Abstract
5-Lipoxygenase (5-LO) is the key enzyme in the biosynthesis of proinflammatory leukotrienes. This study showed that various forms of cell stress, such as chemical stress (sodium arsenite), osmotic stress, or heat shock lead to substantial formation of 5-LO products in freshly isolated human polymorphonuclear leukocytes (PMNLs), when exogenous arachidonic acid (10 μM) was present. In parallel, cell stress led to activation of p38 MAPK (mitogen-activated protein kinase) and mitogen-activated protein kinase-activated protein kinases (MAPKAPKs) kinases, which can phosphorylate 5-LO in vitro. Interestingly, arsenite also caused redistribution of 5-LO from the cytosol to the nuclear membrane. Only minor activation of extracellular signal-regulated kinases and c-jun NH2-terminal kinases was observed, implying that these MAPKs are less important for 5-LO product formation in stress-stimulated PMNLs. Stimulation of 5-LO product formation by Ca++-ionophore A23187 or thapsigargin depended on Ca++; almost no 5-LO product formation was observed in freshly isolated PMNLs when Ca++ was depleted by chelating agents. Also the response toN-formylmethionyl-leucyl-phenylalanine (fMLP) was clearly diminished, but some 5-LO product formation remained. In contrast, stress-induced product formation and translocation of 5-LO, as well as activation of p38 MAPK, occurred also after Ca++ depletion. Moreover, the p38 MAPK inhibitor SB203580 blocked stress-induced 5-LO product formation efficiently, whereas ionophore- or thapsigargin-induced formation of 5-LO products was less sensitive. These data show that cell stress can activate 5-LO in isolated PMNLs by a mechanism that does not involve Ca++ mobilization. This mechanism could function independently of Ca++-mediated 5-LO activation for stimulation of leukotriene biosynthesis under physiologic conditions as well as in inflammatory diseases.
Introduction
Metabolism of arachidonic acid (AA) by 5-lipoxygenase (5-LO) initializes the biosynthesis of biologically active leukotrienes (LTs), which are potent mediators in inflammatory and allergic reactions.1 Whereas LTB4 is considered as a potent chemotactic and chemokinetic agent for phagocytes, the cysteinyl-LTs C4, D4, and E4 cause smooth muscle contraction and increase vascular permeability. Formation of LTs in leukocytes depends on the availability of free AA from either endogenous pools liberated by activated cytosolic phospholipase A2 (cPLA2) or from transcellular migration of AA, released from surrounding cells like platelets or endothelial cells. cPLA2 is regulated by phosphorylation on serine residues and by Ca++, which binds to the C2 domain of the enzyme and induces its translocation and binding to the nuclear membrane (for a review, see Gijon and Leslie2).
In resting cells, depending on the cell type, 5-LO can occur in different soluble loci. On cell stimulation, 5-LO translocates to the nuclear membrane where it colocalizes with 5-lipoxygenase-activating protein (FLAP) and cPLA2, and an orchestrated interplay between these enzymes is of importance for efficient LT formation (for reviews, see Peters-Golden and Brock3 and Rådmark4). The activation of cellular 5-LO can be induced by ligation of specific receptors for naturally occurring soluble and particulate stimuli such asN-formyl-methionyl-leucyl-phenylalanine (fMLP), platelet-activating factor (PAF), cytokines, immune complexes, and microbes, but also by Ca++-mobilizing agents like ionophores and thapsigargin, as well as by urate or phosphate crystals.5-10 Ca++ is important for cellular 5-LO activation (see Hammarberg and Rådmark11 and references therein), and it stimulates translocation and binding of 5-LO to the nuclear membrane.3 Ca++ activates 5-LO also in vitro,11 and it was recently found that the putative N-terminal C2 like domain of 5-LO binds Ca++ and is important for translocation to the nuclear membrane.12 13
Among the 3 major families of the mitogen-activated protein kinases (MAPKs), the p38 MAPKs and c-jun-NH2-terminal kinases (JNKs) are activated by cellular stress, such as UV light, heat shock, hypertonicity, sodium arsenite (SA), and inflammatory cytokines, whereas the extracellular signal-regulated kinases (ERKs) are mainly activated by mitogens.14,15 Particularly, p38 MAPKs have been implicated in a variety of proinflammatory events, like release of tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1) and IL-8, superoxide formation, adhesion, degranulation, and chemotaxis (for a review, see Herlaar and Brown16). Thus, p38 MAPK is an important signaling kinase in inflammation.
The relevance of phosphorylation for cellular 5-LO activation was implicated some time ago.17 A phosphorylated form of 5-LO was demonstrated in ionophore-treated HL-60 cells, and tyrosine kinase inhibitors interfered with 5-LO activity in granulocytic cells.18 Also ERK activity seemed to be involved in 5-LO translocation and activity in ionophore-stimulated HL-60 cells.19 Recently, we found that p38 MAPK-regulated MAPK-activated protein kinases (MAPKAPKs; MKs) from stimulated PMNLs or Mono Mac 6 (MM6) cells can phosphorylate 5-LO in vitro, and we suggested that such a phosphorylation event could increase cellular 5-LO activity, because activation of p38 MAPK by SA enhanced PAF-induced 5-LO product formation in PMNLs.20
In this study, we demonstrate that several stress stimuli (chemical stress, osmotic shock, heat shock) induced 5-LO product formation in freshly isolated PMNLs, in parallel with activation of p38 MAPK. Stimulation by cell stress did not increase intracellular Ca++ levels and the resulting 5-LO product formation was unaffected by Ca++ chelation, indicating an alternative Ca++-independent pathway for 5-LO activation.
Materials and methods
Materials
Human recombinant 5-LO was expressed and purified as described.11 [γ-32P]-adenosine triphosphate (ATP; 110 TBq/mmol) was purchased from Amersham Pharmacia Biotech (Freiburg, Germany). ZM230487 was a gift from Dr R. M. McMillan (Zeneca Pharmaceuticals, Macclesfield, United Kingdom). Ca++-ionophore A23187, thapsigargin, SA, fMLP, and AA were from Sigma (Deisenhofen, Germany); high-performance liquid chromatography (HPLC) solvents were from Merck (Darmstadt, Germany). BAPTA/am, Fura-2/am, and SB203580 were from Calbiochem (Bad Soden, Germany).
Cells
Human PMNLs were freshly isolated from leukocyte concentrates obtained at St Markus Hospital (Frankfurt, Germany). In brief, venous blood was taken from healthy adult donors and subjected to centrifugation at 4000g for 20 minutes at 20°C for preparation of leukocyte concentrates. PMNLs were immediately isolated by dextran sedimentation and underwent centrifugation on Nycoprep cushions (PAA Laboratories, Linz, Austria) with hypotonic lysis of erythrocytes as described previously.21 PMNLs (5 × 106 cells/mL; purity >96%-97%) were finally resuspended in phosphate-buffered saline (PBS) plus 1 mg/mL glucose (PG buffer), or alternatively in PBS plus 1 mg/mL glucose and 1 mM CaCl2 (PGC buffer) as indicated.
