reactions along with quantitative standards. Standards for each gene were established by quantitating a gel-purified, column-purified cDNA template, containing the target region, by Hoechst 33258 binding,22 using a DyNAQuant 200 fluorometer (Amersham Pharmacia). Standard curves were generated by using serial dilutions of the standards, containing from 1 to 7000 molecules/μL target cDNA as PCR templates. All PCRs were done at several cycle numbers to establish the optimal dynamic range. Amplified product values were normalized to GAPDH for intersample comparisons or to standard curves for intergene comparisons within a given sample.
ABCG2 expression construct
pG2-IRES-EGFP is a bicistronic construct driving coexpression of human ABCG2 and enhanced green fluorescent protein (EGFP) from the cytomegalovirus immediate early promoter/enhancer. ABCG2 cDNA was amplified by RT-PCR from human CD34+ bone marrow cells. Total RNA was primed with oligo dT and reverse-transcribed with SuperScript II (Gibco BRL). The complete coding sequence of ABCG2 was amplified with Advantage 2 polymerase mix (Clontech) using primers encoding unique 5′NotI and 3′ EcoRI sites for cloning (5′ primer: GCATTACATGCGGCCGCGATCCTGAGCCTTTGGTTAAGACC, 3′ primer: CAGGAGTTTCCAGAATTCAATTCTCC). The cDNA was digested with NotI and EcoRI, and inserted into pIRES-EGFP (Clontech).
Results
The SP phenomenon of A549 is associated with expression ofABCG2
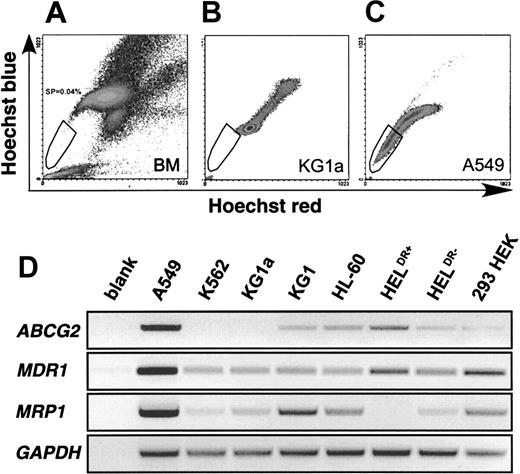
The SP phenotype is characterized by low blue and red fluorescence intensity on a dot-plot displaying dual-wavelength fluorescence of blue versus red detected at 424 and 675 nm, respectively. Figure1A shows a typical SP profile derived from human bone marrow. The SP cells form a tail, which extends from the main body of stained cells to the origin of a blue/red fluorescence dot-plot. Due to the rarity of these cells in bone marrow and to their inherent metabolic and replicative quiescence, we sought a cell line with SP characteristics for preliminary analysis of the phenomenon. A survey of several cell lines for Hoechst efflux activity revealed mostly negative profiles. Figure 1B illustrates a typical negative example, KG1a cells, which exhibit a main body of stained cells and a streak of highly stained cells representing a dividing population with a DNA content more than 2N. Only A549 cells (Figure 1C) exhibit a significant SP of Hoechstlow cells trailing to the origin of the plot and thus displaying low fluorescence in both the blue and red channels.
SP profiles from human bone marrow, KG1a and A549 cells.
(A) Typical SP profile derived from human bone marrow (BM). Vertical axis shows blue Hoechst fluorescence, horizontal axis shows red Hoechst fluorescence. The SP cells are indicated within the gate. (B) SP profile of KG1a cells. (C) A549 cells exhibiting a significant SP. (D) Expression of ABCG2, MDR1, and MRP1 in cell lines. GAPDH-normalized RT reactions from each cell line were subjected to 36 cycles (MDR1, MRP1) and 37 cycles (ABCG2, GAPDH) of amplification with specific primers for each cDNA. The bottom row confirms similarGAPDH levels in the normalized RT reactions.
SP profiles from human bone marrow, KG1a and A549 cells.
