Abstract
Mobilized peripheral blood is increasingly used as the source of hematopoietic stem cells for allogeneic transplantation, currently the only curative approach for sickle cell anemia. However, the safety and feasibility of stem cell mobilization in individuals with sickle cell trait (SCT) has not been documented. This study is a prospective controlled trial to evaluate the safety and feasibility of peripheral blood stem cell (PBSC) mobilization in 8 SCT subjects and 8 control subjects matched for age and race. Mobilization with filgrastim 10 μg/kg subcutaneous daily for 5 days was followed by 12-L apheresis on the fifth day. Filgrastim administration was accompanied by similar symptoms in all subjects; no untoward adverse events occurred in either group, including sickle cell crises. CD34+ cell mobilization response was not significantly different between SCT and control subjects. Median CD34+ cell content was also similar in PBSCs collected from SCT versus control subjects, 6.8 versus 3.9 ×106 CD34+ cells/70 kg,P = .165. Red cell depletion from SCT products was not possible by using hydroxyethyl starch sedimentation but was achievable with ammonium chloride lysis. There was no evidence of gelling of SCT products after thaw, and no difference in cell recovery was seen among red cell–depleted versus nondepleted products. Cryopreservation in 5% dimethyl sulfoxide/6% pentastarch was associated with superior cell recovery (both SCT and control subjects) compared with 10% dimethyl sulfoxide (P = .001). The study concluded that filgrastim mobilization, large volume apheresis, processing, and cryopreservation appears to be safe in donors with SCT, allowing PBSC use for transplantation in patients with sickle cell anemia.
Introduction
Sickle cell anemia (SCA) affects more than 6 million Americans, resulting in significant morbidity and early mortality.1 Allogeneic bone marrow transplantation remains the only currently available curative treatment; however, many patients are ineligible because of either the lack of a suitable donor or their underlying condition. The development of hematopoietic stem cell (HSC) transplantation by using nonmyeloablative conditioning regimens with lower toxicity offers a more favorable risk–benefit ratio and should allow more widespread application of this modality, at least to those with an HLA-matched sibling donor. With nonmyeloablative conditioning, adequate cell doses may be even more important for successful engraftment, and the best available method to obtain high cell numbers is through the use of mobilized peripheral blood stem cells (PBSCs). Related donors of patients with SCA have a probability of at least 50% of having sickle cell trait (SCT) and the safety of granulocyte colony-stimulating factor (G-CSF) administration has not been demonstrated in such donors.
Although SCT is considered a benign disorder, complications associated with this condition include bacteriuria and pyelonephritis in pregnancy, hyposthenuria, hematuria, as well as an increased risk of sudden death with physical exertion.2 There are also numerous reports of patients with SCT experiencing splenic and/or pain crises in situations associated with oxidative stress. Typically, crises have been precipitated by movement to high altitudes, resulting in pulmonary and splenic infarctions and red cell hemolysis.3-6 The use of G-CSF may represent another form of stress. In at least one report, a patient with sickle cell disease given G-CSF for PBSC mobilization developed a pain crisis in association with a white blood cell count of 63.4 ×109/L, suggesting that leukocytosis may cause crisis.7 In fact, it has been suggested that the benefits of hydroxyurea in patients with sickle cell disease can be in part attributed to the reduction in white count.8 More recently, there have been 2 case reports of severe complications including death in patients with sickle cell hemoglobin, and hemoglobin S β-thalassemia receiving G-CSF.9 10 Notably, both patients were relatively asymptomatic with regard to symptoms related to sickling before the GCSF administration. There have been no reports of crises with the use of G-CSF in patients with SCT, yet no formal study has been performed to confirm the safety of its use in this group.
The mechanism of stem cell mobilization in response to exogenous G-CSF is not fully elucidated, nor is it possible to predict an individual's mobilization response. Although several reports have documented the normal range of CD34+ cell mobilization,11 12there are no reports to date that characterize this response specifically among individuals with SCT or among African Americans, the predominant racial group in the United States carrying the sickle hemoglobin genotype. The presence of SCT may also have unanticipated effects on the mobilization response to G-CSF.
Finally, there have been reports of red cell gelling and clumping,13,14 requiring special processing of blood products obtained from SCT donors.13 15 Because there is no urgency with regard to the timing of transplantation for patients with SCA, it should be possible to collect and preserve PBSCs before transplantation. Hence, the behavior of PBSCs from SCT donors during collection, processing, and storage should be determined. We undertook a prospective controlled study to determine the safety and feasibility of mobilization and collection of PBSCs from individuals with SCT, as well as the optimal methods for processing and cryopreservation of these products.