Determination of 5-LO product formation
For assays of intact cells in the presence of Ca++, 5 × 106 freshly isolated PMNLs were finally resuspended in 1 mL PGC buffer. When 5-LO product formation was assayed in the absence of Ca++, 5 × 106PMNLs were finally resuspended in 1 mL PG buffer and 1 mM EDTA or 30 μM BAPTA/am (or both) was added. After preincubation with the indicated stimuli at 37°C, the reaction was started by addition of exogenous AA at the indicated concentrations. Ionophore, thapsigargin, or fMLP was added simultaneously with exogenous AA. After 5 minutes at 37°C, the reaction was stopped with 1 mL methanol and 30 μL 1 N HCl, and 200 ng prostaglandin B1 and 500 μL PBS were added. Formed 5-LO metabolites were extracted and analyzed by HPLC as described.22 5-LO product formation is expressed as nanograms of 5-LO products per 106 cells, which includes LTB4 and its all-trans isomers, 5(S),12(S)-di-hydroxy-6,10-trans-8,14-cis-eicosatetraenoic acid [5(S),12(S)-DiHETE], and 5(S)-hydro(peroxy)-6-trans-8,11,14-cis-eicosatetraenoic acid [5-H(p)ETE]. Cysteinyl LTs (LTC4, D4, and E4) were not detected and oxidation products of LTB4 were not determined.
Measurement of intracellular calcium levels
Freshly isolated PMNLs (1 × 107 in 1 mL PGC buffer) were incubated with 2 μM Fura-2/am for 30 minutes at 37°C. Cells were washed, resuspended in 1 mL PGC buffer, and transferred into a thermally controlled (37°C) fluorimeter cuvette in a spectrofluorometer (Aminco-Bowman series 2) with continuous stirring. The fluorescence emission at 510 nm was measured after excitation at 340 and 380 nm, respectively. Intracellular Ca++ levels were calculated according to the method of Grynkiewicz et al.23 Fmax (maximal fluorescence) was obtained by lysing the cells with 1% Triton-X 100 and Fmin (minimal fluorescence) by chelating Ca++ with 10 mM EDTA.
Subcellular fractionation by detergent lysis
Subcellular localization of 5-LO was investigated as described previously.24 In brief, freshly isolated PMNLs (3 × 107) were resuspended in 1 mL PGC buffer or in 1 mL PG buffer containing 1 mM EDTA or 30 μM BAPTA/am (or both) and preincubated at 37°C for 10 minutes. After addition of the stimuli, samples were incubated for the indicated times and chilled on ice. Nuclear and nonnuclear fractions were obtained after cell lysis by 0.1% NP-40. Aliquots of nuclear and nonnuclear fractions were immediately mixed with the same volume of 2 times sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) sample loading buffer (SDS-b), heated for 6 minutes at 95°C, and analyzed for 5-LO protein by SDS-PAGE and immunoblotting.
MAPK activation
Freshly isolated PMNLs (5 × 106) were resuspended in PGC buffer or in PG buffer containing 1 mM EDTA or 30 μM BAPTA/am (or both); final volume was 100 μL. After addition of the indicated stimuli, samples were incubated at 37°C and the reaction was stopped after the indicated times by addition of 100 μL ice-cold SDS-b, vortexed, and heated for 6 minutes at 95°C. Total cell lysates (20 μL) were analyzed for phosphorylated MAPK by SDS-PAGE and immunoblotting or by in-gel kinase assay.
SDS-PAGE and immunoblotting
Total cell lysates (20 μL) and aliquots of nuclear fractions (25 μL) were mixed with 4 μL glycerol/0.1% bromphenol blue (1:1, vol/vol) and analyzed by SDS-PAGE using a Mini Protean system (Bio-Rad, Munich, Germany) on a 10% gel. After electroblot to nitrocellulose membrane (Amersham Pharmacia), membranes were blocked with 5% nonfat dry milk in 50 mM Tris/HCl, pH 7.4, and 100 mM NaCl (Tris[hydroxymethyl]aminomethane-buffered saline; TBS) for 1 hour at room temperature. Membranes were washed and then incubated with primary antibody overnight at 4°C. Anti–5-LO antiserum (AK7, 1551) was affinity purified on a 5-LO column; antibodies against p38 MAPK were from Santa Cruz, Heidelberg, Germany, phosphospecific antibodies recognizing p38 MAPK (Thr180/Tyr182), ERK 1/2 (Thr202/Tyr204), and JNK (Thr183/Tyr185) were obtained from New England Biolabs (Frankfurt, Germany) and used as 1:2000 dilution. The membranes were washed with TBS and incubated with 1:1000 dilution of alkaline phosphatase (AP)–conjugated IgGs (Sigma, Deisenhofen, Germany) for 2 hours at room temperature. After washing with TBS and TBS plus 0.1% NP-40, proteins were visualized with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate (Sigma) in detection buffer (100 mM Tris/HCl, pH 9.5, 100 mM NaCl, 5 mM MgCl2).
In-gel kinase assay
Total cell lysates of PMNLs corresponding to 0.5 × 106 cells were analyzed for 5-LO kinase activity by in-gel kinase assay using purified 5-LO (0.2 mg/mL) as substrate as described.20 Phosphorylated proteins were visualized using a Fuji Phosphorimager FLA-3000.
Results
SA induces 5-LO product formation and translocation in isolated PMNLs, in parallel with activation of p38 MAPK and downstream 5-LO kinases
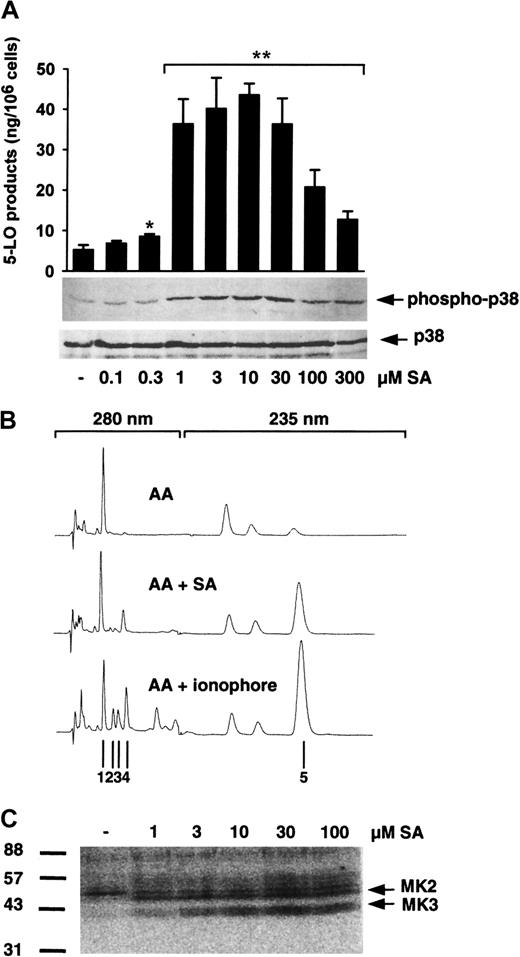
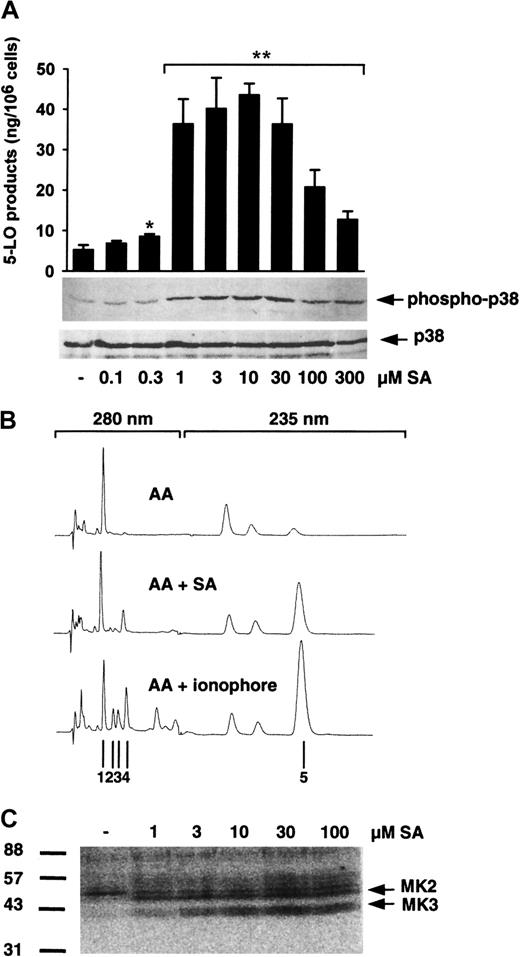
