Abstract
To assess the safety and efficacy of nonmyeloablative allogeneic transplantation in patients with HIV infection, a clinical protocol was initiated in patients with refractory hematologic malignancies and concomitant HIV infection. The results from the first 2 patients are reported. The indications for transplantation were treatment-related acute myelogenous leukemia and primary refractory Hodgkin disease in patients 1 and 2, respectively. Only patient 1 received genetically modified cells. Both patients tolerated the procedure well with minimal toxicity, and complete remissions were achieved in both patients, but patient 2 died of relapsed Hodgkin disease 12 months after transplantation. Patient 1 continues in complete remission with undetectable HIV levels and rising CD4 counts, and with both the therapeutic and control gene transfer vectors remaining detectable at low levels more than 2 years after transplantation. These results suggest that nonmyeloablative allogeneic transplantation in the context of highly active antiretroviral therapy is feasible in patients with treatment-sensitive HIV infection.
Introduction
Allogeneic bone marrow transplantation (BMT) is a well-established approach with curative potential for a number of hematologic malignancies1; however, patients with concomitant HIV infection have generally been excluded from allogeneic BMT trials. Indeed, early allogeneic BMT trials as a primary treatment for HIV infection uniformly failed to control disease progression.2-4 The demonstration by Kolb et al5 that donor lymphocytes alone exert a powerful antileukemic effect, however, challenged the notion that high-dose chemoradiotherapy is necessary. This graft-versus-leukemia effect is most dramatically demonstrated among relapsed allogeneic BMT recipients receiving transplants for chronic myeloid leukemia; in these patients, a simple infusion of donor lymphocytes produces a complete and durable remission in the majority of treated patients6-8 and has led to intense interest and clinical activity investigating the role of nonmyeloablative transplant regimens for both malignant and nonmalignant disorders.9 10 We developed a clinical research trial to evaluate the use of cyclophosphamide/fludarabine–based conditioning in HIV- infected patients with an underlying malignancy, in whom an HIV-negative, HLA-matched sibling donor was available. Using this strategy, we also examined the possibility of efficient gene transfer of an HIV resistance vector carrying a dominant-negative mutant Rev at the level of the self-renewing stem cell.
Study design
The protocol was approved by the Institutional Scientific Review Board and the Institutional Review Board of the National Heart, Lung, and Blood Institute, the Institutional Biosafety Committee, the Center for Biologics Evaluation of the Food and Drug Administration, and the Office of Biotechnology Activities, and patients gave written informed consent. Eligibility included HIV positivity; an HLA-matched, HIV-negative sibling donor; and a hematologic malignancy meeting the standard indications for allogeneic transplantation in HIV-negative subjects.11 No restrictions were made regarding viral load or highly active antiretroviral therapy (HAART) sensitivity. Donor granulocyte colony-stimulating factor–mobilized peripheral blood was collected by apheresis on days 5 and 6. The day 5 apheresis product was CD34 enriched by means of a Nexell 300i (Nexell Therapeutics, Irvine, CA) automated immunomagnetic selection system for the recipient of genetically modified cells. Target CD34+cells were split equally for transduction with either (1) GCsapSL3rd3, containing a dominant-mutant or transdominant Rev (TdRev) engineered to inhibit viral replication through inhibition of wild-type Rev, a key HIV regulatory protein,12,13 or (2) a control vector encoding human GP91phox14 using previously established methods.15 Products not subjected to genetic modification, including the day 6 apheresis product for both patients, were cryopreserved without manipulation.
Patients were conditioned with a nonmyeloablative regimen16consisting of 60 mg/kg intravenous cyclophosphamide (IV) per day on days −7 and −6, followed by 25 mg/m2 fludarabine IV per day over 30 minutes on days −5 through −1. Cyclosporin was begun on day −4. HAART was discontinued 1 week prior to conditioning and was resumed when the patient was able to tolerate intake by mouth. Patients received antibiotic prophylaxis including acyclovir, bactrim, and norfloxacin as well as prophylaxis for mycobacterium aviumcomplex if indicated. Cytomegalovirus (CMV) reactivation was monitored by antigenemia and was treated preemptively with ganciclovir until day 100.17
In vitro transduction efficiency was estimated by polymerase chain reaction (PCR) of DNA isolated from individual clonogenic progenitors derived from the end of transduction for genetically modified cells as previously described.18 Lineage-specific assessment of donor chimerism was determined as previously described.19Lineage-specific engraftment by genetically modified cells was determined by real-time PCR (Perkin Elmer, Branchburg, NJ) as previously described20 with the use of vector-specific primers.
