The morbidity and mortality associated with sickle cell disease (SCD) is caused by hemolytic anemia, vaso-occlusion, and progressive multiorgan damage. Bone marrow transplantation (BMT) is currently the only curative therapy; however, toxic myeloablative preconditioning and barriers to allotransplantation limit this therapy to children with major SCD complications and HLA-matched donors. In trials of myeloablative BMT designed to yield total marrow replacement with donor stem cells, a subset of patients developed mixed chimerism. Importantly, these patients showed resolution of SCD complications. This implies that less toxic preparative regimens, purposefully yielding mixed chimerism after transplantation, may be sufficient to cure SCD without the risks of myeloablation. To rigorously test this hypothesis, we used a murine model for SCD to investigate whether nonmyeloablative preconditioning coupled with tolerance induction could intentionally create mixed chimerism and a clinical cure. We applied a well-tolerated, nonirradiation-based, allogeneic transplantation protocol using nonmyeloablative preconditioning (low-dose busulfan) and costimulation blockade (CTLA4-Ig and anti-CD40L) to produce mixed chimerism and transplantation tolerance to fully major histocompatibility complex–mismatched donor marrow. Chimeric mice were phenotypically cured of SCD and had normal RBC morphology and hematologic indices (hemoglobin, hematocrit, reticulocyte, and white blood cell counts) without evidence of graft versus host disease. Importantly, they also showed normalization of characteristic spleen and kidney pathology. These experiments demonstrate the ability to produce a phenotypic cure for murine SCD using a nonmyeloablative protocol with fully histocompatibility complex–mismatched donors. They suggest a future treatment strategy for human SCD patients that reduces the toxicity of conventional BMT and expands the use of allotransplantation to non–HLA-matched donors.
Introduction
Patients with sickle cell disease (SCD) suffer from both episodic acute complications and chronic, progressive, multisystem decline. Although medical treatments are life-extending, only stem cell transplantation offers an effective cure. There are currently 2 major barriers to stem cell transplantation for SCD: (1) the high morbidity and mortality associated with conventional bone marrow transplantation (BMT) and (2) the scarcity of acceptable stem cell donors.1,2 (1) Conventional BMT can cure SCD but requires toxic myeloablative preconditioning regimens to achieve donor cell engraftment.3,4 These intensive preparative regimens have many toxic adverse effects, including potential organ failure and a long-term risk of malignancy. In heavily pretreated patient populations, the morbidity and mortality of transplantation can outweigh the morbidity and mortality of SCD.2 A dilemma is now developing between early treatment with stem cell transplantation (shown to increase survival and disease-free survival when compared with transplantation after more disease-related complications have occurred) and a delayed approach, during which medical management ameliorates the symptoms of SCD until a later age when definitive therapy can be instituted.1,2 Unfortunately, this latter course may decrease the chance of successful stem cell transplantation. (2) The paucity of matched related donors has severely limited the number of SCD patients eligible for transplantation. In fact, in the Seattle consortium study, only 6.5% of potential SCD patients were found to be eligible for stem cell transplantation based on disease severity, and of these only 14% had an HLA-matched related donor.1 3 The lack of matched donors compounds the problem of transplantation-mediated toxicity due to the aggressive regimens used to gain alloengraftment. Thus, for widespread transplantation to be successful, it is essential to develop methods for the generation of allochimerism that have low levels of morbidity and mortality.
The transplantation experience with SCD suggests that a cure can be achieved even without total replacement of recipient stem cells.3-6 Walters et al reported that 4 of 50 patients treated with conventional myeloablative preconditioning unintentionally developed mixed donor/recipient hematopoiesis.3Importantly, those patients with stable mixed chimerism developed no further sickle-related complications. Recently, Iannone et al achieved varying degrees of mixed normal/sickle hematopoiesis by titrating bone marrow from a sickle mouse into a lethally irradiated normal mouse.7 Increasing levels of sickle red blood cells (RBCs) in the chimeric mice resulted in increasing hematopoietic and solid organ pathology. Importantly, solid organ pathology was reported even at sickle RBC percentages that normalized hematologic parameters, such as the reticulocyte count and hemoglobin level. This implies that relying on partial correction of hematologic indices may not predict true systemic cure of SCD in the setting of moderate levels of mixed RBC chimerism. Although the Iannone study was able to investigate certain pathophysiologic sequelae of mixing normal and sickle red cells, many questions about the effect of mixed chimerism could not be addressed due to the recipient mice lacking the sickle genotype.
Given the results of recent studies, there is expanding interest in protocols that are nonmyeloablative and that intentionally produce stable mixed chimerism.8,9 The problem to overcome is one of tolerance because there must be a coexistence of both host and donor cells for stable mixed chimerism to be achieved. While the initial protocols used relatively nonspecific immunosuppressive agents to induce transplantation tolerance, recent murine studies have focused on blocking T-cell activation pathways as a targeted approach for developing donor-specific tolerance and long-term mixed chimerism.10-15 These studies have shown that disruption of the T-cell costimulation signal mediated by the CD28/B7 or CD40/CD40L pathways at the time of BMT can lead to anergy of donor-reactive host T cells and produce long-term tolerance to the graft.10-15 Our group has developed a novel regimen for murine BMT that employs nonmyeloablative preconditioning with low-dose busulfan coupled with costimulation blockade of the CD28/B7 and CD40L pathways.14 This regimen produces long-term, mixed chimerism and robust tolerance to a fully major histocompatibility complex (MHC)–mismatched allograft without the requirement of myeloablation in a variety of mouse strains.
To rigorously test the ability for such a transplantation regimen to be successful in SCD, we used a transgenic knock-out mouse that lacks all murine hemoglobins and instead produces exclusively human α-globin, γ-globin, and sickle β-globin.16 The development of this and its related model17 were landmarks in SCD research because they represent the most authentic genetic representation of human SCD and replicate much of the complex multiorgan disease characteristics present in human SCD patients.16 17Although this new murine model of SCD is widely held to be one of the most authentic reproductions of human SCD created to date, there has previously never been a demonstration of BMT and engraftment of donor marrow in this model. Here we show for the first time that nonmyeloablative preconditioning with busulfan coupled with costimulation blockade can safely produce stable white blood cell (WBC) mixed chimerism and total replacement of the peripheral red cell compartment, resulting in a phenotypic cure of murine SCD. Furthermore, this cure is accomplished with fully MHC-mismatched donor marrow. Importantly, the hematologic cure that occurred with total replacement of the red cell compartment was accompanied by normalization of characteristic sickle organ pathology, indicating a total-body amelioration of disease. These results point the way to future trials in human SCD patients, with the goal of creating mixed chimerism across MHC barriers without the prohibitive toxicity of the current standard BMT therapy.
Materials and methods
Animals used in the study
Sickle mice16 were generously supplied by Dr Paszty at the Lawrence Berkley National Laboratory and are currently maintained at Emory University. Transplant recipients (males, 7-12 weeks) expressing exclusively human α- and sickle β-globin were bred by selective mating and exist on a mixed genetic background (strains: FVB/N, 129, DBA/2, C57BL/6, and Black Swiss). BALB/c and SJL mice (The Jackson Laboratory, Bar Harbor, ME) were used as bone marrow donors. BALB/c and C3H/HeJ mice were used for tests of donor-specific tolerance, and C57BL/6 mice were used as hematologically normal control mice (Jax).
BMT protocol
Recipient mice received 2 × 107 BALB/c or SJL T-cell–depleted bone marrow (TDBM) on day 0. T-cell depletion was accomplished with anti-CD3, anti-CD4, anti-CD8 antibodies (Miltenyi, Auburn, CA). After depletion, T cells represented less than 0.4% of marrow as determined by flow cytometry. Busulfan (20 mg/kg, intraperitoneally; Busulfex, Orphan Medical, Minnetonka, MN) was administered on day −1, and 500 μg each of hamster antimouse CD40L monoclonal antibody (MR1, BioExpress, Lebanon, NH) and human CTLA4-immunoglobulin (CTLA4-Ig) (Bristol-Myers Squibb, Princeton, NJ) (for costimulation blockade) were administered intraperitoneally on days 0, 2, 4, and 6 relative to the BMT. Control mice received 1 of 4 control protocols: (1) busulfan, 20 mg/kg alone, without bone marrow rescue; (2) TDBM alone, without busulfan or costimulation blockade; (3) TDBM plus busulfan, without costimulation blockade; and (4) TDBM plus costimulation blockade, without busulfan. The baseline hematologic parameters were measured 1 week prior to transplantation, and chimerism was tested 2 weeks, 4 weeks, and at monthly intervals after transplantation.
Flow cytometric analysis
Peripheral blood was analyzed by staining with fluorochrome-conjugated antibodies (anti-CD3, anti-CD5, anti-CD11b, anti-GR1, anti-B220, anti–H-2Kd, anti–H-2Ks, anti–H-2Kb, anti-Vβ5.1/5.2, Pharmingen, San Diego, CA; and anti-CD4 and anti-CD8, Caltag Laboratories, Burlingame, CA) or Ig isotype controls (Pharmingen) followed by RBC lysis and washing with a whole blood lysis kit (R&D Systems, Minneapolis, MN). Stained cells were analyzed either using WinList (Verity Software House, Topsham, ME) or CellQuest (Becton Dickinson, Mountain View, CA) software on either a FACScan or FACSCalibur flow cytometer (Becton Dickinson). WBC chimerism was determined by staining with either donor (anti-H2Kd[BALB/c] or anti-H2Ks [SJL]) or recipient (anti-H2Kb) antibodies and specific lineage markers and analysis by flow cytometry. Background staining with anti-H2Kd or anti-H2Ks was typically less than 1%. Vβ deletion was determined by staining with Vβ5 antibodies and specific lineage markers and analysis by flow cytometry.
