Abstract
Fcγ receptor–mediated phagocytosis is a model for the study of immunoreceptor (immunoreceptor tyrosine-based activation motif [ITAM]) signaling and involves the activation of protein tyrosine kinases, protein tyrosine phosphatases, and downstream effectors including phosphatidylinositol-3 (PI-3) kinase. Relatively little is known of the role of lipid phosphatases in the control of ITAM signaling and inflammation. A heterologous COS7 cell system was used to examine the roles played by PI-3 kinase and the dual-specificity phosphatase, phosphatase and tensin homolog deleted on chromosome 10 (PTEN), in the signal transduction pathway leading to Fcγ receptor IIA–mediated phagocytosis and the activation of Rac. The expression of wildtype PTEN completely abrogated the phagocytosis of immunoglobulin-G–sensitized sheep red blood cells, as compared with the catalytically inactive mutant of PTEN, which had no effect. This is the first direct evidence that PTEN, an inositol 3′ phosphatase, regulates Fcγ receptor–mediated phagocytosis, an ITAM-based signaling event. The data suggest that PTEN exerts control over phagocytosis potentially by controlling the downstream conversion of guanosine diphosphate–Rac to guanosine triphosphate–Rac following ITAM stimulation.
Introduction
Fcγ receptor–mediated phagocytosis in macrophages is an important primary mode of defense in the immune system and represents an excellent model for studying signaling events propagated through the immunoreceptor tyrosine-based activation motif (ITAM). ITAM-based signaling, which involves the YxxLx6YxxL consensus motif, is at the basis for T-cell receptor, B-cell receptor, and Fc receptor signaling in hemapoeitic cells and controls platelet activation through the collagen receptor VI.1 In this study, we use Fcγ receptor signaling in COS7 cells to identify a role for the lipid phosphatase, phosphatase and tensin homolog deleted on chromosome 10 (PTEN), in the physiologic regulation of ITAM signaling.
Signal transduction events associated with Fcγ receptor involve activation of nonreceptor tyrosine kinases Hck, Lyn, Fgr,2,3 and Syk.4,5 This leads to phosphorylation of complex adapter proteins6 and activation of effector molecules, including Rac, Rho, and Rab.7 The role played by tyrosine kinases in this phenomenon has been examined.8-10 There are no reports of involvement of protein, inositol 3′ phosphatases, or dual-specificity phosphatases in the regulation of the Fcγ receptor pathway. Here, we present the first evidence that the protein/lipid phosphatase, PTEN, negatively regulates Fcγ receptor–mediated phagocytosis and controls ITAM signaling leading to the downstream activation of Rac.
Study design
Cells, antibodies, plasmids, and reagents
COS7 cells were grown in Dulbecco modified Eagle medium (DMEM) containing 10% fetal calf serum (FCS) and transfected with episomal plasmids encoding Fcγ receptor IIA (FcγRIIA), enhanced green fluorescent protein (EGFP)–Syk, and PTEN. Anti-PTEN antibody was generated by immunizing rabbits with the N-terminal peptide of PTEN.11 Plasmids encoding PTEN, mutant PTEN (Cys124Ser), EGFP-tagged Syk kinase, kinase-dead mutant EGFP-Syk (Lys396Arg), and FcγRIIA were prepared by standard subcloning methods in pRK5, pEGFP, or pcDNA3.1, respectively, and purified by means of the Qiagen (Valencia, CA) kit as described.12 In all transfections, plasmid DNA concentration and composition were equalized with the use of the appropriate amount of empty vector DNA (ie, pRK5, PTEN; pEGFP, Syk; pcDNA3.1, FcγRIIA). Detection of FcγRIIA expression was by flow cytometry with the use of an allophycocyanin-conjugated anti-CD32 monoclonal antibody (FLI8.26) (Pharmingen, San Diego, CA). Phospho-AKT antibody against S463 was obtained from New England Biolabs (Beverly, MA).