Results and discussion
Two patients have been treated to date. Their characteristics as well as the characteristics of the allograft are given in Table1. Both patients tolerated the procedure well and resumed HAART on day 0; they were discharged on day 9 and 11, respectively. In both patients, 100% donor chimerism was achieved by day 96 in both myeloid and lymphoid lineages. Lymphoid engraftment preceded myeloid engraftment and was similar to HIV-negative allogeneic transplant recipients conditioned with the same regimen.19 Both patients developed CMV antigenemia and were treated preemptively without evidence of disease. Restaging by bone marrow examination in patient 1 as well as computerized tomography and gallium scanning in patient 2 showed no evidence of disease in either. Grade II “acute” skin graft-versus-host disease (GVHD) developed in both beyond day 100, necessitating treatment with oral prednisone and continued cyclosporin. The observation of a clinical remission in concert with the development of acute GVHD in a patient with chemo-refractory Hodgkin is supportive of the concept of a graft-versus-Hodgkin effect.21-23 Control of GVHD in patient 2 was associated with relapsed Hodgkin disease in the retroperitoneum on day 180. Biopsy-proven central nervous system toxoplasmosis also developed and responded to treatment. However, despite withdrawal of immunosuppression, radiation therapy, interferon, and eventual administration of 1 × 107 donor lymphocytes per kilogram, the patient succumbed to progressive disease and died 12 months after transplantation. Patient 1 is currently being treated with cyclosporine and low-dose prednisone for limited chronic GVHD and remains in complete remission more than 2 years after transplantation.
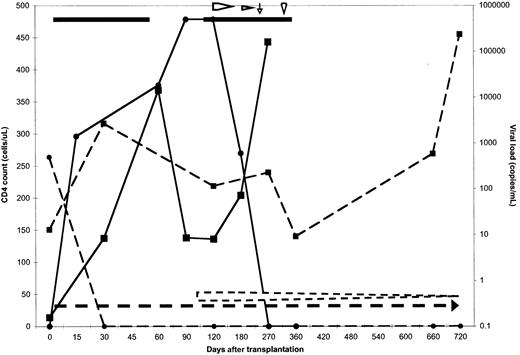
The effect of nonmyeloablative allogeneic transplantation on HIV status is depicted in Figure 1. HIV was undetectable in both patients during extended follow-up with the exception of a brief period in patient 2 during which HAART was interrupted owing to persistent nausea. An acute febrile illness of 2 weeks' duration, with headache, cerebrospinal fluid pleocytosis, and a negative evaluation for infectious causes coincided with a rise in the HIV burden to 106 copies per milliliter. This episode may have represented acute HIV infection of the allograft,24underscoring the importance of continued antiretroviral therapy following transplantation. Reinstitution of HAART was associated with resumed viral control. CD4 counts rose above pretransplantation levels in both patients after transplantation. In patient 1, the CD4 count has risen as high as 450 cells per microliter, and the pattern of recovery is similar to that seen in HIV-negative allogeneic transplant recipients who received the same conditioning regimen (unpublished observations, November 1999).
Effect of transplantation on peripheral blood CD4 count and viral load.
Data for patient 1 are represented by dashed lines and symbols, and for patient 2 by solid lines and symbols. The CD4 count in cells per microliter is represented by squares for both patients and is plotted along the left y-axis. The circles represent the viral load in copies per milliliter, which is plotted along the right y-axis. Both variables are plotted against the days after transplantation. The horizontal lines represent the days of HAART therapy, and the horizontal arrows represent the beginning of the immunosuppression taper. The vertical arrow shows the commencement of interferon, and the arrowhead, the day of donor lymphocyte infusion given to patient 2. Patient 2 died 12 months after transplantation. The CD4 count for both patients remained at or above what it was prior to transplantation. The viral load remained undetectable at the latest time point measured.