Patients, materials, and methods
Protocol design
The protocol was approved by the Institutional Scientific Review Committee and the Institutional Review Board of the National Heart, Lung, and Blood Institute, and all the study subjects gave written informed consent.
Eight healthy subjects aged 18 years and older with a history of SCT were enrolled. Eight healthy subjects who were matched for age and race were also enrolled as control subjects. One additional SCT subject was enrolled subsequently to perform a CD34+ selection on the PBSC product. Eligibility criteria included a negative history for contraindications to the use of G-CSF, a negative history of sickle cell crisis, and a normal physical examination with complete blood count and chemistry panel. SCT was confirmed by using sickle cell prep analysis, and hemoglobin electrophoresis was used to rule out other hemoglobinopathies.
PBSC mobilization and apheresis
All subjects received G-CSF (filgrastim; Amgen, Thousand Oaks, CA) 10 μg/kg per day subcutaneously for 5 days. PBSCs were collected on day 5, starting 1 to 2 hours after the last dose of G-CSF. A single 12-L apheresis procedure was performed by using a CS3000 Plus Blood Cell Separator (Baxter Healthcare, Deerfield, IL), peripheral venous access, and anticoagulation with anticoagulant citrate dextrose solution formula (ACD-A; Travenol; Baxter). All subjects were evaluated before and 9 to 12 days after mobilization and apheresis with a history, physical examination, complete blood count, and chemistry panel. Subjects also completed a standard National Marrow Donor Program (NMDP) questionnaire that listed 8 possible adverse effects of G-CSF, including fatigue, myalgia, headache, fever, chills, nausea, insomnia, and sweats. Any positive response on the NMDP screening form was given a score from 1 to 4 by using the Cancer and Leukemia Group B (CALGB) modified toxicity criteria (NMDP Form 400 V2 4-4, January 1999). A score of 1 corresponds to mild, 2 to moderate in severity, 3 to severe, and 4 to life threatening. Any event receiving a symptom score of 3 or higher was defined as a serious adverse event and required reporting to the institutional review board. The scores for all symptoms experienced by each subject were combined to give a cumulative symptom score. In addition, patients recorded the total number of tablets of acetaminophen required during the mobilization and apheresis. The median cumulative symptom score and the median number of tablets required for each cohort were used for comparison.
Ex vivo processing, cryopreservation, and thawing
For the first 3 subjects with SCT, each PBSC product was split into 2 equal parts. Plasma removal was performed on one part after centrifugation of the PBSC product at 4600g. On the other part, red blood cells were removed by expression of the PBSC product after a 60-minute sedimentation procedure that used hydroxyethyl starch (HES; Hespan; Braun Medical, Irvine, CA). After 3 attempts at HES sedimentation of PBSCs from 3 different SCT subjects proved unsuccessful, we evaluated a procedure that used ammonium chloride (ACK; BioWhittaker, Walkersville, MD) red cell lysis for the PBSC product derived from the next 9 subjects (5 SCT and 4 control subjects). ACK is a guanosine monophosphate reagent produced for laboratory and manufacturing use, and its formulation is NH4Cl 8024 mg/L plus KHCO3 1001 mg/L, plus NaEDTA 3.7 mg/L. The pH varies but is in the 7.2 to 7.4 range, and the osmolality is 290 mOsm/kg H2O. For this procedure, each product was incubated with sterile ACK buffer for 7 minutes followed by 2 wash and centrifugation steps. After the initial plasma removal or red cell removal, each processed PBSC fraction was again divided into 2 parts for evaluation of 2 cryopreservation methods. The freezing solution for method A used 10% dimethyl sulfoxide (DMSO; final vol/vol; Cryoserv; Ben Venue Laboratories, Bedford, OH) and 10% human AB serum, single donor (final vol/vol), and the freezing solution for method B used 5% DMSO (final vol/vol), 6% low molecular weight hydroxyethyl starch (final vol/vol; pentastarch; Braun), and 4% human serum albumin (Hyland; Baxter). For both methods, the final freeze volume of 50 mL was cryopreserved gradually in Cryocyte freezing bags (Nexell, Irvine, CA) by using a controlled rate device. The bags were stored in the liquid phase of liquid nitrogen (LN2) for at least 14 days. For one PBSC product from a donor with SCT, a CD34+ cell selection procedure before cryopreservation was performed, using the Isolex 300i automated immunomagnetic system (Nexell).
