Abstract
Tyk2 is activated in response to interleukin-12 (IL-12) and is essential for IL-12–induced T-cell function, including interferon-γ (IFN-γ) production and Th1 cell differentiation. Because IL-12 is a stimulatory factor for natural killer (NK) cell–mediated cytotoxicity, we examined whether tyk2 is required for IL-12–induced NK cell activity. IL-12–induced NK cell activity in cells from tyk2-deficient mice was drastically reduced compared to that in cells from wild-type mice. IL-18 shares its biologic functions with IL-12. However, the molecular mechanism of IL-18 signaling, which activates an IL-1 receptor–associated kinase and nuclear translocation of nuclear factor-κB, is different from that of IL-12. We next examined whether biologic functions induced by IL-18 are affected by the absence of tyk2. NK cell activity and IFN-γ production induced by IL-18 were reduced by the absence of tyk2. Moreover, the synergistic effect of IL-12 and IL-18 for the production of IFN-γ was also abrogated by the absence of tyk2. This was partially due to the absence of any up-regulation of the IL-18 receptor treated with IL-12, and it might suggest the presence of the cross-talk between Jak-Stat and mitogen-activated protein kinase pathways in cytokine signaling.
Introduction
The nonreceptor tyrosine kinases of the Jak family are associated with the cytokine receptor and play a pivotal role in transducing cytokine signaling.1 Activated Jaks phosphorylate the tyrosine residues of the receptors, thereby recruiting signal transducers and activators of transcription (Stats) and other signaling molecules into the activated receptor complex. Stats are in turn phosphorylated by Jaks, and subsequently these activated Stats translocate to the nucleus to affect gene expression.2,3 There are 4 mammalian Jaks: Jak1, Jak2, Jak3, and tyk2. Tyk2 has been identified as a novel protein kinase to compensate for the interferon-α (IFN-α) nonresponsible mutated fibroblasts,4 although we and others have shown that tyk2 has only a restricted function and does not play a major role in IFN-α signaling using tyk2-deficient mice.5,6Surprisingly, tyk2 was revealed to be essential for interleukin-12 (IL-12)–mediated T-cell function, including IFN-γ production and Th1 cell differentiation. Moreover, tyk2 is redundant for IL-12–induced T-cell proliferation.5 Because IL-12 was initially identified as a stimulatory factor for natural killer (NK) cells that activates NK cell–mediated cytotoxicity,7 8 we examined the NK cell activity of IL-12–induced cells from tyk2-deficient mice.
Interleukin-18 is a cytokine that was originally identified as an IFN-γ–inducing factor,9 and it appears to share its biologic functions with IL-12, including enhancement of the NK cell activity and proliferation of activated T cells.10-12 The IL-18 receptor is composed of 2 chains, a cytokine-binding IL-18 receptor (IL-18R; originally identified as an IL-1R–related protein) and an accessory proteinlike chain, AcPL.13-16IL-18 stimulation activates the DNA binding activity of both nuclear factor-κB (NF-κB) and AP-1,17 and in some cell line cells it also activates Stat3.18
Using previously generated tyk2-deficient mice that specifically lack T-cell responses to IL-12, we show here that tyk2 is also essential for IL-12–induced NK cell activity, and that tyk2 also contributes to the IL-18 biologic functions by in part regulating the IL-18R expression.
Materials and methods
Mice
The generation of tyk2-deficient mice has been previously described.5 Mice were housed and bred in the Kyushu University Animal Center.
Antibodies and cytokines
Anti-CD3 (145-2C11) was purchased from Pharmingen (San Diego, CA). Purified recombinant cytokines were obtained from the following sources: murine IL-12 was purchased from R & D Systems (Minneapolis, MN); human IL-15 and murine IL-18 were purchased from Peprotech (London, United Kingdom); human recombinant IL-2 was obtained from Shionogi (Osaka, Japan).