(A) Typical SP profile derived from human bone marrow (BM). Vertical axis shows blue Hoechst fluorescence, horizontal axis shows red Hoechst fluorescence. The SP cells are indicated within the gate. (B) SP profile of KG1a cells. (C) A549 cells exhibiting a significant SP. (D) Expression of ABCG2, MDR1, and MRP1 in cell lines. GAPDH-normalized RT reactions from each cell line were subjected to 36 cycles (MDR1, MRP1) and 37 cycles (ABCG2, GAPDH) of amplification with specific primers for each cDNA. The bottom row confirms similarGAPDH levels in the normalized RT reactions.
To identify the efflux pump responsible for this phenotype, we interrogated A549 cells and 7 SP− cell lines for expression of 3 ABC transporters, which are well-established efflux pumps related to drug resistance. The MDR1 gene encoding P-gp has already been implicated as a candidate. MRP1 is part of a distinct cellular detoxification pathway and is responsible for the efflux of a variety of lipophilic compounds.23Recently, it was demonstrated that the novel ABC half-transporter, ABCP/BCRP/MXR, herein referred to as ABCG2 as suggested by International Gene Nomenclature (http://www.gene.ucl.ac.uk/users/hester/abc.html), is responsible for a number of multidrug-resistance phenomena that are independent of overexpression of MDR1 or MRP family members.18,24 25 Each of these pumps is known to efflux small lipophilic molecules, rendering them candidates for Hoechst efflux. As shown in Figure 1D, all 3 of these efflux pumps are expressed more highly in A549 cells than in any of the SP−cell lines. Thus, any of them, alone or in combination, could potentially contribute to the SP phenotype.
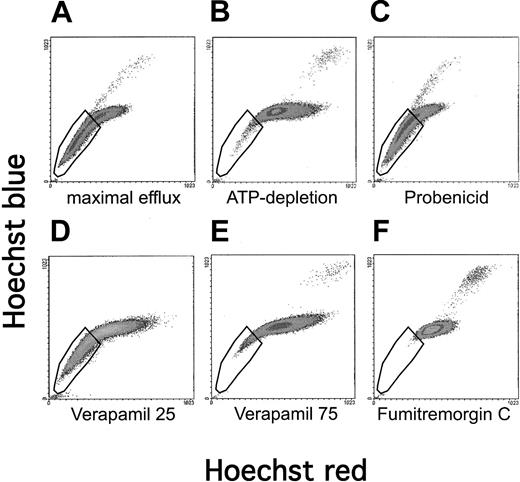
To elucidate which of the expressed ABC transporters contribute to the SP phenotype in this cell line, we conducted inhibition studies on A549 cells (Figure 2). A549 cells that were allowed to efflux in the absence of any inhibitor demonstrated a large proportion of cells fitting the criteria of an SP (Figure 2A). If A549 cells were depleted of ATP for 20 minutes before the staining and efflux period, the SP phenotype was markedly reduced, verifying that this phenomenon is energy dependent (Figure 2B). Incubation at 4°C conferred the same degree of inhibition of the SP phenotype as did ATP depletion (data not shown). These observations are consistent with the role of an ABC transporter in this phenomenon. An inhibitor ofMRP1, probenecid, did not influence the ability to efflux Hoechst, suggesting that the SP phenomenon is independent ofMRP1 (Figure 2C). Verapamil, at concentrations that are sufficient to inhibit MDR1, only moderately inhibited efflux (Figure 2D). These data are consistent with the notion thatMDR1 and MRP1 are not major contributors to the SP phenotype of A549 cells. However, only 3-fold higher concentrations of verapamil (75 μg/mL) strongly inhibited efflux activity (Figure2E). Furthermore, the inhibitor fumitremorgin C, shown to be specific for ABCG2,26 exerted very potent inhibition of the SP phenotype in A549 cells (Figure 2F). Robey et al27have recently established that the fumitremorgin C-inhibitable efflux of the fluorescent chemotherapy agent mitoxantrone is diagnostic forABCG2 activity. We found significant efflux of mitoxantrone in A549 cells, which was sensitive to inhibition by fumitremorgin C, corroborating ABCG2 activity (data not shown). Taken together, these data strongly implicate ABCG2 as the primary contributor to the SP phenomenon in this cell line.