Analysis of hematologic characteristics
Complete blood counts were performed on a Hemavet 1500 blood analyzer (1500 R series, CDC Technologies, Oxford, CT). Reticulocyte counts were performed by flow cytometry of peripheral blood labeled with antibodies specific for RBCs (anti–Ter-119, Pharmingen) and WBCs (anti-CD45, Pharmingen) and a fluorescent label of RNA, thiazole (Sigma, St Louis, MO). Reticulocyte counts were defined as the percent of peripheral blood cells that were Ter-119+, thiazole+, and CD45−. “Stress” reticulocytes18 were also analyzed by labeling with an antibody against the transferrin receptor (CD71, Pharmingen).
Blood smears
Smears of peripheral blood were made under ambient air (ie, oxygenated conditions) and then Wright-stained prior to microscopic analysis.
Tissue histology
Tissues were immersion-fixed in 4% paraformaldehyde prior to embedding and cutting. After hematoxylin and eosin staining, a veterinary pathologist examined the sections in a blinded manner.
RBC studies
RBC population half-life.
Half-life was determined by a pulsed biotinylation experiment performed essentially as previously described.19 Briefly, 50 mg/kg N-hydroxysuccinimide biotin (Calbiochem, San Diego, CA) (initially dissolved at a concentration of 50 mg/mL in N,-N,-dimethylacetamide and diluted into 250 μL normal saline just prior to use) was injected intravenously into engrafted or naive sickle animals. This produced a biotin pulse label to the peripheral blood. Blood was obtained either from the retro-orbital venous plexus or through a tail nick at regular intervals after biotinylation. The percentage of peripheral RBCs that were biotinylated was determined by flow cytometry using fluorescent streptavidin-cychrome (Pharmingen) to identify biotinylated cells and a fluorescent Ter-119–phycoerythrin antibody (Pharmingen) to identify RBCs. The decay of biotinylation is directly related to the clearance of the biotinylated RBCs from the peripheral circulation and thus can be used to determine the half-life of the RBC population.
Plasma-membrane phosphatidylserine exposure.
Exposure was measured by the percentage of cells that were positive in annexin V (Pharmingen) binding assays. Annexin V binding assays were performed by incubating 1 × 106 peripheral blood cells with 5 μL annexin V and appropriate lineage-specific antibodies in annexin binding buffer (Pharmingen) for 30 minutes at room temperature. Cells were then washed once with annexin binding buffer and analyzed by flow cytometry to determine the percentage of annexin V+ cells.
Scramblase assays.
RBC scramblase enzyme assays were performed essentially as previously described.20 21 Briefly, 2 × 106 peripheral blood cells were incubated with 3 mM of the fluorescent phosphatidylcholine analog palmitoyl-C6-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-phosphatidylcholine (NBD-PC; Avanti Polar Lipids, Birmingham, AL) in phosphate-buffered saline containing 1 mM CaCl2 for 30 minutes at 37°C. Cells were then cooled on ice and washed into buffer containing 10 mg/mL defatted bovine serum albumin (Sigma) to back-exchange noninternalized phospholipid. Cells were then incubated with appropriate lineage-specific antibodies (Pharmingen) for 20 minutes at 4°C prior to analysis by flow cytometry.
Analysis of RBC chimerism
RBC chimerism was determined by differential hemoglobin electrophoresis of donor and recipient hemoglobin. Donor β-globin consists of murine “major” and “minor” β-globin isomers, which have different electrophoretic mobilities than recipient human sickle β-globin. Hemoglobin electrophoresis was performed on the Helena Titan III electrophoresis system (Helena Laboratories, Beaumont, TX). Gels were scanned, and percent donor or recipient hemoglobin was determined by densitometry using Kodak 1-D Image Analysis software (Kodak, Rochester, NY).
Determining tolerance to donor antigen by CFSE assays
Splenic and mesenteric lymph node cells were harvested from experimental mice. After RBC lysis and nylon wool passage, cells were incubated in 10 μM of the fluorescent dye, CFSE (Molecular Probes, Eugene, OR). Irradiated (1.8 Gy [1800 rads]) sickle, BALB/c, or C3H mice then received 1 × 107 to 1 × 109 CFSE-labeled cells intravenously. After 66 to 72 hours, splenocytes were harvested from the recipients, the RBCs lysed, and the remaining cells stained with anti-CD4 and anti-CD8 or isotype controls and analyzed by flow cytometry as described above.
Determining hematopoietic balance in recipient hematopoietic organs
Mice were killed, and their splenocytes and bone marrow were harvested with conventional techniques. Hematopoietic balance was specified by determining the percent of bone marrow and spleen cells that were either RBCs (Ter-119+, CD45−), reticulocytes (Ter-119+, CD45−, thiazole+), or WBCs (Ter-119−, CD45+).
Results
Creation of stable chimeras using busulfan and costimulation blockade
Sickle mice16 were generously provided by Paszty and colleagues and treated with a regimen that included busulfan (20 mg/kg) pretreatment on day −1, BMT with TDBM from BALB/c mice on day 0, and costimulation blockade with 500 μg each of anti-CD40L and CTLA4-Ig on days 0, 2, 4, and 6. The T-cell content of transplanted cells was determined by flow cytometry and represented less than 0.4% of donor cells (data not shown). Additional animals received control protocols, including either (1) 20 mg/kg of busulfan without any bone marrow rescue, (2) TDBM alone, (3) TDBM and busulfan but no costimulation blockade, or (4) TDBM and costimulation blockade but no busulfan.
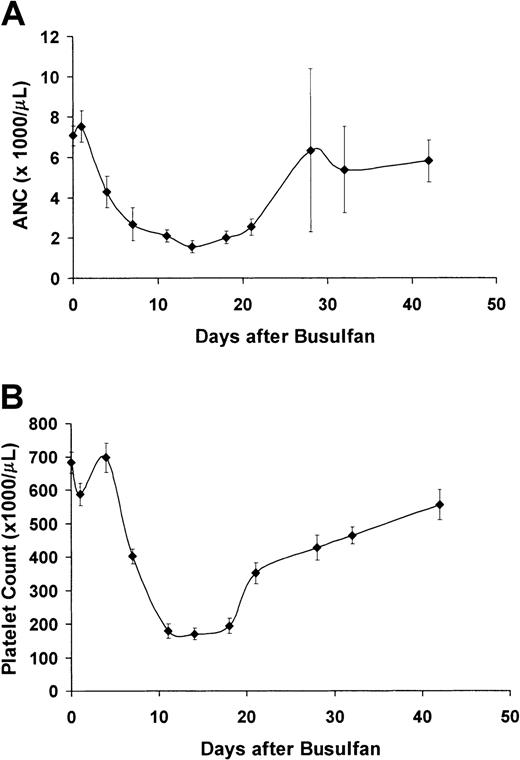
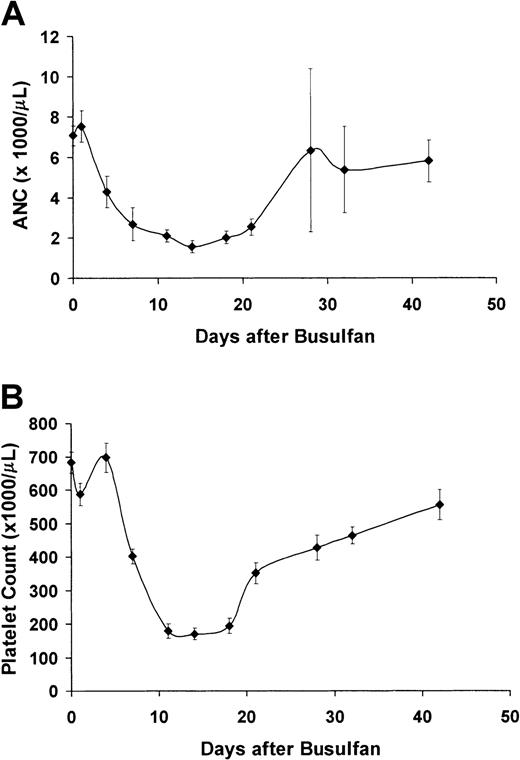
Figure 1 shows the transient myelosuppressive effect of 20 mg/kg busulfan given to 10 sickle mice. All recipients survived the busulfan treatment and recovered hematopoiesis without the need for stem cell rescue (absolute neutrophil count nadir = 1600 at 14 days, platelet nadir = 170 000 at 14 days). Thus, pretreatment with 20 mg/kg busulfan is nonmyeloablative in the sickle mice.
Treatment with 20 mg/kg busulfan is nonmyeloablative in sickle mice.
(A) Average absolute neutrophil count (ANC, from automated complete blood count analysis) of 10 sickle mice treated with 20 mg/kg busulfan. (B) Platelet count (from automated complete blood count analysis) of 10 sickle mice treated with 20 mg/kg busulfan.