Cells were thawed rapidly by immersing each bag, within a plastic overwrap bag, in a 37°C water bath. Product fractions cryopreserved by method B (pentastarch mixture) were subsequently diluted with 12.5 mL PlasmaLyte A (Baxter) to reduce the viscosity of the suspension. The thawed PBSC products were then left undisturbed at monitored room temperature (20°C to 22°C) and sampled after 30 minutes.
Assays
Total nucleated cells were counted by using impedance gating on a CellDyn 3500 automated cell counter (Abbott Diagnostics, Santa Clara, CA). Phenotypic analysis and viability were performed by using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) and CellQuest software (Becton Dickinson). Cells were stained with fluorochrome-conjugated monoclonal antibodies to CD34, CD45, and CD3 surface markers, and 7-amino-actinomycin D (7-AAD) was added for viability determination. CD45+ cells that excluded 7-AAD (and were therefore judged as viable) were analyzed for CD34+ and CD3+. The percentage of viable cells expressing each marker was calculated by dividing the number of viable cells expressing the marker by the total cells in the gated population and multiplying by 100%. The total number of viable CD34+and viable CD3+ cells in each fraction was calculated by multiplying the total nucleated cell content by the percentage of viable cells expressing the relevant marker.
Granulocyte-macrophage colony-forming units (CFUs-GM) and erythroid burst-forming units (BFUs-E) were assayed in a commercially available methylcellulose culture medium containing a combination of recombinant colony-stimulating factors (MethoCult No. 4434; Stem Cell Technologies, Vancouver, BC). Duplicate 1-mL cultures, each containing 1 × 105 mononuclear cells, were plated in 35-mm gridded Petri dishes and incubated at 37°C in a humidified atmosphere in 5% CO2. The total number of CFUs-GM and BFUs-E were enumerated on day 14 by using an inverted microscope. Total colony-forming units (CFUs) were calculated by the following formula: [average number of colonies (CFU-GM ± BFU-E)/105 mononuclear cells from 2 plates] × total mononuclear cells/105.
Recoveries of nucleated cells, CD34+ and CD3+cells, and CFUs were calculated as the number of each population in the thawed product divided by the number in the product before freezing and multiplied by 100%.
Calculations and statistical analysis
Summary statistics were calculated for all numerical data. Student unpaired two-sample t test was used for determining significant differences between groups for all paired data with a presumed normal distribution. Categorical variables were compared by using the Fisher exact test. The Wilcoxon rank sum test was used to compare cumulative symptom scores and analgesic usage between SCT and control subjects, as these data are subject to difficulties with outlying data and asymmetry, making a Student t test less applicable. All statistical analyses were performed 2-sided at a significance level of .05.
Results
Patient characteristics
Table 1 summarizes the baseline characteristics of the 17 study participants. All 17 subjects were of African American background. Of the 17 subjects, 9 had SCT confirmed at our institution by sickle cell prep testing and hemoglobin electrophoresis. The 2 groups had similar baseline characteristics. There were more women than men in the SCT cohort, but this difference was not statistically significant. Baseline laboratory testing results did not differ between the 2 groups, with the exception of the absolute neutrophil count, which was significantly higher in the control subjects than in the SCT subjects (median, 4.2. versus 1.93 × 109/L, P = .04).
Mobilization and collection
All 17 subjects tolerated G-CSF mobilization without any unanticipated adverse effects. Specifically, there was no evidence of crisis in any SCT subject. The median cumulative symptom score was higher in the SCT than the non-SCT group, 3.4 versus 1.2, respectively,P = .014 (Table 2). No subject rated the severity of any one symptom as more than 2. The increased cumulative score in the SCT group reflected an increase in the overall number of reported symptoms and not their severity. There was a trend toward more analgesic use by SCT subjects (13 versus 6.3 tablets), but this trend was not statistically significant (P = .066).