Influence of inhibitors on the SP profile of A549 cells.
A549 cells were stained with Hoechst 33342, allowed to efflux, and then analyzed by flow cytometry for red/blue staining characteristics. The conditions for staining and efflux were designed for (A) maximal efflux (ie, no inhibitors), (B) ATP depletion, or (C-F) inhibition by pump-specific drugs.
Influence of inhibitors on the SP profile of A549 cells.
A549 cells were stained with Hoechst 33342, allowed to efflux, and then analyzed by flow cytometry for red/blue staining characteristics. The conditions for staining and efflux were designed for (A) maximal efflux (ie, no inhibitors), (B) ATP depletion, or (C-F) inhibition by pump-specific drugs.
ABCG2 is sufficient to cause the SP phenotype
To determine whether ABCG2 is sufficient to generate the SP phenotype in a controlled background, we constructed a bicistronic vector, pG2-IRES-EGFP, expressing the ABC transporter and the GFP reporter (Figure 3A). The parent vector pIRES-EGFP, expressing only GFP, was used as a control. We then transiently transfected these constructs into 293 cells. After 48 hours the cells were incubated with Hoechst 33342 and subsequently analyzed by fluorescent microscopy. As shown in Figure 3B, expression of the control plasmid, lacking ABCG2, resulted in simultaneous green (GFP) and blue (Hoechst) fluorescence, indicating transgene expression but no efflux activity. However, 293 cells transfected with the pG2-IRES-EGFP construct exhibited strong efflux of Hoechst, specifically from the expressing (GFP+) cells (Figure 3E). The nonexpressing (GFP−) cells, which served as internal controls, acquired the typical blue Hoechst fluorescence in their nuclei. Thorough analysis of these images indicated an inverse correlation between the intensity of GFP and Hoechst fluorescence. These data established that GFP expression could be used as a reliable reporter for coexpression of ABCG2 efflux activity from the bicistronic vector. We further analyzed the relationship betweenABCG2 expression and Hoechst efflux by flow cytometry. The SP phenotype was observed only in the ABCG2-transfected cells (Figure 3F) and not in the pIRES-EGFP controls (Figure 3C). Moreover, Figure 3G clearly illustrates a quantitative correlation between ABCG2 transgene expression as reported by GFP and efflux activity. Together, these data conclusively demonstrate thatABCG2 is necessary, and sufficient to efflux Hoechst 33342, and to generate the SP phenotype in 293 cells.
ABCG2 is sufficient to generate the SP profile in 293 cells.
(A) Illustration of the bicistronic vectors used to transfect 293 cells. CMV indicates cytomegalovirus immediate early promoter/enhancer; MCS, multiple cloning site; and IRES, internal ribosomal entry site. (B,E) Fluorescent microscopic images of cells transfected with control (B) or pG2-IRES-EGFP vectors (E). Twenty to 30% of the cells express the construct (green). All cells that fail to exclude Hoechst dye exhibit blue nuclei. (C,F) Red/blue FACS plot of these same cell populations. The pG2-IRES-EGFP–transfected cells exhibit a prominent SP tail (arrow). (D,G) FACS plots of the same cell populations comparing EGFP expression (vertical axis) and Hoechst blue staining (horizontal axis).
ABCG2 is sufficient to generate the SP profile in 293 cells.
(A) Illustration of the bicistronic vectors used to transfect 293 cells. CMV indicates cytomegalovirus immediate early promoter/enhancer; MCS, multiple cloning site; and IRES, internal ribosomal entry site. (B,E) Fluorescent microscopic images of cells transfected with control (B) or pG2-IRES-EGFP vectors (E). Twenty to 30% of the cells express the construct (green). All cells that fail to exclude Hoechst dye exhibit blue nuclei. (C,F) Red/blue FACS plot of these same cell populations. The pG2-IRES-EGFP–transfected cells exhibit a prominent SP tail (arrow). (D,G) FACS plots of the same cell populations comparing EGFP expression (vertical axis) and Hoechst blue staining (horizontal axis).