Fluorescence-activated cell sorter analysis
For determination of the proportion of NK cells in spleen or hepatic lymphocytes,19 they were incubated with phycoerythrin (PE)–conjugated anti-DX5 (Pharmingen) and fluorescein isothiocyanate (FITC)–conjugated anti-CD3 (Pharmingen). For determination of IL-18R expression on T and NK cells, after FcR blocking, splenocytes were incubated with antimurine IL-18R monoclonal antibody (mAb) followed by FITC-conjugated antirat IgG1 mAb,19 together with PE-conjugated anti CD4, CD8, or DX5 (Pharmingen). Stained cells were analyzed using a dual laser FACScalibur (Becton Dickinson, Mountain View, CA). Ten thousand cells were analyzed and data were processed with CellQuest (Becton Dickinson).19
Enrichment of NK cells
Spleen cells or hepatic lymphocytes were cultured with 300 ng/mL IL-15 for 10 days.20 Viable cells were then incubated with CD90 MicroBeads (Miltenyi Biotec, Bergisch Gladbach, Germany), followed by passage of cells over negative columns. CD90− cells were then incubated with DX5 MicroBeads for 20 minutes at 4°C, followed by passage of cells over positive columns. To determine the purity of NK cells, the sorted cells were additionally incubated with PE-conjugated anti-DX5 and FITC-conjugated anti-CD3.
NK cell activity
Naive spleen cells, hepatic lymphocytes, or enriched NK cells were incubated with 51Cr-labeled YAC-1 target cells at the indicated effector-to-target (E/T) ratios. After a 4-hour culture, supernatants were counted for 51Cr release by a γ counter. In the case of in vitro activation of NK cells, spleen cells, hepatic lymphocytes, or enriched NK cells were incubated with or without IL-12 (2 ng/mL) or IL-18 (20 ng/mL) for 24 hours. Their cytolytic activity against YAC-1 cells was measured.21
IFN-γ assay
Spleen cells or enriched NK cells (5 × 105 cells) from wild-type or tyk2-deficient mice were activated with medium only, IL-12 (1 ng/mL), IL-18 (50 ng/mL), IL-2 (500 U/mL), or a combination of IL-12 and IL-18. Plates were cultured for 48 hours, and IFN-γ produced in the supernatant was quantified by enzyme-linked immunosorbent assay (ELISA; Genzyme, Cambridge, MA).
Proliferation assay
Spleen cells from wild-type or tyk2-deficient mice were activated with plate-bound anti-CD3 (5 μg/mL) for 48 hours at 2 × 106 cells/mL, washed, and then plated in a 96-well microtiter plate at 1 × 105/mL. Cells were incubated in different concentrations of IL-12 or IL-18 as indicated for 48 hours. Cells were pulsed with 1 μCi (37 kBq)3H-thymidine per well for the last 18 hours before the harvest, and their radioactivities were measured by a γ counter.
Results
NK cell numbers and activity in the absence of Tyk2
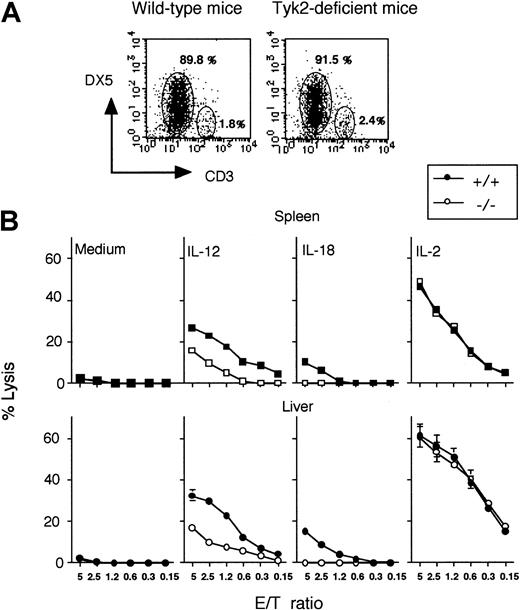
The population of DX5+ cells in the spleen from wild-type or tyk2-deficient mice was 4.2% or 5.3%, respectively, whereas that in hepatic lymphocytes from wild-type or tyk2-deficient mice was 11.8% or 7.2%, respectively. The total numbers of spleen cells or hepatic lymphocytes were almost equivalent between tyk2-deficient mice and wild-type mice. NK cells, characterized by large granular morphology, are functionally defined as immune effector cells that can lyse selected target cells in a major histocompatibility class nonrestricted manner. Naive NK cell activity against YAC-1 NK target cells was examined by using whole splenocytes or hepatic lymphocytes as effector cells. Although there was no difference in naive NK cell activity between spleen cells from wild-type and those from tyk2-deficient mice, hepatic naive NK cell activity was absent in tyk2-deficient mice (Figure 1 A,D). In separate experiments, we observed that tyk2-deficient hepatic lymphocytes killed YAC-1 cells but more weakly at a 20:1 E/T ratio, as compared with wild-type cells (data not shown).