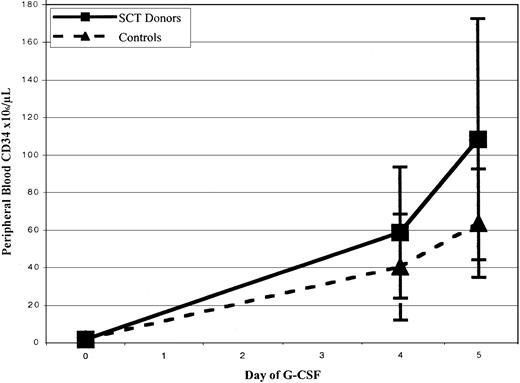
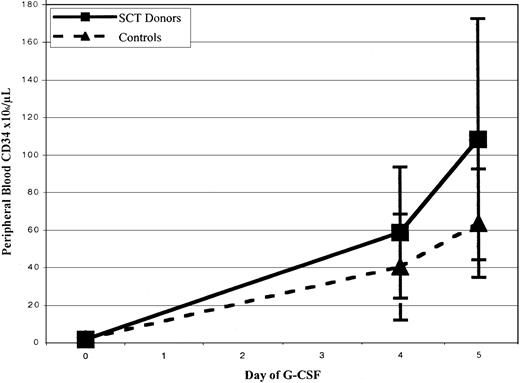
CD34+ cell mobilization response is shown in Figure1 and Table3. There was a trend toward greater peak CD34+ cell counts after filgrastim administration in SCT versus control subjects, with median values of 56 versus 33 × 106 cells/L (P = .27) at day 4 and 77.7 versus 54.5 × 106 cells/L at day 5 (P = .08). Mean mononuclear cell collection efficiency during leukapheresis was significantly greater in control subjects than in SCT subjects, 60% versus 48% efficiency, P = .04. There were, however, no significant differences between the median number of CD34+ cells and CFUs-GM collected from SCT subjects and control subjects (6.8 versus 3.9 × 106CD34+ cells/70 kg, P = .165; 40.1 versus 28 × 106 CD34+ cells/L processed,P = 0.23; and 36.7 versus 14.9 CFUs-GM × 106, P = .065, respectively), as shown in Table 3. There were also no statistically significant differences between the total number of nucleated cells and CD3+ cells collected among the 2 groups (P = .24 and P = .236, respectively).
Processing
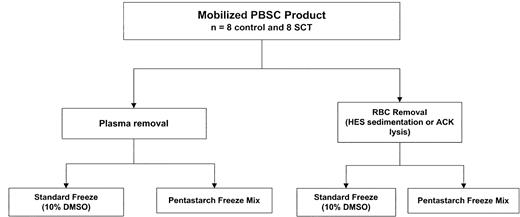
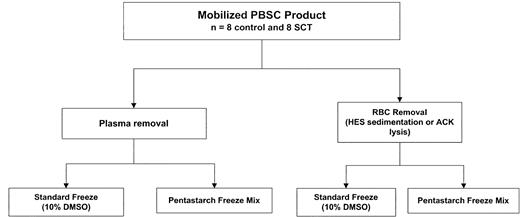
Figure 2 summarizes the experimental design for product processing. All products were initially divided into 2 aliquots and underwent either hard spin to remove plasma or hetastarch sedimentation to remove red cells. In the first 3 consecutive SCT PBSC products, HES sedimentation did not result in a distinct layer of red cells and was unsuccessful. Subsequently, red cell lysis was performed by using ACK lysis in those aliquots undergoing red cell removal. ACK lysis was associated with a small but statistically significant decrease in total nucleated cell content (10.7 versus 9.8 × 109 cells before and after lysis, respectively, P = .0001) largely because of CD3+ cell loss (4.66 versus 3.32 × 109cells, P = .01). There was no associated decrease in CD34+ cell or CFU-GM content. The products were further split for cryopreservation into solutions containing either 5% DMSO/6% pentastarch or 10% DMSO. There was no evidence of gelling of SCT PBSC products on thawing with or without prior red cell removal, and no overall difference was seen in CD34+ cell recovery among components that underwent ACK lysis and those that did not (69.7 versus 66.2 × 106 cells, respectively,P = .06). Table 4 presents the postthaw recoveries of control and SCT PBSCs cryopreserved by the 2 different freezing methods. There were no statistically significant differences between control and SCT PBSC products in postthaw recovery of CD34+ and CD3+ cells or of CFUs-GM for either freeze method. However, when data from control and SCT PBSCs were combined, cryopreservation in 5% DMSO/6% pentastarch was associated with superior recoveries of both CD34+ cells (92.6% versus 24.7% recovery,P = .001) and CD3+ cells (41.2% versus 14.1%, P = .005; Table 5).