Treatment with 20 mg/kg busulfan is nonmyeloablative in sickle mice.
(A) Average absolute neutrophil count (ANC, from automated complete blood count analysis) of 10 sickle mice treated with 20 mg/kg busulfan. (B) Platelet count (from automated complete blood count analysis) of 10 sickle mice treated with 20 mg/kg busulfan.
Mice who received control protocols, including BMT alone (5 mice), BMT after pretreatment with 20 mg/kg busulfan (no costimulation blockade, 5 mice), or BMT and costimulation blockade (no busulfan, 9 mice) showed low to undetectable levels of peripheral WBC chimerism (< 2.5%) throughout the experimental period (Figure2A). In contrast, 10 of 13 mice who received the full protocol of BMT, 20 mg/kg busulfan, and costimulation blockade achieved multilineage WBC mixed chimerism (Figure 2A). Two of these engrafted mice died during anesthesia at 3 months after transplantation (with no signs of graft versus host [GVH] disease prior to their death), and 1 mouse was killed at 2 months for histologic analysis. Chimerism for the remaining 7 mice (mean, 43% ± 10%) peaked 3 months after transplantation and was stable for more than 150 days (Figure 2A). Three mice rejected the graft. These mice displayed transient levels of WBC chimerism at 1 to 2 months after transplantation but fully reverted to the recipient WBC and RBC phenotype by 3 to 4 months after transplantation (data not shown).
Sickle mice treated with BMT after busulfan and costimulation blockade developed stable WBC mixed chimerism in the peripheral blood and the hematopoietic organs.
(A) Mean WBC chimerism in the peripheral blood. Sickle mice (H2-Kb) recipients were treated with 20 mg/kg busulfan (intraperitoneally) on day −1, with 500 μg CTLA4-Ig and anti-CD40L intraperitoneally on days 0, 2, 4, and 6, and underwent transplantation with allogeneic BALB/c (H2-Kd) TDBM (2 × 107 cells intravenously on day 0). The data in this figure represent the mean percent engraftment for 7 engrafted mice that were fully analyzed for more than 150 days (▪). Three mice became transiently engrafted but rejected their grafts on or before 60 days. These mice completely reverted to the host WBC and RBC phenotype by 3 to 4 months after transplantation (data not shown). Control animals received 1 of 3 treatments: TDBM alone (n = 5, ○), TDBM plus busulfan (no costimulation blockade, n = 5, ▵), or TDBM plus costimulation blockade but no busulfan (n = 9, □) Animals in each of the control groups showed less than 2.5% peripheral WBC chimerism. (B) Lineage-specific chimerism over time. The percent of each lineage (granulocytes [GR1], macrophages [CD11b], B cells [B220], and T cells [CD3]) that was donor type (H2-Kd) was identified by flow cytometry over time. Values shown are the mean for each time point from 7 engrafted animals ± SEM. ▪ indicates B220; ●, GR1; ▴, CD11b; ⧫, CD3. (C) Representative flow cytometric analysis. At left is a histogram showing H2-Kd staining of a chimeric animal with both host (H2-Kd-negative) and donor (H2-Kd-positive) WBCs. At right is a dot plot showing a representative quadrant analysis of CD3 cells versus H2-Kd. (D) WBC chimerism in the bone marrow (▪), spleen (■), and thymus (░). Engrafted sickle cell mice (n = 4) showed 45% ± 15% chimerism (H2-Kd-positive cells by flow cytometry) in the splenic compartment, 50% ± 10% chimerism in the bone marrow, and 25% ± 15% chimerism in the thymus.
Sickle mice treated with BMT after busulfan and costimulation blockade developed stable WBC mixed chimerism in the peripheral blood and the hematopoietic organs.
(A) Mean WBC chimerism in the peripheral blood. Sickle mice (H2-Kb) recipients were treated with 20 mg/kg busulfan (intraperitoneally) on day −1, with 500 μg CTLA4-Ig and anti-CD40L intraperitoneally on days 0, 2, 4, and 6, and underwent transplantation with allogeneic BALB/c (H2-Kd) TDBM (2 × 107 cells intravenously on day 0). The data in this figure represent the mean percent engraftment for 7 engrafted mice that were fully analyzed for more than 150 days (▪). Three mice became transiently engrafted but rejected their grafts on or before 60 days. These mice completely reverted to the host WBC and RBC phenotype by 3 to 4 months after transplantation (data not shown). Control animals received 1 of 3 treatments: TDBM alone (n = 5, ○), TDBM plus busulfan (no costimulation blockade, n = 5, ▵), or TDBM plus costimulation blockade but no busulfan (n = 9, □) Animals in each of the control groups showed less than 2.5% peripheral WBC chimerism. (B) Lineage-specific chimerism over time. The percent of each lineage (granulocytes [GR1], macrophages [CD11b], B cells [B220], and T cells [CD3]) that was donor type (H2-Kd) was identified by flow cytometry over time. Values shown are the mean for each time point from 7 engrafted animals ± SEM. ▪ indicates B220; ●, GR1; ▴, CD11b; ⧫, CD3. (C) Representative flow cytometric analysis. At left is a histogram showing H2-Kd staining of a chimeric animal with both host (H2-Kd-negative) and donor (H2-Kd-positive) WBCs. At right is a dot plot showing a representative quadrant analysis of CD3 cells versus H2-Kd. (D) WBC chimerism in the bone marrow (▪), spleen (■), and thymus (░). Engrafted sickle cell mice (n = 4) showed 45% ± 15% chimerism (H2-Kd-positive cells by flow cytometry) in the splenic compartment, 50% ± 10% chimerism in the bone marrow, and 25% ± 15% chimerism in the thymus.
Figure 2B shows the engraftment kinetics of individual cell lineages in the peripheral blood of chimeric mice. As expected, donor granulocytes (GR1) and macrophages (CD11b) appear first in the periphery, followed by donor B cells (B220) and T cells (CD3). Figure 2C shows representative flow cytometric data from which the values shown in Figure 2A,B were drawn. The peripheral chimerism in individual engrafted animals was reflected in their hematopoietic organs. Mean chimerism for bone marrow (50%), spleen (45%), and thymus (25%) is shown in Figure 2D.
The chimerism noted in the animals that received the full protocol of BMT, 20 mg/kg busulfan, and costimulation blockade was not donor strain–specific, because 5 sickle mice undergoing transplantation with busulfan, costimulation blockade, and TDBM from SJL mice (which are of the H-2Ks rather than H-2Kd genotype) showed similar levels of WBC and RBC chimerism (data not shown). Engrafted mice showed no signs of GVH disease: Their individual body weights remained stable (mean pretransplantation weight, 32 ± 1 g; mean posttransplantation weight, 36 ± 4 g) and posttransplantation necropsies, in which organs (including the skin, heart, lungs, spleen, liver, kidney, and intestinal tract) were specifically evaluated for signs of GVH disease, showed normal histology.
Peripheral white cell chimerism was comparable to previous results using busulfan and costimulation blockade in allogeneic wild-type mice,14 whereas peripheral RBC chimerism was strikingly higher in the sickle transplantation recipients. Quantification of donor (normal BALB/c β-globin, major and minor alleles) and recipient (human sickle β-globin) hemoglobins separated by cellulose acetate electrophoresis showed 78% to 90% donor chimerism within 2 weeks that reached 100% by 1 month in all of the engrafted sickle mice (Figure3). The complete replacement of host hemoglobin was stable for the entire experimental duration (> 150 days after transplantation). The higher level of peripheral erythroid chimerism compared with WBC chimerism is consistent with the accumulation and enhanced survival of normal RBCs in an environment with rapid turnover and degradation of sickle RBCs.
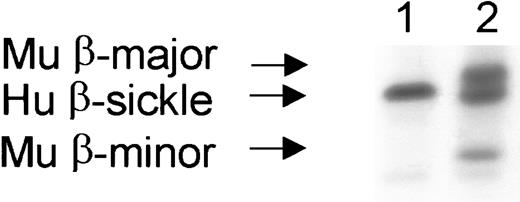
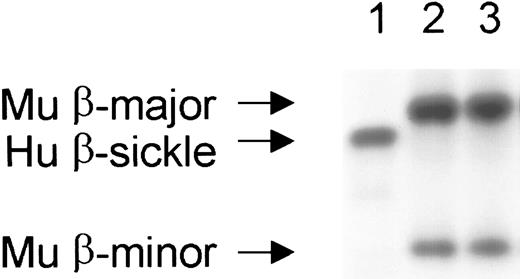
Stable, complete replacement of the peripheral RBC compartment occurred in engrafted mice.
RBC chimerism is determined by hemoglobin electrophoresis. Recipient mice originally possessed only human sickle β-globin (lane 1), while donor mice possessed the major and minor alleles of mouse β-globin (lane 2). Lane 3 shows a representative engrafted mouse with complete peripheral replacement with donor β-globin. Complete replacement of the peripheral blood with donor hemoglobin occurred within 1 month after transplantation and was stable for the entire observation period (> 150 days) in all engrafted mice. Hu indicates human; Mu, murine.
Stable, complete replacement of the peripheral RBC compartment occurred in engrafted mice.