NK cell activity in tyk2-deficient mice.
Freshly isolated spleen cells were analyzed regarding NK lytic activity against 51Cr-labeled YAC-1 target cells (A).51Cr-release in supernatants of cultures at the indicated effector-to-target (E/T) ratios was measured. Spleen cells from wild-type and tyk2−/− mice were incubated with 2 ng/mL IL-12 (B) or 20 ng/mL IL-18 (C) for 24 hours, and their cytotoxic activity against YAC-1 cells was determined. Freshly isolated hepatic lymphocytes from wild-type or tyk2-deficient mice were analyzed regarding NK lytic activity against 51Cr-labeled YAC-1 target cells (D). Hepatic lymphocytes from the mutant or wild-type mice were incubated with IL-12 (E) or IL-18 (F) for 24 hours, and their cytotoxic activity against YAC-1 cells was determined.
NK cell activity in tyk2-deficient mice.
Freshly isolated spleen cells were analyzed regarding NK lytic activity against 51Cr-labeled YAC-1 target cells (A).51Cr-release in supernatants of cultures at the indicated effector-to-target (E/T) ratios was measured. Spleen cells from wild-type and tyk2−/− mice were incubated with 2 ng/mL IL-12 (B) or 20 ng/mL IL-18 (C) for 24 hours, and their cytotoxic activity against YAC-1 cells was determined. Freshly isolated hepatic lymphocytes from wild-type or tyk2-deficient mice were analyzed regarding NK lytic activity against 51Cr-labeled YAC-1 target cells (D). Hepatic lymphocytes from the mutant or wild-type mice were incubated with IL-12 (E) or IL-18 (F) for 24 hours, and their cytotoxic activity against YAC-1 cells was determined.
Activity of NK cells was enhanced in the presence of IL-12 or IL-18. IL-12 was initially identified as a stimulatory factor that activates NK cell–mediated cytotoxicity. Because NK cell activity induced by IL-12 was drastically reduced in Stat4-deficient mice, the Jak-Stat pathway would seem to be involved in the expression of the IL-12–induced genes for enzymes such as perforin and granzyme B. As shown in Figure 1B, spleen cells incubated in IL-12 exerted readily detectable cytolytic activity against YAC-1 NK target cells. In contrast, NK activity was only slightly observed with cells from tyk2-deficient mice.
Interleukin-18 also augments the lytic activity of spleen cells against YAC-1 cells. In tyk2-deficient mice, this lytic activity of spleen cells treated with IL-18 decreased, but did not completely disappear (Figure 1C). As also noted with spleen cells, hepatic cells from tyk2-deficient mice failed to augment the cytolytic activity against target cells in the presence of IL-12 or IL-18 (Figure 1E-F).
The above results were gained by using spleen or hepatic cells that contain T and B cells besides NK cells. To enrich the NK cells, we cultured splenocytes or hepatic lymphocytes with IL-15,22 and CD3−DX5+ NK cells were collected by magnetic-activated cell sorter (MACS; Figure2A). Hepatic NK cells were collected from hepatic lymphocytes by performing the same procedure (data not shown). By using purified NK cells from splenocytes or hepatic lymphocytes, we examined unstimulated, IL-12–induced, IL-18–induced, or IL-2–induced killing activity against YAC-1 cells.