In our previous report, SA activated p38 MAPK-regulated MKs (which phosphorylate 5-LO in vitro) in isolated PMNLs and enhanced 5-LO product generation in PMNLs stimulated with PAF and AA.20To examine whether SA alone is sufficient to activate 5-LO, freshly isolated PMNLs were treated with varying concentrations of SA, prior to addition of 40 μM AA as exogenous substrate (to circumvent the need for phospholipase activity), and 5-LO product formation was determined. Also MAPK activation was assayed. As can be seen in Figure1A, preincubation of PMNLs with SA for 3 minutes caused a dose-dependent induction of 5-LO product formation versus control with AA alone, peaking at 10 μM with a 9-fold up-regulation and a decline at higher SA concentrations. 5-LO product formation induced by SA was qualitatively similar to that induced by the standard stimulus Ca++-ionophore A23187; thus, SA caused no appreciable change in the ratio of LTA4metabolites to 5-H(p)ETE. As observed before, the high activity obtained with ionophore plus exogenous AA led to the appearance of the nonenzymatically formed all-trans isomers of LTB4. These all-trans isomers were relatively less abundant after stimulation of PMNLs with SA plus AA (Figure 1B). In parallel, we found a similar dose response for activation of p38 MAPK after 3 minutes of cell stimulation, as determined by immunoblotting of p38 MAPK with a specific antibody recognizing the active dually phosphorylated form of p38 MAPK (Figure 1A). In contrast, phosphorylation (activation) of ERKs and JNKs was practically undetectable after SA treatment (1-100 μM), and prolonged preincubation up to 30 minutes (10 μM SA) did not increase phosphorylation of these kinases (data not shown). Finally, stimulation of PMNLs by SA (as low as 1 μM) led to activation of p38 MAPK-regulated MKs (probably MK2 and MK3, compare with results from Werz et al20), which phosphorylated 5-LO in in-gel kinase assays (Figure 1C).
SA induces 5-LO product formation and p38 MAPK activation.
(A) To determine 5-LO product formation, freshly isolated PMNLs (5 × 106 in 1 mL PGC buffer) were stimulated with SA for 3 minutes at 37°C. After addition of 40 μM AA the samples were incubated for another 5 minutes and 5-LO products were determined by HPLC. Results are given as mean + SE (n = 3-4). Student t test; *P < .05; **P < .01. To determine activation of p38 MAPK, freshly isolated PMNLs (5 × 106 in 100 μL PGC buffer) were stimulated with SA for 3 minutes at 37°C. Incubations were terminated by addition of the same volume of SDS-b. Samples were electrophoresed and analyzed for dually phosphorylated p38 MAPK by immunoblotting (upper panel); equal amounts of protein were evaluated with anti-p38 MAPK antibodies (lower panel). Results are representative of at least 3 separate experiments. (B) Typical HPLC chromatograms of 5-LO products extracted from PMNLs. Cells were stimulated for 5 minutes at 37°C with 40 μM AA alone (top), 10 μM SA for 3 minutes and subsequent addition of 40 μM AA (middle), or with 2.5 μM ionophore plus 40 μM AA (bottom); 1, prostaglandin B2; 2,3,trans-isomers of LTB4; and 4, LTB4were recorded at 280 nM, and 5, 5-H(p)ETE at 235 nM. (C) Activation of p38 MAPK-regulated 5-LO kinases. Samples prepared for determination of p38 MAPK activation from above were analyzed by in-gel kinase assay as described. Arrows indicate the positions of MK2 and MK3. Results are representative of at least 3 separate experiments.
SA induces 5-LO product formation and p38 MAPK activation.
(A) To determine 5-LO product formation, freshly isolated PMNLs (5 × 106 in 1 mL PGC buffer) were stimulated with SA for 3 minutes at 37°C. After addition of 40 μM AA the samples were incubated for another 5 minutes and 5-LO products were determined by HPLC. Results are given as mean + SE (n = 3-4). Student t test; *P < .05; **P < .01. To determine activation of p38 MAPK, freshly isolated PMNLs (5 × 106 in 100 μL PGC buffer) were stimulated with SA for 3 minutes at 37°C. Incubations were terminated by addition of the same volume of SDS-b. Samples were electrophoresed and analyzed for dually phosphorylated p38 MAPK by immunoblotting (upper panel); equal amounts of protein were evaluated with anti-p38 MAPK antibodies (lower panel). Results are representative of at least 3 separate experiments. (B) Typical HPLC chromatograms of 5-LO products extracted from PMNLs. Cells were stimulated for 5 minutes at 37°C with 40 μM AA alone (top), 10 μM SA for 3 minutes and subsequent addition of 40 μM AA (middle), or with 2.5 μM ionophore plus 40 μM AA (bottom); 1, prostaglandin B2; 2,3,trans-isomers of LTB4; and 4, LTB4were recorded at 280 nM, and 5, 5-H(p)ETE at 235 nM. (C) Activation of p38 MAPK-regulated 5-LO kinases. Samples prepared for determination of p38 MAPK activation from above were analyzed by in-gel kinase assay as described. Arrows indicate the positions of MK2 and MK3. Results are representative of at least 3 separate experiments.
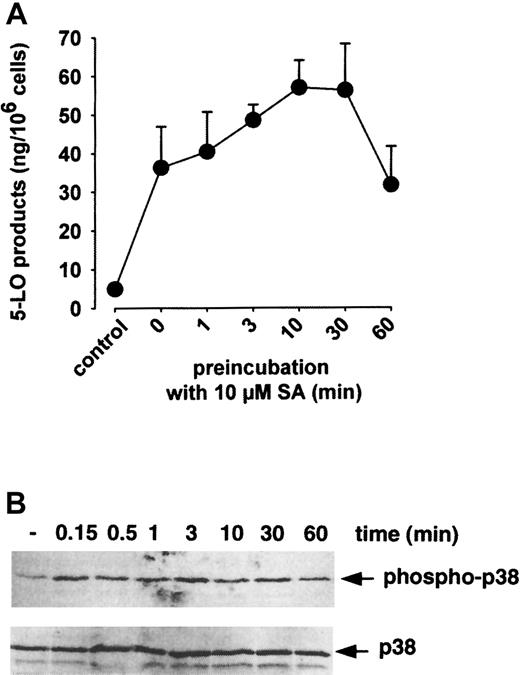
In Figure 2, the time courses of SA-induced activation of p38 MAPK and induction of 5-LO product formation are shown. p38 MAPK was activated within seconds on addition of 10 μM SA (Figure 2B), and simultaneous addition of SA (no preincubation) together with AA resulted in substantial 5-LO product formation (Figure 2A). Also, p38 MAPK-regulated 5-LO kinases became substantially activated within less than 0.5 minute (data not shown). After long preincubations with SA (up to 1 hour) p38 MAPK was still active, and the increased capacity for 5-LO product formation remained. The results indicate that SA stimulated the formation of 5-LO products in isolated PMNLs and that this strongly correlated with activation of p38 MAPK and downstream kinases, which can phosphorylate 5-LO.