Phagocytic assays
COS7 cells were plated at 1 × 105 cells per well on a 6-well plate (Costar, Corning, NY) overnight. Cells were transiently transfected with plasmids by means of lipofectamine reagent. After 4 hours, the medium was changed and the cells were further incubated for 24 to 48 hours. For phagocytosis assays, cells were incubated with sheep red blood cells (sRBCs) coated with immunoglobulin G (IgG) at subagglutinating concentration. The target-to-effector ratio was kept at 200:1. The cells were scrapped after 2 hours, and cytospins were prepared, fixed, and stained with Wright Giemsa stain (Dade AG, Düdingen, Switzerland). The slides were observed under a microscope for sRBC rosette formation, an index of surface FcγRIIA conjugate formation. Cells were subjected to water shock to lyse the uningested sRBCs, suspended in DMEM containing 20% FCS and spun down on glass slide, and then fixed and stained by Wright Giemsa stain. A minimum of 150 cells were counted for each slide, and the phagocytic index was calculated as follows: Phagocytic index = no. sRBCs internalized per 100 COS7 cells randomly sampled.
Expression of PTEN and EGFP-Syk in COS7 cells
COS7 cells were transiently transfected with the use of lipofectamine according to manufacturers' specifications with plasmids encoding FcγRIIA and EGFP-Syk or kinase-dead EGFP-Syk along with PTEN or mutant PTEN to determine if PTEN regulates ITAM signaling in vivo. Briefly, 1 μg plasmid DNA encoding the FcγRIIA, Syk, and/or Cbl was cotransfected with plasmid encoding the wildtype PTEN or C124S mutant of PTEN. After 48 hours of transfection at 37°C, COS7 cells were exposed to IgG-sensitized sRBCs for 2 hours followed by determination of phagocytic index. During all transfections, total plasmid DNA concentration and composition were equilibrated with the use of identical empty vector plasmids to assure that during each condition the levels of FcγRIIA, Syk, and PTEN were equal for comparison of effects of PTEN or Syk. All transfected proteins were quantified in COS7 cells by Western blot and flow cytometry to ensure that each transfection condition generated the predicted and equivalent levels of expression of heterologous proteins, including similar levels of expression of FcγRIIA, PTEN, and EGFP-Syk for each condition (Figure F). COS7 cells were collected and suspended at a concentration of 2 × 106 cells per milliliter of DMEM and stimulated with IgG-coated sRBCs at 37°C for 5 minutes. The samples were centrifuged at 500g in a refrigerated centrifuge, and the supernatant was aspirated. The cell pellet was used for Western analysis as described earlier.13
Effect of PTEN on FcγRIIA-mediated Rac1 activation
COS7 cells were transfected either with FcγRIIA plus Syk plus pRK5, with FcγRIIA plus Syk plus wildtype PTEN, with FcγRIIA plus Syk plus mutant PTEN, or with the corresponding equal amount of empty vectors (pCDNA3, pEGFP, pRK5). At 48 hours after transfection, cells were stimulated with IgG-opsonized sRBCs for different periods of time. Following the termination of the reactions, cells were lysed with lysis buffer (25 mM Hepes, pH7.5; 150 mM NaCl; 1% Igepal Ca-630; 10 mM MgCl2; 5 mM EDTA; 10% glycerol; 10 μg/mL leupeptin; 10 μg/mL aprotonin; 25 mM sodium fluoride; and 1 mM sodium orthovanadate). For in vitro guanine nucleotide binding, for a positive control, cell lysate was incubated for 15 minutes at 30°C in the presence of 10 mM EDTA and 100 μM guanosine thiotriphosphate γS (GTPγS). The loading was stopped by addition of MgCl2 to 60 mM. Binding reactions were initiated by adding 10 μL p21 activated kinase (PAK)–agarose (glutathione-S-transferase fusion protein, corresponding to the p21-binding domain, cds42/rac interacting domain (CRIB) p21 binding domain (PBD) residues 67 through 150, of human PAK-1, expressed in Escherichia coli and bound to glutathione agarose beads from Upstate Biotechnology, Lake Placid, NY) to each sample and incubated for 45 minutes at 4°C. Agarose beads were then washed 3 times with washing buffer and resuspended in 30 μL Laemmli sample buffer. Each sample was resolved on 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and blotted with specific antibodies for Rac1 (1:2000, clone 23A8) (Upstate Biotechnology).