Requirement of tyk2 for up-regulation of NK lytic activity by IL-12 or IL-18.
Spleen cells or hepatic lymphocytes from wild-type or tyk2-deficient mice were incubated with IL-15 for 10 days. CD3−DX5+ NK cells were isolated by MACS. The expressions of CD3 and DX5 were determined by flow cytometry (A). CD3−DX5+ NK cells were incubated with IL-12 (2 ng/mL), IL-18 (20 ng/mL), or IL-2 (500 U/mL) for 24 hours, and their cytotoxicity against YAC-1 was determined (B).
Requirement of tyk2 for up-regulation of NK lytic activity by IL-12 or IL-18.
Spleen cells or hepatic lymphocytes from wild-type or tyk2-deficient mice were incubated with IL-15 for 10 days. CD3−DX5+ NK cells were isolated by MACS. The expressions of CD3 and DX5 were determined by flow cytometry (A). CD3−DX5+ NK cells were incubated with IL-12 (2 ng/mL), IL-18 (20 ng/mL), or IL-2 (500 U/mL) for 24 hours, and their cytotoxicity against YAC-1 was determined (B).
No significant differences were noted in lytic activity against YAC-1 cells of enriched NK cells from wild-type or tyk2-deficient mice when cells were cultured with medium only or with IL-2 (Figure 2B). Although the augmented NK cell activity by IL-12 or IL-18 was observed in wild-type mice when enriched NK cells were used as effector cells, it was reduced in enriched NK cells from tyk2-deficient mice, just as when whole splenocytes or hepatic lymphocytes were used as effector cells (Figures 1B,C,E,F and 2B).
Requirement for Tyk2 in IFN-γ induction by IL-12 or IL-18 on NK cells
Because NK cell activity induced by IL-12 or IL-18 is abrogated in tyk2-deficient NK cells, we next examined the influence of the absence of tyk2 on IFN-γ production from NK cells induced by IL-12 or IL-18. The stimulation of unfractionated spleen cells from tyk2-deficient mice with IL-12 resulted in decreased IFN-γ production compared with that from wild-type mice (Figure 3A). In addition, IL-18 failed to induce IFN-γ production from tyk2-deficient spleen cells (Figure 3A). Because the production of IFN-γ from CD4+ T cells after stimulation with IL-12 or IL-18 alone was not detected (data not shown), the cell source of the IFN-γ production from wild-type splenocytes after IL-12 or IL-18 stimulation would seem to be NK cells. To verify this, we enriched NK cells by MACS sorting of splenocytes that had been cultured with IL-15. We could hardly detect any IFN-γ from tyk2-deficient enriched NK cells following IL-12 or IL-18 stimulation, although IL-12 or IL-18 induced IFN-γ production from wild-type enriched NK cells (Figure4A). This was also the case for hepatic NK cells enriched by the same protocol (Figure 4C). On the other hand, there was no difference in the production of IFN-γ by IL-2 from wild-type and tyk2-deficient enriched NK cells (Figure 4A,C).
IFN-γ production from freshly isolated splenocytes.
(A) Splenocytes were obtained from either wild-type or tyk2-deficient mice and plated in wells with medium alone, IL-12, or IL-18. Supernatants were collected after 48 hours, and the amount of IFN-γ was quantified by ELISA. (B) Splenocytes were stimulated with IL-12 and IL-18. Supernatants were collected after 48 hours, and the amount of IFN-γ was quantified by ELISA.
IFN-γ production from freshly isolated splenocytes.
(A) Splenocytes were obtained from either wild-type or tyk2-deficient mice and plated in wells with medium alone, IL-12, or IL-18. Supernatants were collected after 48 hours, and the amount of IFN-γ was quantified by ELISA. (B) Splenocytes were stimulated with IL-12 and IL-18. Supernatants were collected after 48 hours, and the amount of IFN-γ was quantified by ELISA.
Impaired IFN-γ production in tyk2-deficient NK cells on stimulation with IL-12 or IL-18 but not IL-2.