Time course of SA-induced 5-LO product formation and p38 MAPK activation.
(A) 5-LO product formation. Freshly isolated PMNLs (5 × 106 in 1 mL PGC buffer) were stimulated with 10 μM SA at 37°C. After the indicated times, 40 μM AA was added, the samples were incubated for another 5 minutes, and 5-LO products were determined by HPLC. Results are given as mean + SE (n = 3). (B) Activation of p38 MAPK. Freshly isolated PMNLs (5 × 106 in 100 μL PGC buffer) were stimulated with 10 μM SA at 37°C. After the indicated times, incubations were terminated by addition of SDS-b and samples were analyzed for dually phosphorylated p38 MAPK by immunoblotting (upper panel); equal amounts of protein were evaluated with anti-p38 MAPK antibodies (lower panel). Results are representative of at least 3 separate experiments.
Time course of SA-induced 5-LO product formation and p38 MAPK activation.
(A) 5-LO product formation. Freshly isolated PMNLs (5 × 106 in 1 mL PGC buffer) were stimulated with 10 μM SA at 37°C. After the indicated times, 40 μM AA was added, the samples were incubated for another 5 minutes, and 5-LO products were determined by HPLC. Results are given as mean + SE (n = 3). (B) Activation of p38 MAPK. Freshly isolated PMNLs (5 × 106 in 100 μL PGC buffer) were stimulated with 10 μM SA at 37°C. After the indicated times, incubations were terminated by addition of SDS-b and samples were analyzed for dually phosphorylated p38 MAPK by immunoblotting (upper panel); equal amounts of protein were evaluated with anti-p38 MAPK antibodies (lower panel). Results are representative of at least 3 separate experiments.
Because LT formation in PMNLs is connected to translocation of 5-LO from the cytosol to the nuclear membrane, we determined whether treatment of PMNLs with SA leads to 5-LO redistribution. Figure3 shows that exposure of isolated PMNLs to SA for 5 minutes led to dose-dependent enrichment of 5-LO protein in the nuclear fraction of detergent-lysed (0.1% NP-40) cells. Compared to activation of p38 MAPK and 5-LO product formation, translocation of 5-LO required higher concentrations of SA, starting at 10 μM and with maximum effect at 100 μM SA. Association of 5-LO with the nucleus was a rapid process; it appeared within 3 minutes after SA addition, as observed after cell challenge with ionophore A23187 (O.W., unpublished observation, October 1998) and remained for longer than 30 minutes (Figure 3). When nuclei were further fractionated by sonification and ultracentrifugation, 5-LO was found in the particulate (membrane) fraction of the nucleus after treatment with SA (data not shown).
SA induces rapid translocation of 5-LO to the nucleus.
Freshly isolated PMNLs (3 × 107 in 1 mL PGC buffer) were stimulated with varying concentrations of SA or 2.5 μM ionophore A23187 at 37°C for the indicated times. After cell fractionation by detergent lysis (0.1% NP-40), 5-LO was determined in nuclear fractions by immunoblotting. Results are representative of at least 3 separate experiments.
SA induces rapid translocation of 5-LO to the nucleus.
Freshly isolated PMNLs (3 × 107 in 1 mL PGC buffer) were stimulated with varying concentrations of SA or 2.5 μM ionophore A23187 at 37°C for the indicated times. After cell fractionation by detergent lysis (0.1% NP-40), 5-LO was determined in nuclear fractions by immunoblotting. Results are representative of at least 3 separate experiments.
Heat shock and osmotic stress lead to 5-LO product formation in PMNLs
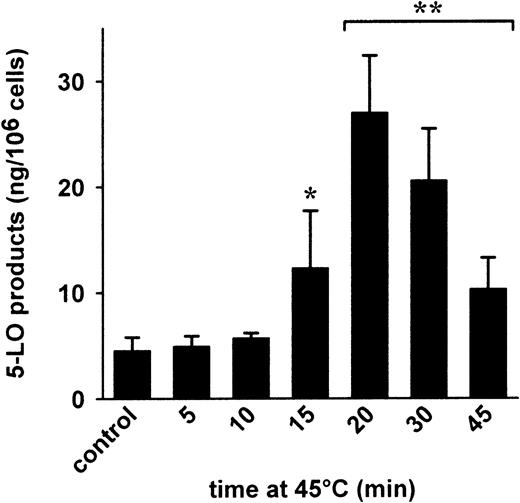
Heat shock (exposure of cells to elevated temperature up to 45°C for 15-45 minutes) activated p38 MAPK and resulted in phosphorylation of heat shock protein 27 by the 5-LO kinase MK2.25 To determine whether heat shock can activate 5-LO, PMNLs were incubated at 42°C or 45°C for various periods and transferred to normal incubation temperature (37°C); 40 μM exogenous AA was added and 5-LO product formation was determined. An about 1.5- to 2-fold enhancement of 5-LO product formation was detectable in PMNLs exposed to 42°C for 15 to 45 minutes (not shown). As shown in Figure4, the formation of 5-LO products increased significantly when PMNLs were subjected to 45°C for at least 10 minutes, peaking at 20 minutes with a 6-fold enhancement versus control. Similarly, a 2- to 3-fold increase of 5-LO product formation was observed when cells were exposed to UV light for 1 to 10 minutes (causes p38 MAPK activation26) prior to addition of substrate (not shown).
Heat shock induces formation of 5-LO products.
Freshly isolated PMNLs (5 × 106 in 1 mL PGC buffer) were preincubated at 45°C for the indicated times. Then, samples were brought to 37°C and 40 μM AA was added. After another 5 minutes at 37°C, 5-LO products were determined by HPLC. Results are given as mean + SE (n = 3-5). Student t test; *P < .05; **P < .01.
Heat shock induces formation of 5-LO products.
Freshly isolated PMNLs (5 × 106 in 1 mL PGC buffer) were preincubated at 45°C for the indicated times. Then, samples were brought to 37°C and 40 μM AA was added. After another 5 minutes at 37°C, 5-LO products were determined by HPLC. Results are given as mean + SE (n = 3-5). Student t test; *P < .05; **P < .01.
Hypertonicity induced p38 MAPK signaling in PMNLs,27,28 and hypertonicity enhanced 5-LO activity in a human B-cell line in a p38 MAPK-dependent manner.29 Thus, we tested whether hypertonicity could activate 5-LO in PMNLs. As can be seen in Figure 5, addition of NaCl to PMNLs resuspended in normotonic PGC led to dose-dependent 5-LO activation (up to 12-fold) in parallel with p38 MAPK activation. JNKs and ERKs were almost not at all phosphorylated (activated) under these conditions (data not shown). These effects were not specific for NaCl, because hypertonicity due to sucrose (0.2-0.8 M) or Na2SO4 (0.2 M) gave comparable 5-LO product formation, accompanied by p38 MAPK activation. Exposure to hypotonic solutions or cell-permeable urea (up to 0.8 M) caused no or only marginal activation of 5-LO or p38 MAPK (data not shown). This indicates that cell shrinkage, rather than cell expansion or osmotic imbalance, induced 5-LO activation. As found for SA, NaCl-induced activation of 5-LO and p38 MAPK was rapid and remained for longer than 30 minutes (not shown).