Effect of transplantation on peripheral blood CD4 count and viral load.
Data for patient 1 are represented by dashed lines and symbols, and for patient 2 by solid lines and symbols. The CD4 count in cells per microliter is represented by squares for both patients and is plotted along the left y-axis. The circles represent the viral load in copies per milliliter, which is plotted along the right y-axis. Both variables are plotted against the days after transplantation. The horizontal lines represent the days of HAART therapy, and the horizontal arrows represent the beginning of the immunosuppression taper. The vertical arrow shows the commencement of interferon, and the arrowhead, the day of donor lymphocyte infusion given to patient 2. Patient 2 died 12 months after transplantation. The CD4 count for both patients remained at or above what it was prior to transplantation. The viral load remained undetectable at the latest time point measured.
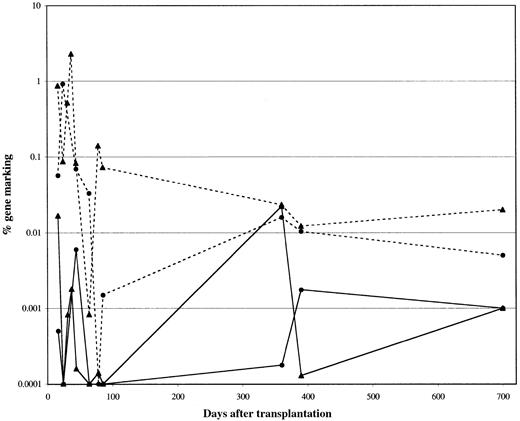
Recent improvements in gene transfer methodologies targeting the hematopoietic stem cell population have resulted in increased levels of genetically modified cells circulating after transplantation in relevant large animals, with levels of 5% to 10% or greater now achievable.25-27 While an equally high gene-transfer efficiency to the progenitor population was obtained as estimated by PCR, with 80% for the TdRev aliquot and 90% for the GP91phox aliquot, in vivo levels of circulating genetically modified cells were lower than those obtained in large animals. Overall marking levels of 0.01% were seen (Figure2). The differences in marking from the TdRev and control vector–transduced fractions did not suggest a survival or proliferative advantage to cells carrying the therapeutic gene; however, the absence of detectable HIV for the duration of the study suggests the absence of selective pressure as one potential explanation. Further, a threshold level of engraftment may be necessary to achieve a therapeutic effect. Finally, an effect by the genetically modified cells cannot be ruled out, as the recipient of genetically modified cells continues to show detectable marking from the TdRev vector with no detectable HIV. The low-level contribution toward hematopoeisis by genetically modified cells may also reflect competition by the unmanipulated graft. In murine and nonhuman primate competitive repopulation experiments, ex vivo cultured cells compete poorly against fresh cells, and cells cultured for longer periods compete poorly against cells cultured for shorter periods.25,28 29
Percentage of transduced cells measured in the peripheral blood.
The gene-marking levels in patient 1 were analyzed by PCR. The percentage of cells positive for the GP91phox transgene is represented by dashed lines, and the percentage positive for TdRev, by solid lines. Marking in myeloid cells is shown as circles and in T cells as triangles. Overall marking averaged about 0.01% for both vectors and in both lineages.
Percentage of transduced cells measured in the peripheral blood.
The gene-marking levels in patient 1 were analyzed by PCR. The percentage of cells positive for the GP91phox transgene is represented by dashed lines, and the percentage positive for TdRev, by solid lines. Marking in myeloid cells is shown as circles and in T cells as triangles. Overall marking averaged about 0.01% for both vectors and in both lineages.
A reluctance to consider allogeneic transplantation in HIV-infected patients may in part explain our slow accrual. However, these preliminary results suggest that allogeneic transplantation after nonmyeloablative conditioning represents a viable option for such patients. Further improvements in both gene transfer technology and allogeneic transplantation may allow application to patients with progressive AIDS without an underlying hematologic malignancy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John F. Tisdale, Bldg 10, Rm 9N116, 9000 Rockville Pike, National Institutes of Health, Bethesda, MD 20892; e-mail:johntis@intra.niddk.nih.gov.