Spleen cells (A,B) or hepatic lymphocytes (C,D) from wild-type or tyk2-deficient mice (Tyk2−/−) were incubated with IL-15 for 10 days. CD3−DX5+ NK cells were isolated as shown in the legend to Figure 2 and incubated with medium alone, IL-12, IL-18, or IL-2 (A,C) or the combination of IL-12 and IL-18 (B,D) for 48 hours. The IFN-γ concentration in each supernatant was determined by ELISA.
Impaired IFN-γ production in tyk2-deficient NK cells on stimulation with IL-12 or IL-18 but not IL-2.
Spleen cells (A,B) or hepatic lymphocytes (C,D) from wild-type or tyk2-deficient mice (Tyk2−/−) were incubated with IL-15 for 10 days. CD3−DX5+ NK cells were isolated as shown in the legend to Figure 2 and incubated with medium alone, IL-12, IL-18, or IL-2 (A,C) or the combination of IL-12 and IL-18 (B,D) for 48 hours. The IFN-γ concentration in each supernatant was determined by ELISA.
Absence of Tyk2 does not affect cytokine-induced cell proliferation
Because the absence of tyk2 impaired NK cell response on stimulation with IL-18 or IL-12 (Figures 1, 2B, 3, and 4), we next examined whether tyk2 was required for IL-18–, IL-12–, or IL-18 plus IL-12–induced T-cell proliferation. Spleen cells had been activated with anti-CD3 for 48 hours, washed, and then stimulated with IL-12 (Figure 5A), IL-18 (Figure 5B), or a combination of IL-12 and IL-18 (Figure 5C) for an additional 48 hours. As shown previously,5 tyk2-deficient activated T cells had no defect in proliferative response to the stimulation with IL-12 (Figure 5A). The proliferation of activated T lymphocytes from tyk2-deficient mice by IL-18 was almost the same as that from wild-type mice (Figure 5B). The absence of tyk2 did not affect the proliferation activity by IL-18, and the same situation was also observed when the cells were stimulated with a combination of IL-12 and IL-18 (Figure 5C).
Proliferative responses of splenocytes from wild-type and tyk2−/− mice.
Splenocytes from wild-type and tyk2-deficient mice were activated with anti-CD3 for 48 hours. Viable cells were washed, replated, and incubated in the absence or the presence of various doses of IL-12 (A), IL-18 (B), or IL-18 in the presence of 1 ng/mL IL-12 (C). Cells were pulsed with 3H-thymidine for the last 18 hours of a 48-hour assay.
Proliferative responses of splenocytes from wild-type and tyk2−/− mice.
Splenocytes from wild-type and tyk2-deficient mice were activated with anti-CD3 for 48 hours. Viable cells were washed, replated, and incubated in the absence or the presence of various doses of IL-12 (A), IL-18 (B), or IL-18 in the presence of 1 ng/mL IL-12 (C). Cells were pulsed with 3H-thymidine for the last 18 hours of a 48-hour assay.
Up-regulation of the IL-18 receptor induced by IL-12 is abrogated in the absence of Tyk2
It was reported that Stat4-deficient T cells had barely any detectable expression of IL-18R.22 To investigate the mechanism of IL-18 unresponsiveness in tyk2-deficient cells, we examined the cell surface IL-18R. The IL-18R expression level on CD4+, CD8+, and DX5+ cells from tyk2-deficient mice was about half that of the cells from wild-type mice (Figure 6). IL-18R on T and NK cells was up-regulated by the treatment of IL-12, and this up-regulation effect by IL-12 was completely abrogated on the cells from tyk2-deficient mice (Figure 6).
IL-18R on T and NK cells.
Splenic nonadherent cells were stained with anti–IL-18R mAb followed by FITC-conjugated antirat IgG1, together with PE-conjugated anti-CD4, anti-CD8, or anti-DX5. The proportion of IL-18R+ cells gated among the CD4+ (A), CD8+ (B), or DX5+ (C) cells is shown before and after culture with 2 ng/mL IL-12 for the indicated days.
IL-18R on T and NK cells.