Hypertonicity induces 5-LO product formation and p38 MAPK activation.
(A) To determine 5-LO product formation, NaCl was added to freshly isolated PMNLs (5 × 106) in PGC buffer to a final volume of 1 mL, 3 minutes prior to stimulation with 40 μM AA. After 5 minutes at 37°C, 5-LO product formation was determined. Results are given as mean + SE (n = 3). Studentt test; **P < .01. (B) To determine the activation of p38 MAPK, NaCl was added to freshly isolated PMNLs (5 × 106) in PGC buffer to a final volume of 100 μL. After 3 minutes at 37°C, incubations were terminated and samples were analyzed for dually phosphorylated p38 MAPK by immunoblotting (upper panel); equal amounts of protein were evaluated with anti-p38 MAPK antibodies (lower panel). Results are representative of at least 3 separate experiments.
Hypertonicity induces 5-LO product formation and p38 MAPK activation.
(A) To determine 5-LO product formation, NaCl was added to freshly isolated PMNLs (5 × 106) in PGC buffer to a final volume of 1 mL, 3 minutes prior to stimulation with 40 μM AA. After 5 minutes at 37°C, 5-LO product formation was determined. Results are given as mean + SE (n = 3). Studentt test; **P < .01. (B) To determine the activation of p38 MAPK, NaCl was added to freshly isolated PMNLs (5 × 106) in PGC buffer to a final volume of 100 μL. After 3 minutes at 37°C, incubations were terminated and samples were analyzed for dually phosphorylated p38 MAPK by immunoblotting (upper panel); equal amounts of protein were evaluated with anti-p38 MAPK antibodies (lower panel). Results are representative of at least 3 separate experiments.
Heat shock or hypertonicity could possibly destroy PMNLs. However, experiments with ZM230487, a selective nonredox 5-LO inhibitor that blocks enzyme activity at low concentrations (<1 μM) only in intact cells,30 revealed that cell stress-induced 5-LO product formation occurred in intact cells and was not related to crude 5-LO activity after cell destruction (data not shown).
Requirement of exogenous AA for stress-induced 5-LO activation
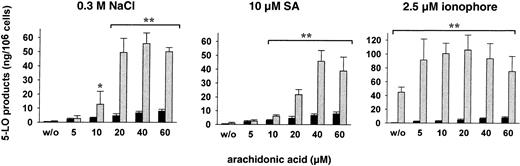
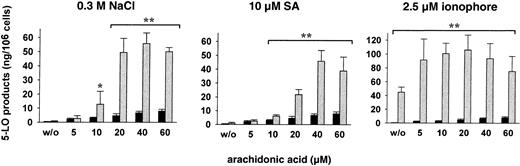
When isolated PMNLs were treated with SA or NaCl alone (in absence of exogenous AA), 5-LO product formation was practically not detectable (Figure 6). PMNLs, stimulated with AA alone at 5 to 60 μM, showed low product formation (Figure 6). Pretreatment of cells with 10 μM SA or 0.3 M NaCl for 3 minutes and subsequent addition of less than 10 μM AA gave no significant change in 5-LO product formation compared to controls. However, pretreatment with SA or NaCl caused a substantial increase in 5-LO products at 10 to 20 μM AA and maximal product formation was found at about 40 μM. A distinct pattern was found when PMNLs were stimulated with ionophore. In the absence of exogenous AA, ionophore by itself caused strong 5-LO product formation, which was further increased by simultaneous addition of AA with maximal activation at 20 μM, but declined continuously with higher concentrations of AA (Figure 6). Preincubation with SA or NaCl failed to increase the formation of 5-LO products on subsequent stimulation with physiologic agonists (PAF, fMLP) in the absence of exogenous AA (not shown).
Dose response of exogenous AA on stress-induced 5-LO product formation.
Freshly isolated PMNLs (5 × 106 in 1 mL PGC buffer) were either stimulated with 2.5 μM ionophore A23187 plus the indicated concentrations of AA, or preincubated with 0.3 M NaCl or with 10 μM SA for 3 minutes at 37°C, prior to addition of AA at the indicated concentrations. The reaction was terminated 5 minutes after addition of AA and 5-LO products were determined. Results are given as mean + SE (n = 3-4). Student t test; *P < .05; **P < .01. ░, AA + stimulus; ▪, AA alone.
Dose response of exogenous AA on stress-induced 5-LO product formation.
Freshly isolated PMNLs (5 × 106 in 1 mL PGC buffer) were either stimulated with 2.5 μM ionophore A23187 plus the indicated concentrations of AA, or preincubated with 0.3 M NaCl or with 10 μM SA for 3 minutes at 37°C, prior to addition of AA at the indicated concentrations. The reaction was terminated 5 minutes after addition of AA and 5-LO products were determined. Results are given as mean + SE (n = 3-4). Student t test; *P < .05; **P < .01. ░, AA + stimulus; ▪, AA alone.
Stimulus-dependent requirement of calcium for activation of 5-LO and p38 MAPK
The effects of established stimuli for 5-LO activation (ionophore A23187, thapsigargin, fMLP, and PAF) were compared with the stress inducers SA, NaCl, and heat shock. Exogenous AA (40 μM) was added to all incubations. As seen in Table 1, ionophore caused the most prominent formation of 5-LO products (2-3 times the effect of stress stimuli), whereas thapsigargin, fMLP, and PAF were less efficient than SA, NaCl, and heat shock. For the different stimuli, no appreciable differences were observed regarding the ratio of LTA4 metabolites to 5-H(p)ETE.
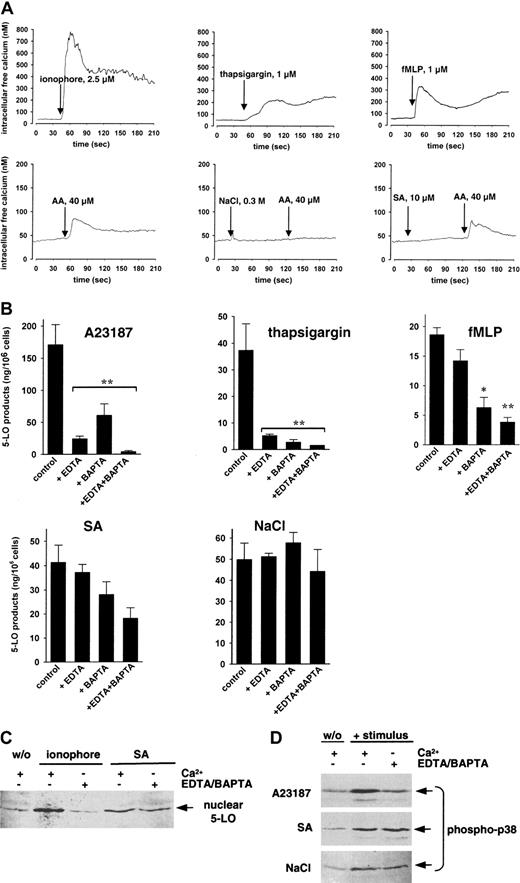
Increased intracellular Ca++ is assumed to activate cellular 5-LO for LT synthesis after stimulation of PMNLs by ionophore and thapsigargin, but also by natural ligands, such as PAF or fMLP.4 We measured Ca++ influx in freshly isolated PMNLs using the Fura-2 method. The intracellular Ca++ concentration in PMNLs not exposed to agonists was 40 to 50 nM. Treatment of PMNLs with ionophore (2.5 μM), thapsigargin (1 μM), or fMLP (1 μM) caused a substantial rise in intracellular Ca++ (Figure 7A). As observed by others,31,32 addition of 40 μM exogenous AA caused a moderate and more transient elevation of intracellular Ca++from 40 to 50 nM to 70 to 80 nM. In contrast, SA (10 μM) or NaCl (0.3 M) failed to elevate cellular Ca++ levels by themselves, in agreement with previous reports,27,33 34 and SA or NaCl did not enhance the subsequent Ca++ influx induced by 40 μM AA (Figure 7A). Actually, NaCl rather prevented the AA-induced Ca++ response. Thus, increased intracellular Ca++ is apparently not the intracellular mediator for 5-LO activation induced by stress stimuli.