Processing of components obtained from SCT and control subjects.
Study schema for processing and cryopreservation of PBSC products collected from 8 SCT donors and 8 control subjects. PBSC products from each donor were split. Half of each product was centrifuged to remove plasma, and the other half was treated for red blood cell removal, either by hydroxyethyl starch (HES) sedimentation (3 SCT and 4 control subjects) or ammonium chloride (ACK) lysis (5 SCT and 4 control subjects). Red blood cell depletion in SCT products could not be accomplished by HES sedimentation. The plasma or red cell–depleted products were again divided, and each half was cryopreserved in either a conventional 10% DMSO solution or in a solution composed of 5% DMSO/6% pentastarch/4% human serum albumin.
Processing of components obtained from SCT and control subjects.
Study schema for processing and cryopreservation of PBSC products collected from 8 SCT donors and 8 control subjects. PBSC products from each donor were split. Half of each product was centrifuged to remove plasma, and the other half was treated for red blood cell removal, either by hydroxyethyl starch (HES) sedimentation (3 SCT and 4 control subjects) or ammonium chloride (ACK) lysis (5 SCT and 4 control subjects). Red blood cell depletion in SCT products could not be accomplished by HES sedimentation. The plasma or red cell–depleted products were again divided, and each half was cryopreserved in either a conventional 10% DMSO solution or in a solution composed of 5% DMSO/6% pentastarch/4% human serum albumin.
A single CD34+ cell selection was performed by using the Isolex 300i immunomagnetic system. The product required no unusual processing and yielded 100% recovery of CD34+ cells.
Discussion
To determine the feasibility of performing allogeneic PBSC transplantation for patients with SCA, we compared PBSC mobilization and collection in subjects with SCT to subjects matched for age and ethnic background. Our cohorts were well matched for all baseline characteristics with the exception of absolute neutrophil counts, which were significantly lower in subjects with SCT. In a study of 297 healthy volunteers, individuals of African descent were found to have lower neutrophil counts than age-matched Caucasian Western Europeans, but there was no difference between those with or without SCT.16 No studies have been done to suggest that neutrophil counts at or below the established lower limit of normal predict a poor response to G-CSF. In fact, there are currently no markers to predict which patients may or may not mobilize well, and there are also no studies documenting the expected range of mobilization in African American donors, hence the need for our control cohort. The age range of our subjects was consistent with the expected ages of possible sibling donors, and, thus, age is unlikely to be a factor in clinical mobilization efforts. All subjects responded to G-CSF administration with the expected increase in blood CD34+ cell counts. In a study of 96 G-CSF–mobilized family donors, there was a suggestion that both women and older donors did not mobilize as well as men and younger donors.17 Despite the presence of more female donors in the SCT cohort of our study, there was a trend toward better mobilization of CD34+ cells in our SCT cohort, although this difference was not statistically significant.
Although there is a theoretical risk that G-CSF mobilization may precipitate a crisis in donors with SCT,3-7 none of the subjects in this study experienced unexpected adverse effects, including symptoms resembling a sickle crisis. White cell counts in our SCT subjects exceeded those recorded for the patients with sickle cell disease who experienced complications from G-CSF administration.7,9,10 The SCT subjects did, however, report a greater number of symptoms, reflected in a significantly higher cumulative symptom score than in the control group. The reason for the increased number of symptoms is unclear. Although it may be hypothesized that patients with SCT have a more active marrow at steady state, thereby resulting in more symptoms with mobilization, the character of the excess symptoms does not support this notion. Moreover, red cell life span is normal in individuals with SCT,18 suggesting no increase in hematopoietic drive. Despite the higher cumulative score, individual symptom severity was not different between the groups, with no scores of more than 2 reported. That the overall severity of these combined symptoms was not worse for the subjects with SCT is also suggested by the similar amount of analgesic use by both groups; however, the sample size may not permit the detection of a small but meaningful difference in the severity of symptoms experienced by donors with SCT. As well, there was a higher reporting of symptoms other than pain including nausea in the SCT subjects, suggesting that more liberal symptom control may be necessary in such donors. All subjects except one were able to perform their usual activities during the week of G-CSF administration, and no subject required stopping of the G-CSF because of his or her symptoms. Additionally, all symptoms resolved within 2 days of discontinuing G-CSF. Finally, although sickle cell crisis did not occur in any SCT volunteers, the sample size does not permit the conclusion that G-CSF is completely without such risk. Our study, however, suggests that the risk is limited, and continued study of SCT donors in the context of allogeneic transplantation is warranted.