Splenic nonadherent cells were stained with anti–IL-18R mAb followed by FITC-conjugated antirat IgG1, together with PE-conjugated anti-CD4, anti-CD8, or anti-DX5. The proportion of IL-18R+ cells gated among the CD4+ (A), CD8+ (B), or DX5+ (C) cells is shown before and after culture with 2 ng/mL IL-12 for the indicated days.
Discussion
Tyk2 was the first member of the Jak family kinases to be cloned as an essential molecule for the transduction of IFN-α signaling.23 Using tyk2-deficient mice, we and others previously reported that tyk2 is redundant for most IFN-α–inducing biologic responses, except for nitrous oxide (NO) production and some kinds of viral resistance. Surprisingly, tyk2 was revealed to be essential for IL-12–mediated T-cell function, including IFN-γ production and Th1 cell differentiation. Moreover, tyk2 is redundant for IL-12–induced cell proliferation. Because IL-12 was initially identified as a stimulatory factor for NK cells that activates NK cell–mediated cytotoxicity,7 8 we examined the NK cell activity of naive and IL-12–induced cells from tyk2-deficient mice.
First, we examined the number of NK cells in splenocytes or hepatic lymphocytes. No difference was observed with regard to DX5+CD3− NK cell numbers, although the number of NK cells in the liver from tyk2-deficient mice was a little smaller than that from wild-type mice. This may result in the absence of NK cell activity of freshly isolated hepatic lymphocytes (Figure 1A,D).
The killing activity of IL-12–induced NK cells in tyk2-deficient spleen cells or hepatic lymphocytes was less than that in wild-type cells (Figure 1B,E). This was also the case for enriched NK cells (Figure 2B). When the cells are treated with IL-12, Jak2 and Tyk2 are initially phosphorylated, followed by Stat4 activation.24NK cell activity in Stat4-deficient mice was drastically reduced when spleen cells were treated with IL-12.25,26 Accordingly, Stat4 is thought to regulate the genes for enzymes such as perforin and granzyme B.27 Because Stat4 activation was decreased in tyk2-deficient cells with IL-12,5 the IL-12–induced Tyk2-Stat4 pathway would seem to play a major role in NK cells, with this pathway being essential for T-cell function mediated by IL-12.
Interleukin-18 is a cytokine that was originally identified as an IFN-γ–inducing factor.9,10 In addition, IL-18 enhances NK cell activity and proliferation of activated T cells.10,11 Thus, IL-18 appears to share its biologic functions with IL-12. We therefore examined IL-18–induced NK cell activity in tyk2-deficient mice. As shown in Figure 1, panels C and F, and Figure 2, panel B, the killing activity of NK cells from tyk2-deficient mice was drastically reduced compared with that from wild-type mice. This is surprising because IL-18 induces the activation of the IL-1 receptor–associated kinase and nuclear translocation of NF-κB.17 28
Tyk2 is also deeply involved in the production of IFN-γ by NK cells following stimulation with IL-12 or IL-18. Tyk2-deficient NK cells did not produce any IFN-γ after stimulation with IL-12 or even IL-18, whereas wild-type NK cells produced the IFN-γ on the same stimulation (Figure 4). This allows us to assume that IL-18–induced IFN-γ production by NK cells might require endogenous tyk2-dependent signaling components. In contrast, tyk2-deficient spleen cells, which are composed of T cells, B cells, macrophages, and NK cells, produced a small amount of IFN-γ after culture with IL-18 as well as IL-12 (Figure 3A). These results suggest that cells that produce IFN-γ other than NK cells, presumably T cells,22 may have the capacity to produce a small amount of IFN-γ on such single stimulation, in a tyk2-independent manner. However, tyk2-deficient NK cells could produce IFN-γ, but a smaller amount than produced by wild-type NK cells, following the combined stimulation with IL-12 and IL-18 (Figures 3B and 4B,D), in contrast with the fact that there was no production of IFN-γ by tyk2-deficient NK cells on single stimulation with IL-12 or IL-18. This may suggest the presence of tyk2-independent activation of some IFN-γ promoter-activating factor(s) other than Stat4, on stimulation with IL-12 plus IL-18. Further studies are required for clarification of the molecular mechanisms of the synergistic IFN-γ–inducing action of IL-12 and IL-18.