Role of calcium in 5-LO product formation, 5-LO translocation, and p38 MAPK activation.
(A) Ca++ influx. To Fura-2–loaded PMNLs (1 × 107/ mL PGC buffer) the indicated stimuli were added and the fluorescence was measured. Intracellular free Ca++ was calculated as described. The monitored curves show one typical experiment of 3 to 4. (B) 5-LO product formation. CaCl2 (1 mM), EDTA (1 mM), and BAPTA/am (30 μM) were added to 5 × 106 freshly isolated PMNLs in PG buffer as indicated. After 10 minutes at 37°C, SA (10 μM) and NaCl (0.3 M) were added 3 minutes prior to addition of 40 μM AA. Ionophore A23187 (2.5 μM), thapsigargin (1 μM), or fMLP (1 μM) was added simultaneously with AA. After another 5 minutes, 5-LO products were determined. Results are given as mean + SE (n = 3). Student t test; *P < .05; **P < .01. (C) 5-LO translocation. Freshly isolated PMNLs (3 × 107 in 1 mL PG buffer) were preincubated for 10 minutes at 37°C with 1 mM CaCl2 or with 1 mM EDTA and 30 μM BAPTA/am as indicated. Then, 100 μM SA or 2.5 μM ionophore was added to the samples and incubated for another 5 minutes at 37°C. 5-LO was detected in nuclear fractions by immunoblotting after subcellular fractionation. Similar results were obtained in 2 additional independent experiments. (D) Activation of p38 MAPK. Freshly isolated PMNL (5 × 106 in 100 μL PG buffer) were treated with 1 mM CaCl2 or with 1 mM EDTA and 30 μM BAPTA/am as indicated for 10 minutes at 37°C. Ionophore (2.5 μM), SA (10 μM), or NaCl (0.3 M) was then added and after 3 minutes at 37°C samples were analyzed for dually phosphorylated p38 MAPK. Results are representative of at least 3 separate experiments.
Role of calcium in 5-LO product formation, 5-LO translocation, and p38 MAPK activation.
(A) Ca++ influx. To Fura-2–loaded PMNLs (1 × 107/ mL PGC buffer) the indicated stimuli were added and the fluorescence was measured. Intracellular free Ca++ was calculated as described. The monitored curves show one typical experiment of 3 to 4. (B) 5-LO product formation. CaCl2 (1 mM), EDTA (1 mM), and BAPTA/am (30 μM) were added to 5 × 106 freshly isolated PMNLs in PG buffer as indicated. After 10 minutes at 37°C, SA (10 μM) and NaCl (0.3 M) were added 3 minutes prior to addition of 40 μM AA. Ionophore A23187 (2.5 μM), thapsigargin (1 μM), or fMLP (1 μM) was added simultaneously with AA. After another 5 minutes, 5-LO products were determined. Results are given as mean + SE (n = 3). Student t test; *P < .05; **P < .01. (C) 5-LO translocation. Freshly isolated PMNLs (3 × 107 in 1 mL PG buffer) were preincubated for 10 minutes at 37°C with 1 mM CaCl2 or with 1 mM EDTA and 30 μM BAPTA/am as indicated. Then, 100 μM SA or 2.5 μM ionophore was added to the samples and incubated for another 5 minutes at 37°C. 5-LO was detected in nuclear fractions by immunoblotting after subcellular fractionation. Similar results were obtained in 2 additional independent experiments. (D) Activation of p38 MAPK. Freshly isolated PMNL (5 × 106 in 100 μL PG buffer) were treated with 1 mM CaCl2 or with 1 mM EDTA and 30 μM BAPTA/am as indicated for 10 minutes at 37°C. Ionophore (2.5 μM), SA (10 μM), or NaCl (0.3 M) was then added and after 3 minutes at 37°C samples were analyzed for dually phosphorylated p38 MAPK. Results are representative of at least 3 separate experiments.
We addressed whether Ca++ was required for cell stress-induced 5-LO activation. Extracellular Ca++ was removed by 1 mM EDTA and for depletion of intracellular Ca++ cell-permeable BAPTA/am (30 μM) was added to the incubations. As found by others,35 chelation of Ca++ by EDTA or BAPTA/am dramatically reduced 5-LO product formation in PMNLs stimulated with ionophore or thapsigargin (Figure 7B), and depletion of intracellular Ca++ by BAPTA/am clearly suppressed 5-LO product formation in PMNLs challenged with fMLP (Figure 7B) or with PAF (P < .01; not shown). In contrast, 5-LO product formation induced by NaCl was unaffected by addition of EDTA or BAPTA/am, and there was no significant change in the 5-LO product pattern. For SA, removal of either extracellular (EDTA) or intracellular (BAPTA/am) Ca++ gave slight inhibition of 5-LO product formation, and the combination of EDTA and BAPTA/am reduced 5-LO products to about 45% of control. Thus, the effects of Ca++ chelation on 5-LO activation by ionophore, as compared to stress stimuli, were strikingly different. When PMNL had been treated with 30 μM BAPTA/am in presence of 1 mM EDTA to remove intracellular and extracellular Ca++, Ca++ levels of PMNLs not exposed to agonists were practically undetectable by the Fura-2 method (<10 nM) and none of the above stimuli (including ionophore) caused any elevation of intracellular Ca++ (not shown).
Also, 5-LO translocation to the nucleus in PMNLs was determined in the presence of or after depletion of Ca++. Cell stimulation with 2.5 μM ionophore in the presence of Ca++ led to a considerable 5-LO translocation, but also 100 μM SA gave a clear response (Figure 7C). Ca++ depletion by EDTA plus BAPTA/am abrogated ionophore-induced 5-LO redistribution; however, the chelators did not significantly affect SA-induced translocation of 5-LO. In parallel, activation of p38 MAPK was examined with respect to Ca++. As shown in Figure 7D, activation of p38 MAPK by ionophore (also by thapsigargin, not shown) was abolished after depletion of Ca++, whereas p38 MAPK activation induced by SA or NaCl was similar in presence or absence of Ca++.