Expression of ABCG2 in hematopoiesis
To determine whether the aforementioned observations are pertinent to the biology of human hematopoietic stem cells, that is, whetherABCG2 is expressed in early hematopoietic progenitor/stem cells, we interrogated several relevant flow-sorted hematopoietic cell subpopulations from human bone marrow by RT-PCR. SP cells were isolated as a putative stem cell population. Given the controversial stem cell potential of human bone marrow SP cells,8 we also used the established surface markers, CD34+CD38−,28 and the reported stem cell markers, CD34+KDR+,29 to purify cell populations enriched for primitive hematopoietic progenitors. CD34+CD38+ cells were isolated to represent the CD34+ progenitor population depleted of the CD38− putative stem cell population. CD34+CD33+ and CD34+CD10+ populations were isolated to represent committed myeloid and lymphoid progenitors, respectively.
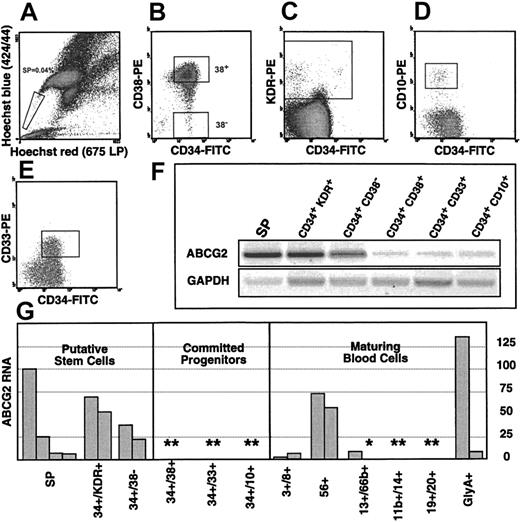
The FACS gates used to isolate these cells are shown in Figure4, panel A to E. The relative expression levels for ABCG2 in these cell populations was analyzed by semiquantitative GAPDH-normalized RT-PCR. As shown in the representative gel in Figure 4F,ABCG2 transcripts are relatively high in SP cells as well as in CD34+/CD38− and CD34+/KDR+ cells. In CD34+/CD38+ cells, thought to represent a general population of committed progenitors,28 30 ABCG2 transcript levels are clearly much lower than in any of the “stem cell” populations. This holds true for specific myeloid and lymphoid committed progenitor cells, represented by CD34+/CD33+ and CD34+/CD10+ populations, respectively. Quantitative analysis of replicate isolates for each cell population (Figure 4G), demonstrated at least a 5- to 10-fold difference between the putative stem cell and committed progenitor populations. Similar analysis of maturing peripheral blood populations reveals high transcript levels in natural killer cells (CD56+) and probably erythroblasts (glycophorin A+). These data indicate that the ABCG2 gene is sharply down-regulated at the time of myeloid/lymphoid lineage commitment and reinduced in specific lineages later.
Expression of
ABCG2 in human hematopoiesis. (A-E) FACS diagrams illustrate the sorting gates used to isolate putative stem cells and committed progenitors. (A) SP cells, (B) CD34+/CD38− and CD34+/CD38+ cells, (C) CD34+/ KDR+, (D) CD34+/CD10+ cells, and (E) CD34+/CD33+ cells. (F) RT-PCR forABCG2 expression in the representative sorted cell populations shown above. Both ABCG2 and GAPDHwere amplified for 36 cycles. (G) Semiquantitative analysis ofABCG2 expression in sorted cell populations. Each bar represents an independent sort. All values are taken from 36 cycle amplifications and are normalized to GAPDH values from the same sample. All values below 50 were in the linear range of the PCR, as determined by an ABCG2 cDNA standard curve. Values above 50 are probably slight underestimates. The asterisk indicates normalized ABCG2 values below 1 on this arbitrary scale. The double asterisks indicate duplicate samples with values below 1.