RBC chimerism is determined by hemoglobin electrophoresis. Recipient mice originally possessed only human sickle β-globin (lane 1), while donor mice possessed the major and minor alleles of mouse β-globin (lane 2). Lane 3 shows a representative engrafted mouse with complete peripheral replacement with donor β-globin. Complete replacement of the peripheral blood with donor hemoglobin occurred within 1 month after transplantation and was stable for the entire observation period (> 150 days) in all engrafted mice. Hu indicates human; Mu, murine.
Mice receiving TDBM alone or TDBM plus busulfan (no costimulation blockade) showed no donor RBC (hemoglobin electrophoresis) or WBC (flow cytometry) engraftment. Thus, in the absence of costimulation blockade, the sickle mice were able to totally reject the donor graft. However, in the setting of BMT plus costimulation blockade (but no busulfan), 5 of 9 control mice developed significant red cell chimerism (10%-54%, Figure 4) despite minimal WBC chimerism (< 2.5%, Figure 2A). This probably reflects very low levels of stem cell engraftment and massive expansion of healthy red cell precursors, leading to significant peripheral read-out of donor hemoglobin.
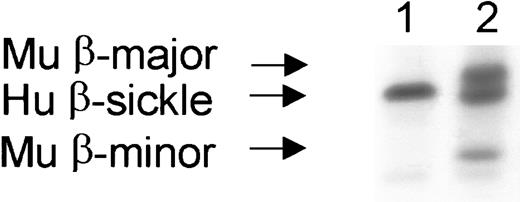
A subset of 5 of 9 mice who received only costimulation blockade (no busulfan) developed significant RBC chimerism.
Lane 1 shows a representative mouse without engraftment, with only human β-sickle hemoglobin. Lane 2 shows a representative mouse 3 months after transplantation with RBC chimerism, having both recipient (human β-sickle hemoglobin) and donor (mouse major and minor β-hemoglobin alleles) RBCs. Hu indicates human; Mu, murine.
A subset of 5 of 9 mice who received only costimulation blockade (no busulfan) developed significant RBC chimerism.
Lane 1 shows a representative mouse without engraftment, with only human β-sickle hemoglobin. Lane 2 shows a representative mouse 3 months after transplantation with RBC chimerism, having both recipient (human β-sickle hemoglobin) and donor (mouse major and minor β-hemoglobin alleles) RBCs. Hu indicates human; Mu, murine.
Chimeric mice are specifically tolerant to donor and recipient antigen
BALB/c mice express I-E and therefore delete Vβ5-bearing T cells, whereas sickle mice do not express I-E and specifically utilize Vβ5.1/2 on about 2% to 3% of CD4+ T cells.22 23 As anticipated, the 9 animals that received BMT and costimulation blockade but no busulfan (and who showed low to undetectable WBC engraftment, Figure 2A) failed to delete donor-reactive Vβ5+CD4+ T cells (Figure5A). In contrast, engrafted animals that received TDBM along with busulfan and costimulation blockade developed near complete deletion of CD4+Vβ5+ T cells by day 60. Part of this decrease in total Vβ5+CD4+ T cells could have been due to the mixture of donor (Vβ5−) and recipient (Vβ5+) CD4+ T cells even without deletion. However, given that 82% of CD4+ T cells showed Vβ5 deletion at 150 days (Figure 5A), while CD4+–T-cell chimerism was only 33% ± 8% at this same time point (data not shown), most of the reduction of Vβ5+CD4+ T cells is likely due to specific Vβ5+–T-cell deletion. The percentage of Vβ8-bearing CD4+ T cells, normally expressed on 15% to 25% of BALB/c and sickle CD4+ T cells, was similar in all groups, indicating that the T-cell deletion was donor-specific in nature (data not shown). These results suggest that the bone marrow–derived I-E–bearing donor cells influence the selection of the T-cell repertoire in engrafted mice, ultimately conferring robust long-term donor-specific tolerance.
Engrafted mice showed specific tolerance to donor T cells.
(A) CD4+ T cells from engrafted animals (■) ceased to utilize Vβ5.1/2, similarly to the donor (BALB/c, ░). Nonengrafted mice (▪) utilized Vβ5.1/2 throughout the observation period. Data show the average of 7 representative mice from each group at 150 days after transplantation. (B) T cells were examined for their proliferative capacity against donor, recipient, and third party using an in vivo alloproliferation model with CFSE-labeled T cells from engrafted and nonengrafted animals. The concentration of CFSE within the cell decreases by 50% after each division. Labeled T cells from engrafted and nonengrafted animals were adoptively transferred into lethally irradiated (1.8 Gy [1800 rads]) BALB/c (donor), sickle (recipient), and C3H (third-party) mice. Histograms of representative animals demonstrate that CD4+ and CD8+ T cells from engrafted recipients are essentially unresponsive to BALB/c (donor) and sickle (recipient) hosts. Both subsets of T cells, however, when transferred into C3H mice (third party) undergo maximal division (up to 8 divisions). Tolerant animals, therefore, show no proliferation to donor or recipient but a normal proliferative response to third party (C3H, H-2k).
Engrafted mice showed specific tolerance to donor T cells.
(A) CD4+ T cells from engrafted animals (■) ceased to utilize Vβ5.1/2, similarly to the donor (BALB/c, ░). Nonengrafted mice (▪) utilized Vβ5.1/2 throughout the observation period. Data show the average of 7 representative mice from each group at 150 days after transplantation. (B) T cells were examined for their proliferative capacity against donor, recipient, and third party using an in vivo alloproliferation model with CFSE-labeled T cells from engrafted and nonengrafted animals. The concentration of CFSE within the cell decreases by 50% after each division. Labeled T cells from engrafted and nonengrafted animals were adoptively transferred into lethally irradiated (1.8 Gy [1800 rads]) BALB/c (donor), sickle (recipient), and C3H (third-party) mice. Histograms of representative animals demonstrate that CD4+ and CD8+ T cells from engrafted recipients are essentially unresponsive to BALB/c (donor) and sickle (recipient) hosts. Both subsets of T cells, however, when transferred into C3H mice (third party) undergo maximal division (up to 8 divisions). Tolerant animals, therefore, show no proliferation to donor or recipient but a normal proliferative response to third party (C3H, H-2k).
Coincident with the long-term chimerism seen in the busulfan-treated animals was the development of specific tolerance to the allogeneic BALB/c bone marrow graft without GVH disease (Figure 5B). A rigorous in vivo alloproliferation assay using CFSE dye was performed to test for the presence of alloreactivity.24 T cells were harvested from spleens and mesenteric lymph nodes of animals that were at least 100 days after transplantation. After labeling with 10 μM CFSE, T cells were transferred into recipient mice (BALB/c, sickle, or C3H) previously supralethally irradiated. Splenocytes were harvested 72 hours later and analyzed via flow cytometry. While CD4+ and CD8+ T cells from nonengrafted groups underwent extensive cell division in response to both BALB/c and third-party (C3H) hosts but not to sickle hosts (data not shown), T cells from the engrafted mice generated no proliferative response to either donor (BALB/c) or recipient (sickle) hosts (Figure 5B). As expected, clear proliferative responses were present when naive BALB/c T cells were transferred into sickle hosts (data not shown) or when chimeric T cells were transferred to a third party (C3H, Figure 5B). These results confirm the specific absence of donor and recipient alloreactive CD4+ or CD8+ T cells capable of cell division in chimeric animals.
Chimeric mice are cured of sickle cell disease
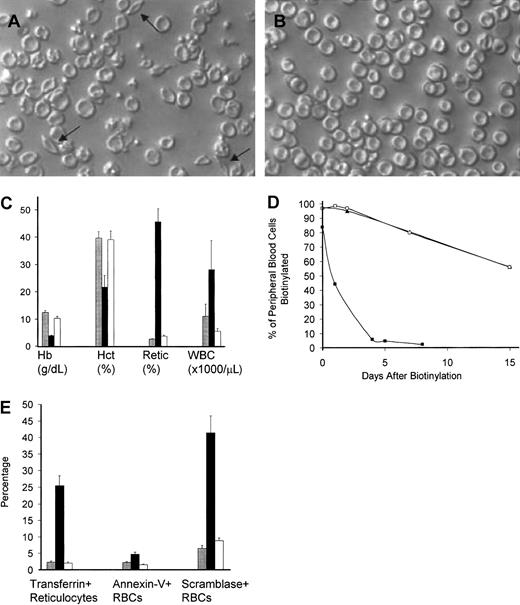
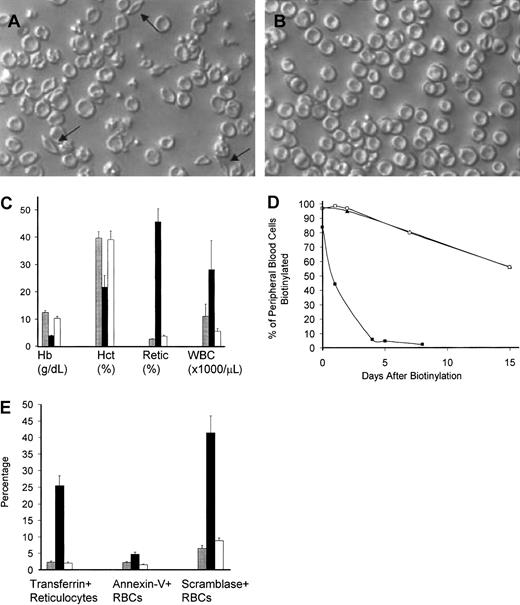
Engrafted sickle mice demonstrated a phenotypic cure of their SCD by a variety of parameters. As seen in Figure6A,B, a striking absence of irreversibly sickled cells in peripheral blood smears occurred after transplantation in mice that were conditioned with busulfan and costimulation blockade. Engrafted mice also demonstrated normalization of their hematologic abnormalities (Figure 6C), including hemoglobin (45 g/L corrected to 100 g/L [4.5 g/dL corrected to 10 g/dL]), hematocrit (0.16 corrected to 0.40 [16% corrected to 40%]), and peripheral thiazole+ reticulocyte percentage (49% corrected to 3.5%), consistent with a reversal of their hemolytic anemia. Furthermore, the abnormally elevated WBC count seen in naive sickle mice was corrected in engrafted mice (20 × 109/L to 5.1/ × 109/L [20 000/μL to 5100/μL]).