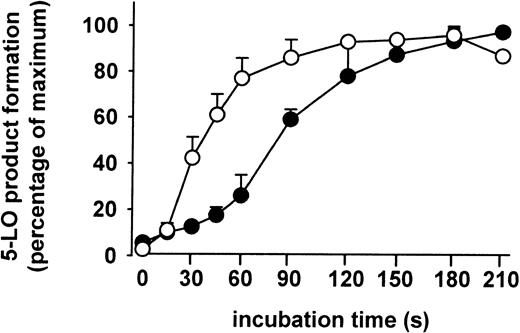
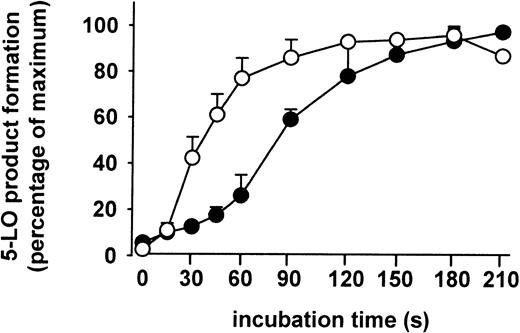
The kinetics of 5-LO product formation induced by ionophore and by chemical stress (SA) were compared. The formation of 5-LO products in stimulated PMNLs was determined after the times indicated in Figure8. Ionophore and SA resulted in half-maximal formation of 5-LO metabolites at about 45 seconds and 90 seconds, respectively. Thus, a lag phase of about 1 minute was observed with SA as stimulus. Finally, when PMNLs were stimulated with combinations of ionophore (or thapsigargin) plus stress stimuli (SA, heat shock), no further elevation of 5-LO product formation was observed.
Kinetics of 5-LO product formation.
Freshly isolated PMNLs (5 × 107 in 10 mL PGC buffer) were stimulated either with SA (10 μM) 3 minutes prior to addition of 40 μM AA (●), or with ionophore A2387 (2.5 μM) together with AA (○). After the indicated times at 37°C, aliquots of these incubations (1 mL) were added to the same volume of ice-cold methanol to stop the 5-LO reaction, and 5-LO products were determined. Results are given as mean + SE (n = 3).
Kinetics of 5-LO product formation.
Freshly isolated PMNLs (5 × 107 in 10 mL PGC buffer) were stimulated either with SA (10 μM) 3 minutes prior to addition of 40 μM AA (●), or with ionophore A2387 (2.5 μM) together with AA (○). After the indicated times at 37°C, aliquots of these incubations (1 mL) were added to the same volume of ice-cold methanol to stop the 5-LO reaction, and 5-LO products were determined. Results are given as mean + SE (n = 3).
Effect of p38 MAPK inhibitor SB203580 on 5-LO product formation in PMNLs depends on the stimulus
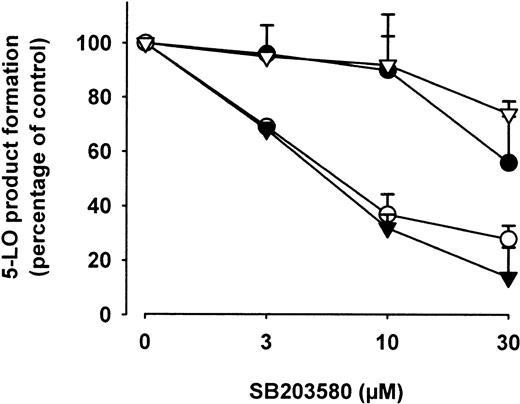
We determined the effects of the p38 MAPK inhibitor SB203580 on 5-LO product formation in PMNLs challenged by stress stimuli or by Ca++-mobilizing agents. The inhibitory concentration of 50% (IC50) for 5-LO inhibition in ionophore- or thapsigargin-stimulated PMNLs was about 30 μM (Figure9). This is similar to our previous study, where we also determined that ionophore-induced 5-LO kinase activation was almost completely abolished at 3 μM SB203580.20 Also, an IC50 value of about 30 μM SB203580 was determined in cells challenged with fMLP (not shown). Thus, 5-LO activation mediated by Ca++ mobilization (ionophore, thapsigargin) or by fMLP appears not dependent on p38 MAPK activity. On the other hand, 5-LO product formation induced by stress stimuli was more sensitive to SB203580, with IC50 values close to 5 μM, suggesting an involvement of p38 MAPK. Inhibition of MEK-1/2 by U0126 (1-10 μM) and PD98059 (3-30 μM) did not suppress 5-LO product formation under these conditions (not shown), again excluding a potential role of MEKs or ERKs in stress-induced 5-LO activation.
Effects of SB203580 on 5-LO product formation.
Freshly isolated PMNLs (5 × 106 in PGC buffer) were preincubated with the indicated concentrations of SB203580 for 30 minutes at 37°C. SA (○, 10 μM) and NaCl (▾, 0.3 M) were added 3 minutes prior to addition of 40 μM AA; ionophore A23187 (●, 2.5 μM) and thapsigargin (thaps, ▿, 1 μM) were added simultaneously with AA, final volume was 1 mL. After 5 minutes at 37°C, 5-LO products were determined. Results are given as mean + SE (n = 3).
Effects of SB203580 on 5-LO product formation.
Freshly isolated PMNLs (5 × 106 in PGC buffer) were preincubated with the indicated concentrations of SB203580 for 30 minutes at 37°C. SA (○, 10 μM) and NaCl (▾, 0.3 M) were added 3 minutes prior to addition of 40 μM AA; ionophore A23187 (●, 2.5 μM) and thapsigargin (thaps, ▿, 1 μM) were added simultaneously with AA, final volume was 1 mL. After 5 minutes at 37°C, 5-LO products were determined. Results are given as mean + SE (n = 3).
Discussion
In this study we found that different types of cell stress (chemical stress, osmotic stress, heat shock) activated 5-LO in PMNLs with comparable efficacy, yielding about 30% to 50% of 5-LO products compared to cells stimulated with ionophore, without a significant change in the metabolite pattern. SA (chemical stress) led to a dose- and time-dependent induction of 5-LO product formation that significantly correlated with the activation of p38 MAPK and downstream 5-LO kinases. Treatment of PMNLs with SA (albeit at higher concentrations) also led to association of 5-LO with the nuclear membrane, a process that contributes to substantial 5-LO product formation. SA has previously been reported to induce apoptosis of PMNLs, which was accompanied by an increase in the respiratory burst.36 Different MAPK pathways were activated by SA (1-30 μM), depending on the cell type.37-42 In our hands, SA predominantly activated p38 MAPK in PMNLs, whereas ERKs and JNKs were less affected, and the p38 inhibitor SB203580 suppressed SA-induced 5-LO product formation. Collectively, p38 MAPK appears to be the MAPK involved in SA-induced 5-LO activation. However, arsenite signaling is complex and may also involve other pathways, in addition to phosphorylation events.
Cell stress induced by hypertonicity led to dose- and time-dependent 5-LO product formation that correlated with the activation of p38 MAPK. Isolation of PMNLs includes hypotonic lysis of erythrocytes, osmotic stress conditions that might activate PMNL. However, in contrast to hypertonic stress, hypotonicity or cell permeable urea did not enhance 5-LO product formation, indicating that cell shrinkage rather than changed osmolarity is responsible for 5-LO activation. In agreement with others, we found that hypertonicity gave a rapid activation of p38 MAPK (within minutes) and that hypertonicity gave no significant activation of JNK or ERK in PMNLs.27,28 The p38 MAPK inhibitor SB203580 reduced hypertonicity-induced 5-LO product formation in a dose-dependent manner. These results agree with our recent study using a human B-cell line, where we also found that hypertonicity (together with ionophore) resulted in substantial 5-LO product formation and p38 MAPK-regulated MK activation.29 Finally, heat shock and UV light, other forms of cell stress that activate p38 MAPK and MKs,25,26 38 also induced formation of 5-LO products in PMNLs. Thus, it appears that cell stress activates 5-LO via p38 MAPK pathways.