Expression of
ABCG2 in human hematopoiesis. (A-E) FACS diagrams illustrate the sorting gates used to isolate putative stem cells and committed progenitors. (A) SP cells, (B) CD34+/CD38− and CD34+/CD38+ cells, (C) CD34+/ KDR+, (D) CD34+/CD10+ cells, and (E) CD34+/CD33+ cells. (F) RT-PCR forABCG2 expression in the representative sorted cell populations shown above. Both ABCG2 and GAPDHwere amplified for 36 cycles. (G) Semiquantitative analysis ofABCG2 expression in sorted cell populations. Each bar represents an independent sort. All values are taken from 36 cycle amplifications and are normalized to GAPDH values from the same sample. All values below 50 were in the linear range of the PCR, as determined by an ABCG2 cDNA standard curve. Values above 50 are probably slight underestimates. The asterisk indicates normalized ABCG2 values below 1 on this arbitrary scale. The double asterisks indicate duplicate samples with values below 1.
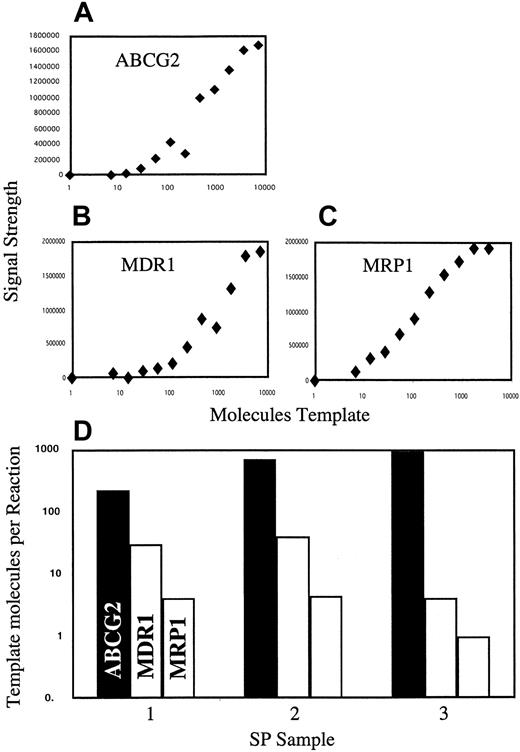
To address the potential contributions of ABCG2 and 2 other well-established multidrug-resistant efflux pumps (MDR1 andMRP1) to the Hoechst efflux activity of SP cells, we analyzed the relative expression levels of these 3 genes in bone marrow SP isolates. To control for differences in amplification characteristics for different cDNAs and to allow PCR signals to be directly related to molecules of template, a set of cDNA dilutions was prepared for each gene. A PCR standard curve for each gene was generated by amplification of these dilution series (Figure 5A-C), and the RT-PCR values from the SP samples were normalized to the standard curves (Figure 5D). The results of this analysis show thatABCG2 transcript levels in SP cells are at least 5-fold higher than those of MDR1 and 50-fold higher than those ofMRP1. Thus, among the efflux pumps tested, ABCG2is probably the predominant form in SP cells.
Relative expression of ABCG2, MDR1,and MRP1 in SP cells.
Three independent SP isolates from 3 independent bone marrow donors were analyzed by RT-PCR. Values for each gene were normalized to a cDNA standard curve generated at the same time, under the same amplification conditions. (A-C) Standard curves for ABCG2, MDR1, and MRP1, respectively. (D) Relative transcript levels for the 3 genes in each of 3 SP isolates. Note that because the standards do not control for reverse transcriptase efficiency, they cannot be used to determine absolute mRNA levels.
Relative expression of ABCG2, MDR1,and MRP1 in SP cells.
Three independent SP isolates from 3 independent bone marrow donors were analyzed by RT-PCR. Values for each gene were normalized to a cDNA standard curve generated at the same time, under the same amplification conditions. (A-C) Standard curves for ABCG2, MDR1, and MRP1, respectively. (D) Relative transcript levels for the 3 genes in each of 3 SP isolates. Note that because the standards do not control for reverse transcriptase efficiency, they cannot be used to determine absolute mRNA levels.
Discussion
The present study shows direct evidence that humanABCG2 can efflux Hoechst 33342 and that this activity can generate an SP phenotype. Previous studies addressing the mechanism of Hoechst exclusion from cells have relied on a correlation between dye exclusion and high transporter expression in cell lines that were selected for high drug resistance. Given that most cells express an assortment of ABC transporters, and that drug selection can induce genes that are unknown or untested, such a correlation can be misinterpreted. Here we demonstrate that the Hoechst efflux activity of A549 cells correlates with high expression of at least 3 ABC transporters but that its inhibition profile is most consistent with activity of ABCG2. Furthermore, transient expression ofABCG2 in the controlled background of a cell line that does not exhibit Hoechst efflux or the SP phenotype, is sufficient to generate these activities.