Results and discussion
Syk, PI-3 kinases, and FcγRIIA phagocytosis
To confirm a role for the nonreceptor tyrosine kinase Syk in our COS7 cell system for FcγRIIA-mediated phagocytosis, we expressed a dominant-negative (kinase-dead) form of Syk in COS7 cells by transient transfection (data not shown). This Syk mutant encodes a mutated kinase dead form of Syk (K396R). It is expected to dock with the ITAM but not transmit signals. Our results demonstrate that the expression of wildtype Syk augments phagocytosis whereas the expression of catalytically dead Syk in COS7 cells inhibits phagocytosis of IgG-coated sRBCs (not shown). Our results using dominant-negative Syk are consistent with other data in the literature, including studies on the Syk knockout mice9 and chimeric CD16-Syk receptors in COS7 cells,10 and strongly support a role for Syk in propagating signals required for IgG-mediated phagocytosis in this COS7 cell system. The treatment of reconstituted cells with increasing concentrations of a PI-3 kinase inhibitor, LY294002, resulted in the dose-dependent inhibition of phagocytosis, confirming the work of Indik et al14 that PI-3 kinase is required for the FcγRIIA phagocytic response in COS7 cells (data not shown).
PTEN regulates FcγRIIA phagocytosis and activation of Rac
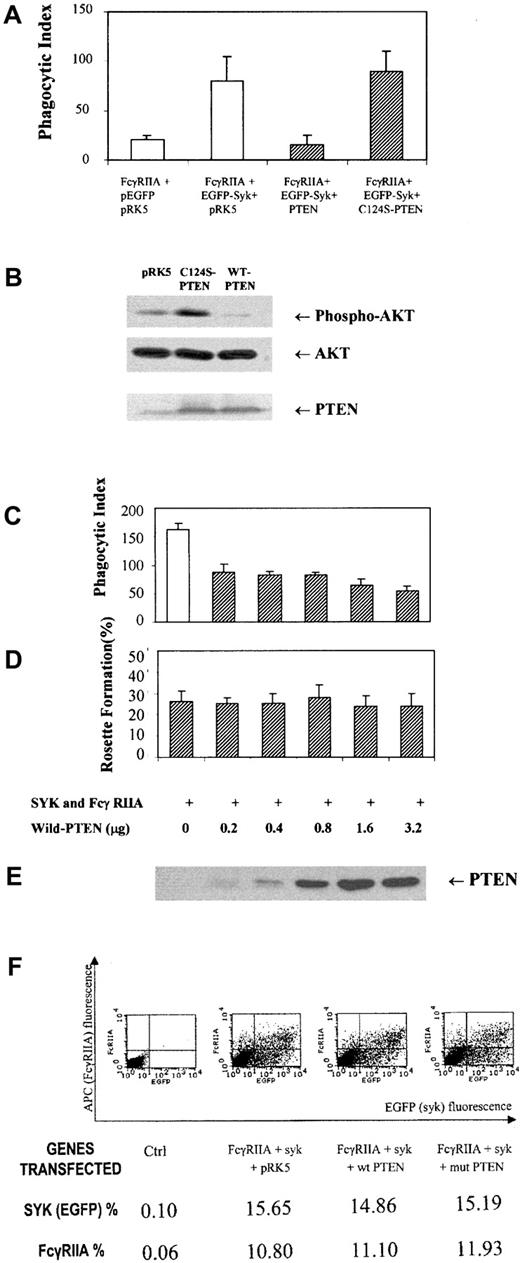
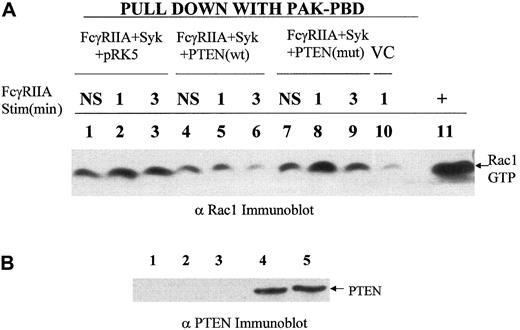
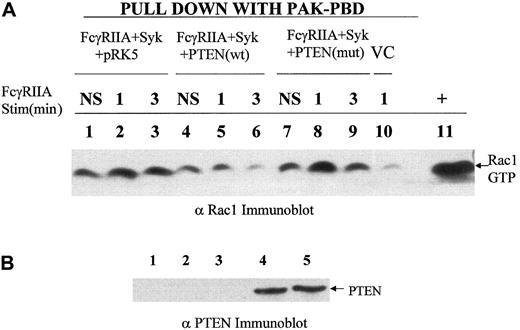
Previous reports using pleiotrophic inhibitors of the PI-3 kinase family, wortmannin and LY294002, suggested an important role for the products of PI-3 kinase in phagocytosis.14 Therefore, we reasoned that dephosphorylation of phosphatidylinositol 3,4,5-trisphosphate, also known as PtdIns(3,4,5)P3, at the D3 position may negatively regulate this response. To address this question genetically and more specifically, we overexpressed a physiologic regulator of the PI-3 kinase pathway, PTEN, a D3 phosphatase, in COS7 cells reconstituted with FcγRIIA and Syk kinase. The overexpression of PTEN in COS7 cells markedly suppressed phospho-AKT levels (Figure 1B) and abrogated phagocytosis of IgG-coated sRBCs (Figure 1A) without inhibiting the binding sRBCs to the Fcγ receptor (Figure 1D) or affecting levels of Syk or FcγRIIA expression (Figure 1F). The effect of PTEN on the phagocytic index was shown to effect a 95% suppression of phagocytosis. In contrast, the C124S mutant of PTEN augments phospho-AKT levels and did not significantly affect ITAM signaling. Importantly, in all experiments