Engrafted mice demonstrated a phenotypic cure of SCD.
Peripheral blood smear from representative untreated (A) and engrafted (B) animals, prepared under ambient air. Arrows point to representative irreversibly sickled cells in the untreated blood. Engrafted animals have a normal peripheral smear. Original magnification, × 250. (C) Hematologic parameters are normalized in engrafted mice. Normalization of hematologic parameters was present within 1 month after transplantation and was stable for the entire experimental period (> 150 days). Shown are the mean ± SEM for C57BL/6 controls (n = 6, ░), nonengrafted mice (n = 4, ▪), and engrafted mice (n = 7, ■) in a representative experiment performed 3 months after transplantation. Hb indicates hemoglobin; Hct, hematocrit; Retic, thiazole+ reticulocyte percent; WBC, white blood cell count. (D) Engrafted mice have a normal RBC half-life. Peripheral blood was biotinylated at time 0, and the decay of biotinylation in RBCs (identified as Ter-119+, CD45−, biotinylated cells) was monitored by flow cytometry. Untreated sickle animals (▪) had an exceedingly short RBC half-life, while engrafted animals (■) had an RBC half-life that was indistinguishable from normal C57BL/6 controls (▴). These data represent the average of 2 animals that were biotinylated 3 months after transplantation. (E) The engrafted RBC population is healthy as determined by normalization of the percentage of transferrin+ stress reticulocytes, PS exposure as measured by annexin V binding, and scramblase activity as measured by NBD-PC internalization. C57BL/6 control (░, n = 6), nonengrafted (▪, n = 4), and engrafted (■, n = 7) animals were analyzed. Shown are the mean ± SEM of animals analyzed 3 months after transplantation. Normalization of RBC parameters in engrafted animals occurred within 1 month after transplantation and was stable for the entire observation period (> 150 days).
Engrafted mice demonstrated a phenotypic cure of SCD.
Peripheral blood smear from representative untreated (A) and engrafted (B) animals, prepared under ambient air. Arrows point to representative irreversibly sickled cells in the untreated blood. Engrafted animals have a normal peripheral smear. Original magnification, × 250. (C) Hematologic parameters are normalized in engrafted mice. Normalization of hematologic parameters was present within 1 month after transplantation and was stable for the entire experimental period (> 150 days). Shown are the mean ± SEM for C57BL/6 controls (n = 6, ░), nonengrafted mice (n = 4, ▪), and engrafted mice (n = 7, ■) in a representative experiment performed 3 months after transplantation. Hb indicates hemoglobin; Hct, hematocrit; Retic, thiazole+ reticulocyte percent; WBC, white blood cell count. (D) Engrafted mice have a normal RBC half-life. Peripheral blood was biotinylated at time 0, and the decay of biotinylation in RBCs (identified as Ter-119+, CD45−, biotinylated cells) was monitored by flow cytometry. Untreated sickle animals (▪) had an exceedingly short RBC half-life, while engrafted animals (■) had an RBC half-life that was indistinguishable from normal C57BL/6 controls (▴). These data represent the average of 2 animals that were biotinylated 3 months after transplantation. (E) The engrafted RBC population is healthy as determined by normalization of the percentage of transferrin+ stress reticulocytes, PS exposure as measured by annexin V binding, and scramblase activity as measured by NBD-PC internalization. C57BL/6 control (░, n = 6), nonengrafted (▪, n = 4), and engrafted (■, n = 7) animals were analyzed. Shown are the mean ± SEM of animals analyzed 3 months after transplantation. Normalization of RBC parameters in engrafted animals occurred within 1 month after transplantation and was stable for the entire observation period (> 150 days).
The health of the newly emerging chimeric red cells was assessed by 3 physiologic markers. First, RBC population half-life was determined through a pulsed biotinylation experiment19 (Figure 6D). Untreated and engrafted sickle mice were injected intravenously with N-hydroxysuccinimide biotin to label the peripheral blood with biotin as previously described.19 RBC half-life was determined by the decay of the biotinylated RBCs over time by flow cytometry. Consistent with previous work from this laboratory,25 RBCs from the naive sickle animals had exceedingly short peripheral half-lives (0.8 days) compared with normal control C57BL/6 mice (half-life 18 days). Engrafted animals had a RBC half-life indistinguishable from that of normal mice, consistent with replacement of the diseased red cell compartment with normal RBCs. Second, we measured the production of transferrin+ “stress” reticulocytes in engrafted and nonengrafted mice. These cells are an indication of overactive erythropoiesis and are thought to contribute to the increased adhesion of sickle reticulocytes in the microvasculature.18,26,27 Figure 6E shows that the percent of these cells decreases from 27% to 3% in engrafted mice, consistent with normalization of red cell turnover in these animals. Third, we examined plasma membrane phosphatidylserine (PS) exposure, which is known to be increased in sickle red cells.28,29 PS is thought to contribute to increased clearance of these cells by macrophages and monocytes and may also contribute to abnormal endothelial adhesion.30 Two assays were used: annexin V binding, which measures exposed PS residues directly,31and NBD-PC internalization, which measures the scramblase enzyme that leads to PS exposure on the plasma membrane.20,21 32Figure 6E shows that sickle mice consistently show a high PS exposure prior to transplantation (measured by annexin V binding), but engrafted mice demonstrate a significant decrease in this PS exposure. Figure 6E also shows that a dramatic decrease in the number of RBCs with active scramblase occurs after engraftment, consistent with the decline in PS exposure described above.
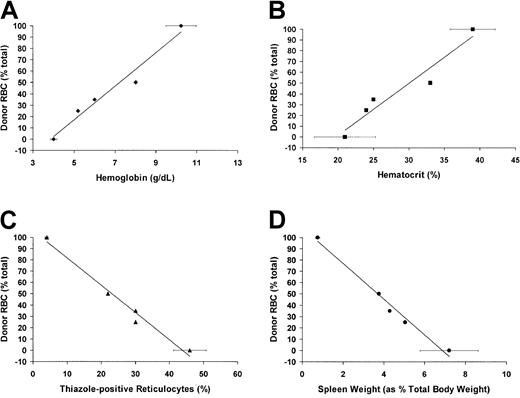
Although the focus of this study is the effect of full RBC replacement in the face of partial WBC engraftment, as noted above, a subset of mice (5 of 9) receiving BMT and costimulation blockade (but not busulfan) did develop partial RBC chimerism. Of those 5 mice, 2 died unexpectedly (1 at 3 months, 1 at 4 months of age, neither with signs of GVH disease) and thus were not fully analyzed. The remaining mice were analyzed for the effects of mixed RBC chimerism on murine SCD. As shown in Figure 7, there was a progressive normalization of both hematologic (hemoglobin and hematocrit) and physiologic (reticulocyte count and spleen size) parameters in mice with increasing RBC engraftment. Further analysis also showed partial correction of NBD-PC internalization, annexin V binding, and splenic hematopoeitic balance in mice with mixed donor and host RBCs (data not shown). A separate study designed to rigorously determine the effect of progressively increasing amounts of normal RBCs in the sickle background is currently underway (L.S.K. et al, in preparation).
Hematologic parameters are partially normalized in mice with partial RBC chimerism.
(A) Hemoglobin concentration (g/dL, as determined by automated complete blood count analysis) versus percent peripheral RBC engraftment. (B) Hematocrit (percentage, as determined by automated complete blood count analysis) versus percent peripheral RBC engraftment. (C) Reticulocyte count (percent thiazole+, Ter-119+ cells determined by flow cytometry) versus percent peripheral RBC engraftment. (D) Spleen size (as percentage of total body weight) versus percent peripheral RBC engraftment. For all panels, age-matched, unengrafted sickle mice were used for 0% engraftment controls (n = 4), and engrafted animals (n = 4) were used as 100% engrafted controls. Each of the other points represents individual mice with varying levels of peripheral RBC engraftment.
Hematologic parameters are partially normalized in mice with partial RBC chimerism.
(A) Hemoglobin concentration (g/dL, as determined by automated complete blood count analysis) versus percent peripheral RBC engraftment. (B) Hematocrit (percentage, as determined by automated complete blood count analysis) versus percent peripheral RBC engraftment. (C) Reticulocyte count (percent thiazole+, Ter-119+ cells determined by flow cytometry) versus percent peripheral RBC engraftment. (D) Spleen size (as percentage of total body weight) versus percent peripheral RBC engraftment. For all panels, age-matched, unengrafted sickle mice were used for 0% engraftment controls (n = 4), and engrafted animals (n = 4) were used as 100% engrafted controls. Each of the other points represents individual mice with varying levels of peripheral RBC engraftment.