The RT-PCR analysis of ABCG2 mRNA in early hematopoietic cells reveals an expression pattern that is appropriate for pluripotent stem cells. We found high expression of ABCG2 in putative human stem cell populations isolated by 3 independent criteria including the SP phenotype. The enrichment for ABCG2transcripts by all 3 isolation protocols suggests that the SP, 34+38−, and 34+KDR+populations are truly overlapping populations. Expression drops by at least 5-fold in the stem cell–depleted CD34+CD38+ subfraction and in committed myeloid and lymphoid progenitors, represented by the CD33+ and CD10+ subfractions of CD34+ cells. This drop may be underestimated due to the large variability observed in SP cells. In our experience, the fraction of human bone marrow cells falling in the SP gate can vary widely, and we infer that the purity of stem cells in this fraction varies accordingly. We suggest that the higher ABCG2 values in some SP preps, which correspond better to the values for the other putative stem cell fractions, represent the more realistic values. Thus, ABCG2 appears to be expressed in pluripotent stem cells, then sharply down-regulated at the point of commitment to lineage-specific development. These data not only implicate a role for ABCG2 in the SP phenomenon of primary hematopoietic cells, but more importantly, the obvious developmental regulation suggests an important role for this ABC transporter in the earliest stages of hematopoietic development.
Most studies of dye efflux by hematopoietic stem cells has either implicated14 or assumed4,16 thatMDR1 plays the primary role in these activities.MDR1 expression is well established in murine bone marrow stem cells.14,15 However, the verapamil inhibition profile of bone marrow SP cells is not entirely consistent with this model. The concentration needed to inhibit the SP phenomenon in bone marrow is variable, but generally higher than that required to block theMDR1-encoded pump.6 31 In light of these observations, our data are consistent with a model in whichABCG2, or perhaps a combination of transporters includingMDR1 and ABCG2, generates the SP phenotype. Because our RT-PCR data indicate that ABCG2 mRNA levels are much higher than those of MDR1 or MRP1 in SP cells, we favor a model in which ABCG2 rather thanMDR1 is the predominant cause of the SP phenotype associated with hematopoietic stem cells.
The question remains as to why hematopoietic stem cells use these efflux pumps. One possibility, consistent with the broad spectrum of toxic substrates effluxed by these pumps, is that they provide a protective function, because these cells must persist for the lifetime of the individual.32 A second possible function, consistent with the lipophilic substrate range of these pumps, could be the efflux of a variety of small lipophilic regulatory molecules, such as steroids, that could trigger growth, differentiation, or apoptotic pathways. Thus, ABCG2 could function to help maintain the unique properties of these immature cells or play a regulatory role in initiating these pathways. The recent findings of Bunting et al,33 of in vivo and ex vivo expansion of multidrug-resistant overexpressing murine stem cells, suggests that these pumps can play fundamental roles in regulation of stem cells. It is possible that ABCG2, MDR1, and perhaps other transporters share overlapping roles in such a function. This hypothesis is currently under investigation.
The authors would like to thank Jonathan Cooper and Mark Groudine for critical reading of the manuscript; Andrew Burger, Michelle Black, and Brian Hall for their assistance with flow cytometry; Adrian Quintanilla for assistance with the Deltavision microscope; Susan Bates and Rob Robey for providing FTC and many helpful discussions; and Jeff Vierra and Marilyn Cornwell for supplying 293 and A549 cells.
Supported in part by grants HL62923, DK56465 from the National Institutes of Health, Department of Health and Human Services, Bethesda, MD; C.W.S. was also supported by a grant from the Stifterverband fuer die Deutsche Wissenschaft (Kind Philipp).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Beverly Torok-Storb, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mailstop D1-100, Seattle, WA 98109; e-mail: btorokst@fhcrc.org.