performed, we determined levels of FcγRIIA and EGFP-Syk expression in all experimental groups using flow cytometry to confirm that transfection of PTEN had no effect on expression of FcγRIIA or Syk kinase (Figure 1F). From these data we conclude that PTEN negatively regulates signaling through the FcγRIIA ITAM required for phagocytosis. To investigate downsteam events that PTEN may control, we examined the effects of PTEN overexpression on ITAM-induced conversion of guanosine diphosphate (GDP)–Rac to GTP-Rac (Figure 2). The expression of PTEN and not the catalytically dead mutant of PTEN abrogated the conversion of GDP-Rac to GTP-Rac under conditions of ITAM stimulation (Figure2A).
PTEN control of ITAM signaling.
(A) Phagocytosis of IgG-sensitized sRBCs by COS7 cells transfected with FcγRIIA receptor, Syk, PTEN, or a mutant (C124S) of PTEN. Phagocytosis of IgG-coated sRBCs by COS7 cells was quantified as described in “Study design.” Bars represent standard deviation of mean. (B) Western blot analysis for PTEN, AKT, and phospho-AKT in COS7 cells after transfection prior to sRBC stimulation. (C) Phagocytic index in COS7 cells transfected with equal amounts of FcγRIIA and EGFP-Syk and different amounts of wildtype PTEN plasmid DNA. (D) Rosette formation in COS7 cells transfected with different amounts of PTEN complementary DNA (cDNA). (E) Western blot analysis for PTEN expression in COS7 cells from panels C and D. (F) Flow cytometric analysis to determine levels of FcγRIIA and EGFP-Syk kinase expression in different transfected populations of COS7 cells shown in Figure 1A.
PTEN control of ITAM signaling.
(A) Phagocytosis of IgG-sensitized sRBCs by COS7 cells transfected with FcγRIIA receptor, Syk, PTEN, or a mutant (C124S) of PTEN. Phagocytosis of IgG-coated sRBCs by COS7 cells was quantified as described in “Study design.” Bars represent standard deviation of mean. (B) Western blot analysis for PTEN, AKT, and phospho-AKT in COS7 cells after transfection prior to sRBC stimulation. (C) Phagocytic index in COS7 cells transfected with equal amounts of FcγRIIA and EGFP-Syk and different amounts of wildtype PTEN plasmid DNA. (D) Rosette formation in COS7 cells transfected with different amounts of PTEN complementary DNA (cDNA). (E) Western blot analysis for PTEN expression in COS7 cells from panels C and D. (F) Flow cytometric analysis to determine levels of FcγRIIA and EGFP-Syk kinase expression in different transfected populations of COS7 cells shown in Figure 1A.