Although there are reports of gelling after thawing of cryopreserved red blood cell products obtained from donors with SCT,13,15 19 no such gelling was observed in our study, regardless of whether or not products from SCT donors underwent red cell removal before cryopreservation. However, special processing requirements of products obtained from SCT subjects were identified. These requirements were related to red cell removal, which has important implications in addition to standard ABO considerations, as patients with sickle cell disease are often alloimmunized to multiple red cell antigens. Red cell removal was not feasible with hetastarch sedimentation. Instead, ACK lysis proved adequate for red cell depletion with minimal mononuclear cell loss. However, further validation of this procedure will be required, including analyzing products for residual NH4Cl before using this method clinically. Red blood cells from SCT subjects displayed different sedimentation properties than those from control subjects, possibly related to altered density. This difference appeared to affect the separation characteristics of PBSCs in the apheresis machine and may explain the inability to perform HES sedimentation as well as the lower apheresis collection efficiency. The lower collection efficiency was offset by a vigorous mobilization response, resulting in equivalent or better CD34+ cell yields in SCT subjects compared with non-SCT control subjects.
Achieving adequate CD34+ cell yields is crucial in allogeneic transplantation. A CD34+ cell dose of more than 2 × 106 cells/kg is associated with lower transplantation-related mortality, and higher overall and event-free survival after allogeneic transplantation. Adequate yields may be even more important when manipulations such as T-cell depletion are used. In patients receiving T-cell–depleted allografts at our institution, those receiving fewer than 1 × 106 CD34+cells/kg experienced higher transplantation-related mortality, whereas recipients of more than 2 × 106 cells/kg experienced faster hematologic recovery with lower transplantation-related mortality.20 Moreover, the faster neutrophil and platelet engraftment as well as the overall survival advantage shown for recipients of PBSC as compared with bone marrow transplant recipients may be related to the higher CD34+ cell doses achievable with mobilization and apheresis.21-23 In disorders such as sickle cell anemia, higher cell doses achievable by PBSC harvests may allow for more complex manipulation of the product, such as CD34+ cell selection and T-cell depletion, which may lead to a decrease in the incidence of graft-versus-host disease, thereby shifting the risk–benefit ratio.24 25
In an attempt to decrease the amount of DMSO infused into transplant recipients, we also compared a freeze solution containing 5% DMSO and pentastarch to the standard 10% DMSO solution. Although there was no difference between our normal control and SCT subjects, cryopreservation in 5% DMSO and 6% pentastarch resulted in markedly higher recoveries of CD34+ and CD3+ cells for both groups when compared with cryopreservation that used 10% DMSO. Because flow cytometric and clonogenic assays for hematopoietic progenitor cells on cryopreserved, thawed samples are known to be problematic in terms of accuracy and precision, the absolute values of the differences may not reliably reflect differences in their in vivo repopulating ability. Nevertheless, our in vitro results suggest the superiority of the pentastarch-containing freeze method, and we are currently exploring the use of this method for clinical application in the context of ongoing allogeneic transplantation trials. Interestingly, there was significant CD3+ cell loss with either method of cryopreservation, an observation that suggests that the delivered CD3+ cell doses may be significantly lower than those calculated before cryopreservation. We, therefore, now routinely confirm CD3+ cell content of all cryopreserved PBSC products by assaying a postthaw sample.
Given the advantages of PBSC grafts along with the potential to lower procedural mortality and morbidity by using novel transplantation-conditioning regimens, PBSC transplantation may be considered in patients with more indolent hematologic disorders such as SCA. Products from SCT donors require only minor changes in ex vivo cell processing, allowing for the use of mobilized peripheral blood as a potential source of stem cells for transplantation in sickle cell disease. Our study suggests that donors with SCT tolerate PBSC mobilization using G-CSF; however, given the increased number of symptoms and the concerns of more serious adverse events, closer monitoring of such donors may be warranted, especially in a pediatric population.
We thank Bob Wesley, PhD, for his assistance with the statistical analysis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John F. Tisdale, Molecular and Clinical Hematology Branch, National Institute of Diabetes, and Digestive, and Kidney Disorders, National Institutes of Health, 9000 Rockville Pike, Bldg 10, Rm 9N116, Bethesda, MD 20892; e-mail: johntis@intra.niddk.nih.gov.