The spleens in the engrafted mice exhibit signs of reversal of characteristic sickle pathology
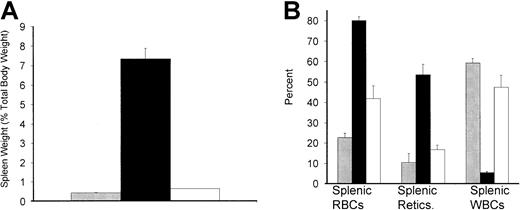
One of the hallmarks of murine sickle cell pathophysiology is the dramatic increase in spleen size compared with that of normal animals.16,17,25 This is related to the immense requirement for erythropoiesis in order to replenish the rapid destruction of peripheral sickle RBCs.16,17 25 As shown in Figure 8A, the spleen undergoes a significant decrease in size in engrafted mice (from 7.3% total body weight in age-matched naive sickle mice to 0.7% total body weight in engrafted mice measured 5 months after transplantation). Figure 8B shows that while the spleen functions as a largely erythropoietic organ in untreated sickle mice, it undergoes a reprogramming in the engrafted cohort and resumes a more normal balance between white and red cell hematopoiesis. Figure 9A,B shows a histologic comparison of the spleens from naive and engrafted mice. In engrafted mice there is a striking resolution of the characteristic hyperactive hematopoiesis and red cell pooling characteristic of SCD and the acquisition of normal splenic architecture.
The spleen demonstrated reversal of pathophysiology in engrafted animals.
(A) Spleen weight expressed as percent total body weight (mean ± SEM) in C57BL/6 control (░, n = 5), untreated sickle (▪, n = 7), and engrafted (■, n = 4) animals. Spleen weights in engrafted animals were measured 5 months after transplantation. Spleen weights for control mice were age-matched to the engrafted animals. (B) The balance of hematopoiesis in the spleen was also normalized in engrafted mice. The percent RBCs, reticulocytes, and WBCs (mean ± SEM) all approach C57BL/6 control values (░, n = 5) in engrafted mice (■, n = 4), while untreated sickle animals (▪, n = 7) are highly abnormal. Splenic hematopoiesis in engrafted animals was measured 5 months after transplantation. Control mice were age-matched to the engrafted animals.
The spleen demonstrated reversal of pathophysiology in engrafted animals.
(A) Spleen weight expressed as percent total body weight (mean ± SEM) in C57BL/6 control (░, n = 5), untreated sickle (▪, n = 7), and engrafted (■, n = 4) animals. Spleen weights in engrafted animals were measured 5 months after transplantation. Spleen weights for control mice were age-matched to the engrafted animals. (B) The balance of hematopoiesis in the spleen was also normalized in engrafted mice. The percent RBCs, reticulocytes, and WBCs (mean ± SEM) all approach C57BL/6 control values (░, n = 5) in engrafted mice (■, n = 4), while untreated sickle animals (▪, n = 7) are highly abnormal. Splenic hematopoiesis in engrafted animals was measured 5 months after transplantation. Control mice were age-matched to the engrafted animals.
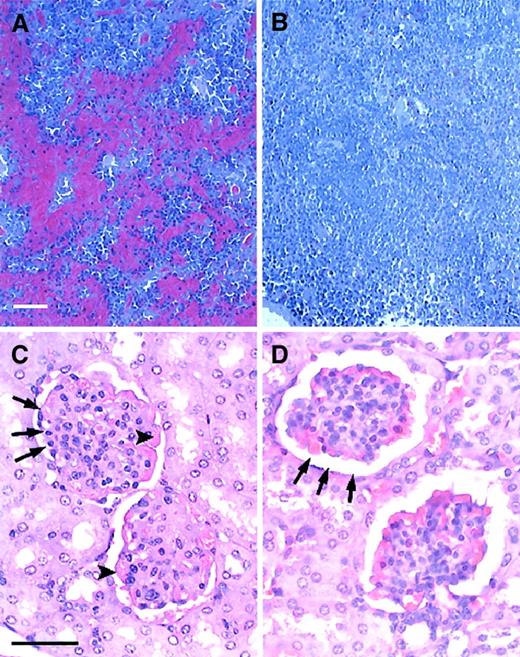
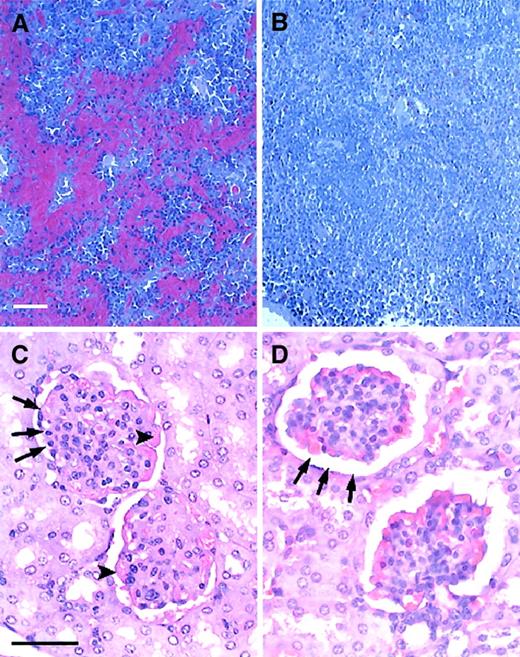
Engrafted mice showed no evidence of characteristic sickle splenic and renal pathology.
(A,B) Histology of the spleen in untreated and engrafted mice. (A) Untreated sickle spleen. Splenic architecture is highly abnormal with pooling of sickled RBCs and areas of increased hematopoiesis. (B) Spleen from an engrafted mouse killed 5 months after transplantation. Splenic architecture is now normal. No RBC pooling is present. (C,D) Histology of the kidney in untreated and engrafted mice. (C) Untreated sickle kidney. The sickle kidney shows evidence of membranoproliferative glomerulonephritis. Arrowheads point to thickened glomerular membrane. Arrows point to narrowed glomerular space. (C) Kidney from an engrafted mouse killed 5 months after transplantation. Glomerular architecture is now normal. Sickle mice were age-matched to engrafted mice for this analysis. Sections were stained with hematoxylin and eosin. Bars = 50 μm.
Engrafted mice showed no evidence of characteristic sickle splenic and renal pathology.
(A,B) Histology of the spleen in untreated and engrafted mice. (A) Untreated sickle spleen. Splenic architecture is highly abnormal with pooling of sickled RBCs and areas of increased hematopoiesis. (B) Spleen from an engrafted mouse killed 5 months after transplantation. Splenic architecture is now normal. No RBC pooling is present. (C,D) Histology of the kidney in untreated and engrafted mice. (C) Untreated sickle kidney. The sickle kidney shows evidence of membranoproliferative glomerulonephritis. Arrowheads point to thickened glomerular membrane. Arrows point to narrowed glomerular space. (C) Kidney from an engrafted mouse killed 5 months after transplantation. Glomerular architecture is now normal. Sickle mice were age-matched to engrafted mice for this analysis. Sections were stained with hematoxylin and eosin. Bars = 50 μm.
Renal histology is normal in engrafted mice
In addition to the defects observed in both the peripheral blood and the hematopoietic organs, sickle mice also demonstrate solid organ pathology similar to that seen in patients with SCD. 16,17As in the original description of this murine SCD model,16 17 we have noted pathologic changes in many organs, including the kidney, liver, lung, and heart in untreated sickle animals. To determine the effect of BMT on organ structure and histology, necropsies were performed on age-matched naive and engrafted animals and tissues were prepared for histologic analysis. We found that engrafted animals had normal histology of all organs tested, including the kidney, liver, heart, and lungs. Representative of the histologic normalization that occurred in these animals, Figure9C,D shows a comparison of renal histology in age-matched untreated and engrafted mice. Figure 9C shows the membranoproliferative glomerulonephritis consistently observed in untreated sickle mice. Figure 9D shows that engrafted animals had normal renal histology, including normalization of glomerular capsular space and glomerular membrane thickness.
Discussion
Stem cell transplantation is the only current treatment that offers an effective cure for SCD.3,4,33,34 However, the availability of this treatment is limited by the lack of HLA-matched related donors and the toxicity of the myeloablative preconditioning regimens.1,2 While alternative stem cell sources are being explored, such as unrelated cord blood stem cells and partially matched relatives, the use of these types of donor cells has required strong, toxic, preparative regimens to myeloablate all residual host cells. High induction-related morbidity/mortality is not acceptable in SCD, because good medical support can extend life to 40 to 60 years without transplantation.2 Indeed, the first 2 adults undergoing transplantation by the Seattle consortium died of toxicity-related complications, which halted the adult clinical trial. Moreover, Vermylen et al showed that survival and disease-free survival after BMT were consistently worse in those children who met the Seattle criteria for severe disease than in those who were relatively asymptomatic.4 BMT will be available to most SCD patients only when a minimally toxic regimen can be established. Furthermore, given the scarcity of matched-related donors, a truly successful approach will need to cross allogeneic barriers.