PTEN control of RAC activation.
(A) A recombinant PAK-CRIB domain–binding assay was used to determine levels of GTP-Rac 1 in cell lysates of COS7 cells transduced with FcγRIIA, Syk in the presence or absence of PTEN or mutant PTEN under conditions of FcγRIIA stimulation. The transfection condition for each group is shown above the lane. Lanes 1, 4, and 7 show no stimulation (NS); lanes 2, 5, 8, and 10, sRBC stimulation for 1 minute at 37° C; lanes 3, 6, and 9, stimulation of transfected COS7 cells for 3 minutes. Lane 10 shows vector control (VC), COS7 cells transfected with empty vectors pcDNA, pEGFP, and pRK5 and stimulated for 1 minute with sensitized sRBCs. Lane 11 shows positive control for GTP-Rac, a COS7 cell lysate incubated with GTPγS. (B) Anti-PTEN Western blot analysis of transfected COS7 cells analyzed for activation of Rac following FcγRIIA ligation with IgG-sensitized sRBCs. Lane 1 shows COS7 cells with no transfection; lane 2, transfection with all 3 empty vectors, pcDNA, pEGFP, and pRK5; lane 3, transfection with FcγRIIA, Syk, and pRK5; lane 4, transfection with FcγRIIA, Syk, and wildtype PTEN; and lane 5, transfection with FcγRIIA, Syk, and mutant PTEN (C124S).
PTEN control of RAC activation.
(A) A recombinant PAK-CRIB domain–binding assay was used to determine levels of GTP-Rac 1 in cell lysates of COS7 cells transduced with FcγRIIA, Syk in the presence or absence of PTEN or mutant PTEN under conditions of FcγRIIA stimulation. The transfection condition for each group is shown above the lane. Lanes 1, 4, and 7 show no stimulation (NS); lanes 2, 5, 8, and 10, sRBC stimulation for 1 minute at 37° C; lanes 3, 6, and 9, stimulation of transfected COS7 cells for 3 minutes. Lane 10 shows vector control (VC), COS7 cells transfected with empty vectors pcDNA, pEGFP, and pRK5 and stimulated for 1 minute with sensitized sRBCs. Lane 11 shows positive control for GTP-Rac, a COS7 cell lysate incubated with GTPγS. (B) Anti-PTEN Western blot analysis of transfected COS7 cells analyzed for activation of Rac following FcγRIIA ligation with IgG-sensitized sRBCs. Lane 1 shows COS7 cells with no transfection; lane 2, transfection with all 3 empty vectors, pcDNA, pEGFP, and pRK5; lane 3, transfection with FcγRIIA, Syk, and pRK5; lane 4, transfection with FcγRIIA, Syk, and wildtype PTEN; and lane 5, transfection with FcγRIIA, Syk, and mutant PTEN (C124S).
One possible interpretation of these data is that PTEN's regulation of Rac contributes to its control over phagocytosis. Using PTEN Western blot analysis, we confirmed that the PTEN and PTEN (C234S) mutant were expressed at equivalent levels in COS7 cells (Figure 2B). Interestingly, the effects of mutant PTEN to augment the phosphorylation of AKT seen in Figure 1C are correlated in Figure 2with a slight increase in Rac2 activation in response to FcγRIIA engagement. The fact that this augmented activation of AKT and Rac in the C124S mutant–transfected COS7 cells does not significantly augment the phagocytic index (Figure 1) is consistent with the notion that while Rac activation and the generation of inositol lipids phosphorylated at the D3 position of the inositol ring are essential for phagocytosis and Rac activation, they are not rate limiting in this COS7 cell FcγRIIA-dependent phagocytic response. These data provide the first genetic evidence that PtdIns(3,4,5)P3 is essential for Fcγ receptor–induced phagocytosis and the activation of Rac.