Ca++ strongly stimulates 5-LO catalytic activity in vitro and can also induce membrane binding and translocation of soluble 5-LO to the nuclear membrane (for reviews, see Samuelsson et al1and Rådmark4). However, Ca++ is not obligatory for 5-LO catalysis, a basal activity is readily detectable in vitro (see Aharony and Stein43 and Reddy et al44 and references therein). Moreover, it was reported that extracellular Ca++ was only partially required for 5-LO product formation in PMNLs when exogenous AA was present.35 Also, it was suggested that when LT biosynthesis in mast cells was stimulated via IgE receptors, another unidentified pathway could act in conjunction with Ca++ to activate 5-LO.45 In a model for AA-induced LT biosynthesis in PMNLs, it was implied that an initial burst of 5-LO activity was Ca++ independent.32Finally, a recent study provides evidence that Mg++ (which is abundant in cells) can bind to 5-LO and stimulate 5-LO catalytic activity in vitro,44 and might substitute for Ca++ in vivo. Thus, there are several indications that 5-LO activation in intact cells may not necessarily be connected with elevated Ca++ levels. Our findings show that cell stress-induced activation of 5-LO proceeds at very low concentration of Ca++ (<50 nM; Figure 7A). For half-maximal activation of 5-LO in vitro 1 to 2 μM Ca++ was required, and theKd for Ca++ binding of 5-LO was 6 μM (see Hammarberg and Rådmark11 and references therein). Interestingly, a lower concentration of Ca++ (200 nM) seemed sufficient for activation of 5-LO in intact PMNLs.35 However, a clearly lower Ca++concentration (<10 nM) was monitored for PMNLs treated with EDTA and BAPTA/am, indicating that stress-induced 5-LO product formation under such conditions is independent of Ca++. Another finding supporting that stress-induced 5-LO activation does not involve Ca++ is the different effects of exogenous AA. When PMNLs were stimulated with ionophore, exogenous AA increased the activity already at 5 μM, and activity was decreased at the highest concentrations (Figure 6). This is in accordance with Ca++lowering the Km of 5-LO for AA, and causing substrate inhibition.43 When PMNLs were stimulated by cell stress, higher concentrations of AA were required, and substrate inhibition was less apparent. In this context, it is interesting that for 5-LO stimulated with Mg++,Km for AA was not lowered, as observed with Ca++.44
In contrast to PMNLs stimulated with ionophore and thapsigargin, it was necessary to add exogenous AA (≥10 μM) for cell stress-induced 5-LO product formation (Figure 6). This could be due to the inability of cell stress to activate cPLA2 in PMNLs, which liberates AA as substrate for 5-LO. However, it has been shown that AA can also be provided by platelets for metabolism in PMNLs by transcellular routes.46
It was suggested that activation of 5-LO in cells involves phosphorylation events17 and phosphorylated 5-LO was isolated from the nucleus of ionophore-stimulated HL-60 cells. Also, various protein kinase inhibitors could interfere with cellular 5-LO activity.18 Recently, we found that p38 MAPK-regulated MKs can phosphorylate 5-LO in vitro, and a MK motif is present in the 5-LO sequence.20 Also, cell stimulation by SA or hyperosmolar sorbitol increased 5-LO product formation in PAF-stimulated PMNLs and ionophore-activated B cells, respectively.20,29Furthermore, priming with lipopolysaccharide (LPS) or phorbol esters, which activated MKs in PMNLs20 led to up-regulation of LT synthesis.24 47 One may speculate that the latter priming effects resemble cell stress-induced 5-LO activation mediated by p38 MAPK. Taken together it seems reasonable that phosphorylation of 5-LO is one determinant for its activation in intact cells and that cell stress activates 5-LO via p38 MAPK cascades.
For cPLA2 it was found that Ca++ and/or phosphorylation can activate the enzyme.2,48 Thus, stimuli that lead to activation of MAPKs (phorbol myristate acetate [PMA], okadaic acid) and cPLA2 phosphorylation caused AA release also at basal Ca++ levels; on the other hand, Ca++-mobilizing agents (ionophore) stimulated AA release when phosphorylation of cPLA2 was blocked.48 However, phosphorylation was found to be essential when there was only a transient Ca++ mobilization (induced by zymosan).49 From our data, it is tempting to speculate that similar mechanisms exist for 5-LO. First, depletion of Ca++ almost completely suppressed 5-LO product formation induced by ionophore or thapsigargin, and removal of intracellular Ca++ clearly reduced 5-LO product synthesis in PMNLs activated by fMLP, whereas stress-induced 5-LO activation (mediated by p38 MAPK and downstream 5-LO kinases) was only partially affected. Second, 5-LO translocation to the nucleus stimulated with Ca++-mobilizing agents was blocked by Ca++depletion, but not so for the p38 MAPK stimulus SA. Third, the kinetics in LT formation differed between cell stress (SA) and Ca++-mediated pathways (ionophore; Figure 8). Finally, SB203580 efficiently inhibited 5-LO product formation induced by cell stress not leading to increased intracellular Ca++ (SA, hypertonicity), whereas 5-LO activation stimulated by agents leading to increased intracellular Ca++ as well as to activation of p38 MAPK (ionophore, thapsigargin, fMLP) was much less sensitive to SB203580.
Originally, p38 MAPK was cloned as a specific target of pyridiyl imidazoles such as SB203580, compounds which inhibited the production of proinflammatory cytokines in monocytes,50 and reduced the severity of murine collagen-induced arthritis and rat adjuvant arthritis, as well as hyperangiogenesis associated with murine air pouch granuloma (see references in Herlaar and Brown16). In PMNLs, p38 MAPK was implicated in TNF-α–induced superoxide release, IL-8 production induced by granulocyte-macrophage colony-stimulating factor (or LPS), and transforming growth factor β-induced chemotaxis (for reviews, see Herlaar and Brown16 and Werz et al20). Therefore, p38 MAPK signaling cascades are considered important in inflammatory diseases.16 LTs are mediators of inflammatory reactions, and p38 MAPK-regulated LTB4 synthesis in PMNLs (presumably via 5-LO phosphorylation) may be another contribution of this kinase within the inflammatory response. p38 MAPK- mediated activation of 5-LO could function independently, or it could function in concert with Ca++-mediated 5-LO activation, for stimulation of LTB4 biosynthesis under physiologic conditions as well as in inflammatory disease states, as asthma. Anti-inflammatory drugs directed against p38 MAPK may benefit from this connection to LT biosynthesis.
We thank Astrid Neuss, Agneta Nordberg, and Dagmar Szellas for expert technical assistance.
Supported by grants from the Fonds der Chemischen Industrie, from the Swedish Medical Research Council (03X-217), from the European Union, and from the Verum Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Oliver Werz, Institute of Pharmaceutical Chemistry, University of Frankfurt, Marie-Curie Str 9, D-60439 Frankfurt, Germany; e-mail: o.werz@pharmchem.uni-frankfurt.de.