Walters et al reported that a subset of patients who were treated with a myeloablative preparative regimen and BMT (designed to produce total replacement with donor cells) developed stable mixed chimerism.3 These mixed-chimeric patients had no further sickle cell–related clinical events. This clinical experience leads to 2 major lines of experimental inquiry: (1) Can a regimen be developed to intentionally produce tolerance and mixed WBC chimerism, and will this result in a reliable cure of SCD? (2) What is the minimal level of RBC chimerism that can lead to both a hematologic and systemic cure of SCD? The first step toward the ultimate goal of answering these questions in a clinical setting is to perform controlled studies in a bona fide animal model of SCD. The recent creation of transgenic mice in which human sickle β-globin exists in a background of normal human α-globin and a complete knock-out of the endogenous murine α- and β-globin genes represent the latest advances toward such a model.16,17,35 These mice recapitulate much of the hematologic pathology seen in human SCD, including hemolytic anemia (with resulting low hemoglobin concentration, low hematocrit, and high reticulocyte count) and high WBC count. Their RBCs do differ from human sickle RBCs in that they have a lower mean corpuscular hemoglobin concentration, likely resulting from a mild α/β chain imbalance, and subsequent mild thalassemic component to their anemia.16,17,35 It is speculated that this slight α/β chain imbalance is just enough to prevent this model from being lethal35 and that the overall authenticity of this model of SCD is not seriously impaired by this difference.16,17Importantly, these knock-out models of SCD also show total-body pathology reminiscent of human SCD, including splenic sequestration and enhanced splenic erythropoiesis, renal cortical and glomerular pathology, and liver infarction.16 17 The creation of this latest generation of murine SCD models thus allows investigation of both the hematologic consequences of mixed hematopoietic chimerism and the effect that engraftment has on organ pathology and the systemic disease.
In this study we have focused on the first question posed above and have intentionally induced mixed WBC chimerism in the transgenic sickle mice with a well-tolerated, nonmyeloablative preconditioning regimen employing low-dose busulfan. Furthermore, we have added costimulation blockade of the CD40/CD40L and CD28/B7 pathways to induce stable donor-specific tolerance to fully MHC-mismatched bone marrow without GVH disease. This protocol resulted in WBC mixed chimerism and complete replacement of the peripheral RBC compartment in 10 of 13 animals within 1 month of transplantation that was stable throughout the observation period (> 150 days). The fact that engrafted mice demonstrated a significantly higher level of donor RBCs in the peripheral blood compared with donor WBCs (100% vs 43%, respectively) is likely due to the dramatically increased clearance of sickle RBCs from the circulation, with RBC read-out reflecting the lineage-specific survival of donor RBCs. Thus, even with partial stem cell engraftment and mixed WBC chimerism, complete normalization of the peripheral RBC defect occurred in engrafted sickle mice.
The replacement of the RBC compartment with normal cells resulted in a clinical cure of murine SCD by all parameters tested, including reversal of hemolytic anemia, disappearance of sickled forms on peripheral blood smears, reduction of RBC PS exposure, and normalization of the hematopoietic balance in the spleen. Furthermore, engrafted mice showed a reduction in peripheral WBC count, which is elevated in the untreated sickle cohort. This reduction in peripheral WBC count is particularly interesting given that several lines of evidence indicate that leukocytes play a major role in sickle cell pathophysiology.36,37 Indeed, elevated WBC count is an epidemiologic risk factor for increased morbidity and mortality in SCD,38 including pulmonary complications,39stroke,40 and silent brain infarction.41Moreover, decline in WBC count was the hematologic parameter most strongly correlated with clinical response to hydroxyurea in reducing the severity of SCD.42 Importantly, in addition to the correction of the sickle hematologic abnormalities noted above, engrafted mice also showed normalization of solid organ structure and histology. Again, this is a strong indication that whole-body cure of murine SCD was accomplished with our transplantation protocol.
All sickle mice treated with TDBM alone or TDBM plus busulfan (no costimulation blockade) fully rejected their bone marrow graft and developed no WBC or RBC chimerism. In contrast, significant peripheral RBC chimerism (10%-54%) was obtained in a subset of mice (5 of 9 animals) that did not receive busulfan, but that did receive TDBM and costimulation blockade, despite minimal WBC chimerism (< 2.5% by flow cytometry). The higher level of donor RBCs compared with WBCs in the peripheral blood again likely reflects their survival advantage over the rapidly cleared sickle cells. Given the recent discovery of common lymphoid and myeloid precursors,43,44 the intriguing possibility also exists of a common erythroid/megakaryocyte precursor and that enhanced engraftment of such a cell may occur in sickle mice. Clinically, the ability to engraft RBCs without any busulfan treatment may be very important. Given the extreme fragility of adult SCD patients subjected to conventional BMT, a regimen that would produce RBC chimerism without any chemotherapy may allow even the sickest patients an option of long-term disease amelioration. This development of mixed RBC chimerism in a subset of mice given BMT and costimulation blockade without busulfan pretreatment applies to the second question posed above: What is the minimal level of RBC chimerism that can lead to both a hematologic and systemic cure of SCD? Although beyond the scope of the present study, the small number of animals who serendipitously developed mixed RBC chimerism (Figure 7) does give us an indication that progressive hematologic and physiologic cure can result with increasing levels of normal RBCs. This is to be expected given the vast clinical experience with acute and chronic transfusion programs. It is also in agreement with the study by Iannone et al, wherein normal mice underwent transplantation with sickle bone marrow in such a way as to produce progressively increasing levels of sickle RBCs.7 In that study, mice with increasing levels of sickle RBCs had progressively more abnormal hematologic and organ pathology. We are currently modifying our transplantation regimen to consistently produce varying levels of mixed donor/recipient RBC chimerism in the sickle background (L.S.K. et al, in preparation). Long-term studies with large numbers of mice will be required to produce insights into the total-body effect of mixed RBC chimerism and may indicate whether less than total RBC replacement is a reasonable goal for clinical cure.
Our results demonstrate that a phenotypic cure for murine SCD can be achieved without the significant risks associated with myeloablative BMT regimens. In the murine SCD model, BMT after nonmyeloablative conditioning and tolerance induction leads to stable, robust mixed WBC chimerism across allogeneic barriers. In this study, complete replacement of abnormal RBCs was accomplished in the setting of mixed WBC chimerism, and this complete replacement with donor RBCs led to hematologic and systemic cure of murine SCD. Many questions still exist: Is complete replacement of sickle RBCs required for a full systemic cure, or is there a level of mixed RBC chimerism in which sickle hematologic and organ pathology is consistently and stably corrected? What are the factors that mediate acceptance or rejection of the bone marrow graft in the setting of tolerance induction and nonmyeloablative conditioning? Is the inflammatory state that exists in the background of both sickle mice and sickle patients a contributor to graft rejection? Perhaps most importantly, how will these results be translated to affect human SCD patients? Clearly, this is just the first step in the long road toward developing nonmyeloablative transplantation and tolerance induction for SCD patients. However, although allogeneic barriers are much more significant in man than in mouse, our successful use of costimulation blockade to cross allogeneic barriers suggests that in the future related techniques may be clinically feasible for human SCD and other nonmalignant hematologic disorders.
We thank Diane Hollenbaugh, Robert Peach, and Alejandro Aruffo (Bristol-Myers Squibb) for providing CTLA4-Ig. We thank J. Wylie Nichols (Emory University) for providing NBD-PC. We thank Sonji Webb for assistance with mouse necropsies and Laura Brown and Daniel Jack Jr for excellent technical support.
Supported in part by research grants DK/AI40519 (C.P.L.), CA74364-03 (C.P.L.), AI44644 (C.P.L.), and R29HL60127 (D.R.A.) from the National Institutes of Health and by CURE Childhood Leukemia (D.R.A.), the Jill Andrews/Phil Niekro Celebrity Classic (D.R.A.), the ERC Program of the National Science Foundation under award number EEC9731643 (C.P.L.), and the Carlos and Marguerite Mason Trust (C.P.L.).
L.S.K. and M.M.D. contributed equally to the work and are co–first authors. C.P.L. and D.R.A. should be considered co–senior authors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David R. Archer, Div of Hematology, Oncology Blood and Marrow Transplantation, Dept of Pediatrics, Emory University School of Medicine, Atlanta, GA 30322; e-mail: darcher@emory.edu.