PTEN is a 55-kd, ubiquitously expressed, dual-specificity phosphatase involved in multiple signaling pathways to control cell division, apoptosis, and angiogenesis.11,15-17 Relatively little is known regarding the role of PTEN in hematopoietic functions or in the regulation of inflammation. PTEN first came into focus when it was identified by positional cloning as a tumor suppressor gene disrupted at locus 10q23.18-20 PTEN was subsequently defined as a lipid phosphatase with specificity for the D3 position of the inositol ring to control the phosphorylation state of PtdIns(3,4,5)P3.21 As far as hematopoiesis is concerned, one report suggests a role for PTEN in Fas signaling.22 There are no reports of a role for PTEN in the control of immunoreceptor or ITAM signaling in myeloid cells. There are a few reports indirectly linking PI-3 kinase to ITAM signaling with the use of inhibitors of PI-3 kinase, wortmannin, and LY294002.23-25 Wang et al26 reported an effect of PTEN on Erk kinase activation in T cells.
To directly investigate the role played by PTEN and PI-3 kinase in ITAM signal transduction, we overexpressed wildtype PTEN, along with FcγRIIA and Syk kinase, in a COS7 cell system. Overexpression of PTEN led to the complete abrogation of FcγRIIA/ITAM receptor–mediated phagocytosis of sensitized sRBCs. In parallel, heterologous expression of catalytically dead EGFP-Syk in COS7 cells significantly inhibited phagocytosis (not shown). The Src-specific protein tyrosine kinase inhibitor PP1 and wortmannin, a potent inhibitor of PI-3 kinase, were shown to inhibit the phagocytic phenomenon in a similar manner (data not shown). Expression of a cysteine (position 124) to serine catalytically dead “trap” mutant of the PTEN phosphatase led to an augmentation of phospho-AKT levels and increased activation of Rac but only to small increases in Fcγ receptor phagocytosis (ITAM signaling). These data support a role for PTEN in the negative regulation of phagocytosis. PTEN exerts an important control over the ITAM–protein tyrosine kinase signalsome to quench downstream ITAM signaling. It is possible that the pharmacologic control of PTEN may allow for immunomodulation of ITAM signaling for therapeutic gain.
In this study, we report that the overexpression of PTEN in a COS7 cell system leads to an abrogation of phagocytosis, an ITAM-dependent signaling event in vivo (Figure 1A-F). Our results clearly indicate an inhibitory role for PTEN in the regulation of IgG-mediated phagocytosis and provide the first evidence that links PTEN to the control of Rac in response to ITAM receptor engagement. Other investigators have previously reported a role for Rac in Fcγ receptor–mediated phagocytosis.27 More recently, a 5′ inositol lipid phosphatase, Src homology 2 domain–containing inositol 5′-phosphatase (SHIP), was implicated in the control of Fcγ and β2 integrin–mediated phagocytosis,28 suggesting that 5′ inositol phospholipids are also required for the phagocytic response. Currently it is clear that the inositol phospholipids are essential for such complex membrane/cytoskeletal actions as phagocytosis, but the mechanisms underlying the function of specific lipids phosphorylated in the D3, D4, or D5 position of the inositol ring remain an enigma. Our data establish an essential role for PtdIns(3,4,5)P3 in the control of phagocytosis and provide the first genetic evidence that the PI-3 kinase pathway is required for phagocytosis.
We thank Drs Mary C. Dinauer, Brian Seed, and Jack E. Dixon for providing cDNA constructs used in these experiments. We thank Drs Michael P. Myers and Nicholas K. Tonks for providing anti-PTEN antisera.
Supported by American Cancer Society grant RPG 98-244-01 to D.L.D. and National Institutes of Health grant CA37372 to R.L.G.
J.S.K. and X.P. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Donald L. Durden, Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 W Walnut St, Rm 468, Indianapolis, IN 46202; e-mail: ddurden@iupui.edu.