![Fig. 2. Sickle mice treated with BMT after busulfan and costimulation blockade developed stable WBC mixed chimerism in the peripheral blood and the hematopoietic organs. / (A) Mean WBC chimerism in the peripheral blood. Sickle mice (H2-Kb) recipients were treated with 20 mg/kg busulfan (intraperitoneally) on day −1, with 500 μg CTLA4-Ig and anti-CD40L intraperitoneally on days 0, 2, 4, and 6, and underwent transplantation with allogeneic BALB/c (H2-Kd) TDBM (2 × 107 cells intravenously on day 0). The data in this figure represent the mean percent engraftment for 7 engrafted mice that were fully analyzed for more than 150 days (▪). Three mice became transiently engrafted but rejected their grafts on or before 60 days. These mice completely reverted to the host WBC and RBC phenotype by 3 to 4 months after transplantation (data not shown). Control animals received 1 of 3 treatments: TDBM alone (n = 5, ○), TDBM plus busulfan (no costimulation blockade, n = 5, ▵), or TDBM plus costimulation blockade but no busulfan (n = 9, □) Animals in each of the control groups showed less than 2.5% peripheral WBC chimerism. (B) Lineage-specific chimerism over time. The percent of each lineage (granulocytes [GR1], macrophages [CD11b], B cells [B220], and T cells [CD3]) that was donor type (H2-Kd) was identified by flow cytometry over time. Values shown are the mean for each time point from 7 engrafted animals ± SEM. ▪ indicates B220; ●, GR1; ▴, CD11b; ⧫, CD3. (C) Representative flow cytometric analysis. At left is a histogram showing H2-Kd staining of a chimeric animal with both host (H2-Kd-negative) and donor (H2-Kd-positive) WBCs. At right is a dot plot showing a representative quadrant analysis of CD3 cells versus H2-Kd. (D) WBC chimerism in the bone marrow (▪), spleen (■), and thymus (░). Engrafted sickle cell mice (n = 4) showed 45% ± 15% chimerism (H2-Kd-positive cells by flow cytometry) in the splenic compartment, 50% ± 10% chimerism in the bone marrow, and 25% ± 15% chimerism in the thymus.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/5/10.1182_blood.v99.5.1840/5/m_h80522171002.jpeg?Expires=1769248050&Signature=fgcJdOIQf2bowMT6aSDa1iXt23PPGIRK53d-IUZICYmGVxfN3zG4I~BLB4NJHEYcBO~jb7fi8inwME3vJbumilBxbLoAqBfi-KU9I9ccTT8Ks6Vs6VfJ5GHcfAsoWHZ8UvOuA0s3VKlTVj4XA6PfN901pgEf2HbUF-0dp00VaTvYRlDhxCvxuKKrsG0n7rvseYaXWKFzXSwnerDm1dM5O4G3NuUjzHNPiq0L11ODHnUHY2fopmE8QfZrVhthenvjwkccE8K5y9o-M79C1dlIci9nN9E-y5P7ktK6XTORdCiwf98RV60gaAmSbisyOFNaTYte~IMa0GtmCNWsGmCa7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 5. Engrafted mice showed specific tolerance to donor T cells. / (A) CD4+ T cells from engrafted animals (■) ceased to utilize Vβ5.1/2, similarly to the donor (BALB/c, ░). Nonengrafted mice (▪) utilized Vβ5.1/2 throughout the observation period. Data show the average of 7 representative mice from each group at 150 days after transplantation. (B) T cells were examined for their proliferative capacity against donor, recipient, and third party using an in vivo alloproliferation model with CFSE-labeled T cells from engrafted and nonengrafted animals. The concentration of CFSE within the cell decreases by 50% after each division. Labeled T cells from engrafted and nonengrafted animals were adoptively transferred into lethally irradiated (1.8 Gy [1800 rads]) BALB/c (donor), sickle (recipient), and C3H (third-party) mice. Histograms of representative animals demonstrate that CD4+ and CD8+ T cells from engrafted recipients are essentially unresponsive to BALB/c (donor) and sickle (recipient) hosts. Both subsets of T cells, however, when transferred into C3H mice (third party) undergo maximal division (up to 8 divisions). Tolerant animals, therefore, show no proliferation to donor or recipient but a normal proliferative response to third party (C3H, H-2k).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/5/10.1182_blood.v99.5.1840/5/m_h80522171005.jpeg?Expires=1769248050&Signature=PH7FhpEgy6JFDH8eSFvFEFvx8XaZwSjdOcRUw689~G1EJA361VmzCAsE84G7hUu~xIvWG7qX~f9oVapA-sYYVUN0o-qVdLLvj4F~MuXkEbpXvVLuVgrgcvQkbcTPsjBJfqgRzBWofJdGcOmwEkuUaAhHqNyDltQfZjI1kmbebtTVHy08ZBZabcx6Ji62aBr5Q96beX~6IzIYegC8vMJSpHx7KFSALepQZlPu~xU-G9g4h-kWWS6QawAhFpnHNGdlTKYgEpOkorjpKHY50usy5K~Le7qgUPOjzHxanfP8xOJfdK17afQrDlyQ9VaUVq8suFLIBRbwCU2DJSj~Xy7nTA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 2. Sickle mice treated with BMT after busulfan and costimulation blockade developed stable WBC mixed chimerism in the peripheral blood and the hematopoietic organs. / (A) Mean WBC chimerism in the peripheral blood. Sickle mice (H2-Kb) recipients were treated with 20 mg/kg busulfan (intraperitoneally) on day −1, with 500 μg CTLA4-Ig and anti-CD40L intraperitoneally on days 0, 2, 4, and 6, and underwent transplantation with allogeneic BALB/c (H2-Kd) TDBM (2 × 107 cells intravenously on day 0). The data in this figure represent the mean percent engraftment for 7 engrafted mice that were fully analyzed for more than 150 days (▪). Three mice became transiently engrafted but rejected their grafts on or before 60 days. These mice completely reverted to the host WBC and RBC phenotype by 3 to 4 months after transplantation (data not shown). Control animals received 1 of 3 treatments: TDBM alone (n = 5, ○), TDBM plus busulfan (no costimulation blockade, n = 5, ▵), or TDBM plus costimulation blockade but no busulfan (n = 9, □) Animals in each of the control groups showed less than 2.5% peripheral WBC chimerism. (B) Lineage-specific chimerism over time. The percent of each lineage (granulocytes [GR1], macrophages [CD11b], B cells [B220], and T cells [CD3]) that was donor type (H2-Kd) was identified by flow cytometry over time. Values shown are the mean for each time point from 7 engrafted animals ± SEM. ▪ indicates B220; ●, GR1; ▴, CD11b; ⧫, CD3. (C) Representative flow cytometric analysis. At left is a histogram showing H2-Kd staining of a chimeric animal with both host (H2-Kd-negative) and donor (H2-Kd-positive) WBCs. At right is a dot plot showing a representative quadrant analysis of CD3 cells versus H2-Kd. (D) WBC chimerism in the bone marrow (▪), spleen (■), and thymus (░). Engrafted sickle cell mice (n = 4) showed 45% ± 15% chimerism (H2-Kd-positive cells by flow cytometry) in the splenic compartment, 50% ± 10% chimerism in the bone marrow, and 25% ± 15% chimerism in the thymus.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/5/10.1182_blood.v99.5.1840/5/m_h80522171002.jpeg?Expires=1769502782&Signature=0pa3csPADto5wz3VTEklgLpmkaVDzIbtZnPaK1UWHn71yNps88PiF5cLIBP9T8zhMva-PXBiDGFxk-LPj3utiej2FCGJT0K6onqAw~cMfKxYkxJtDrL8l7-j1Ni7gwxvcRHcbuKhLOcpu3LL7WSaprc9gHBDbjlILSv60ldrC5F77NhOd9tkDqPlSx40Cl3W-qjAddbD6hCI3kWPShpoUF9PFoV07kzxYzeFugdzUDFD1FpfczgTjDccQd65KQFbhQNlLxyw1hGn6vKI3OkaXkKYEUfPEjZehSRJ1eMyN9Rq~GDLmRuUICPwDE2gS2uEHDAZLXAOfTf4ZVUMNHQbUw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Engrafted mice showed specific tolerance to donor T cells. / (A) CD4+ T cells from engrafted animals (■) ceased to utilize Vβ5.1/2, similarly to the donor (BALB/c, ░). Nonengrafted mice (▪) utilized Vβ5.1/2 throughout the observation period. Data show the average of 7 representative mice from each group at 150 days after transplantation. (B) T cells were examined for their proliferative capacity against donor, recipient, and third party using an in vivo alloproliferation model with CFSE-labeled T cells from engrafted and nonengrafted animals. The concentration of CFSE within the cell decreases by 50% after each division. Labeled T cells from engrafted and nonengrafted animals were adoptively transferred into lethally irradiated (1.8 Gy [1800 rads]) BALB/c (donor), sickle (recipient), and C3H (third-party) mice. Histograms of representative animals demonstrate that CD4+ and CD8+ T cells from engrafted recipients are essentially unresponsive to BALB/c (donor) and sickle (recipient) hosts. Both subsets of T cells, however, when transferred into C3H mice (third party) undergo maximal division (up to 8 divisions). Tolerant animals, therefore, show no proliferation to donor or recipient but a normal proliferative response to third party (C3H, H-2k).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/5/10.1182_blood.v99.5.1840/5/m_h80522171005.jpeg?Expires=1769502782&Signature=gpRg0OAfIP8wwaRLjNiamJ08iS~uF4KKVFKvyjntu6p43tNtbqwYMc4hdmroHlJFJFRpdirk-vZbw0y8VAHxfSi35FX0kQrDx~rZvL-fd0OBJQCDNC5QLjQiUJ9B4Xckc8~TUktz9cR8UnugYlH9dcKqUVQ0G2JtsMawgPu65soONySXF0Xc3o8pTK3Yhh9-rGPpDO8IyVzI4lDWZYPxv5Oi4QFx-erWOEddcVv0zU3tgtCPqHE4rfg-hjkgvGoTL6gouSeSBYPmSwGr1o4dPXrXZgp6Ct5NHDFMYBpXGHC1~I3fI1URDMA4BJsTRJ3U3aOy5OhyGinR7JzUSR09fA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)