The depletion of tyk2 did not affect IL-2 function on NK cells. IL-2 stimulation up-regulated the lytic activity of tyk2-deficient NK cells against YAC-1 (Figure 2B) and induced the IFN-γ production by tyk2-deficient NK cells (Figure 4A,C), to the same extent as wild-type cells. This is plausible, because Stat5 phosphorylation, a major signaling event after activation with IL-2, is not impaired in tyk2-deficient cells.5
Interleukin-18 failed to induce IFN-γ production from tyk2-deficient spleen cells (Figure 3A), although IL-18–induced T-cell proliferation was not affected by the absence of tyk2 (Figure 5B). Recently, Lawless and coworkers reported that IFN-γ production and T-cell proliferation induced by IL-18 are abrogated in Stat4-deficient mice.22Because the expression level of IL-18R messenger RNA is only a trace on splenocytes from Stat4-deficient mice, we next examined the expression level of IL-18R on cells from tyk2-deficient mice. Freshly isolated T and NK cells from tyk2-deficient mice expressed about half the levels of IL-18Rs compared with those from wild-type mice (Figure 6). This decrease in IL-18R on cells from tyk2-deficient mice may partly explain the reason why IL-18–induced NK cell activity or IL-18–induced IFN-γ production is abrogated in cells from tyk2-deficient mice. However, this reduced level of IL-18R is sufficient to transduce the migration activity by IL-18 in tyk2-deficient T cells (Figure 5). Because the functional defects for the induction of IFN-γ are much more significant than the reduced level of IL-18R on NK cells in tyk2-deficient mice, there is the possibility that tyk2 is directly involved in the IL-18 signaling pathway to induce IFN-γ in NK cells. In addition to the activation of IL-1 receptor–associated kinase and the nuclear translocation of NF-κB by IL-18,17,28 the phosphorylation of Stat3 by IL-18 stimulation has been reported in some cell line cells.18
Interleukin-18 acts synergistically with IL-12 on NK cells to produce IFN-γ. This synergistic effect is also affected by the absence of tyk2 (Figures 3B and 4B,D). Some reports have suggested that synergy results from the induction of IL-18R by IL-12.29,30Certainly, the IL-18R on NK cells from wild-type mice is up-regulated by the treatment with IL-12, and this up-regulation of IL-18R by IL-12 is abrogated on NK cells from tyk2-deficient mice (Figure 6). This might be the reason for the decrease in the synergistic effect of IL-12 and IL-18 in tyk2-deficient mice. If this were the only reason, then the additional effect of IL-12 and IL-18, not the synergistic effect, on IFN-γ production should be observed when the cells from tyk2-deficient mice are treated with IL-12 and IL-18, because the IL-18R on the cells from tyk2-deficient mice is not affected by the treatment with IL-12 (Figure 6). However, costimulation with both IL-12 and IL-18 induced the production of IFN-γ from tyk2-deficient cells more than 100-fold of that expected if only the additional effect occurred, even though the amount of the augmented IFN-γ production was much less than that from wild-type mice (Figures 3B and 4B,D). This means that other factors besides the induction of IL-18R by the treatment of IL-12 are important for the synergistic effect of IL-12 and IL-18 in the induction of IFN-γ. Indeed, the IFNG gene has multiple promoter regions that bind to corresponding transcription factors, including Stat4 and NF-κB.31,32Recently Yang et al33 reported that the synergy of IL-12 and IL-18 is involved in GADD45β induction. GADD45β activates the mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinase kinase 4 (MEKK4), and it was induced by IL-18 and augmented by IL-12. This augmentation of GADD45β induction by IL-12 and IL-18 was not observed in the splenocytes from tyk2-deficient or wild-type mice by reverse transcription–polymerase chain reaction (data not shown). Together with the absence of the up-regulation of IL-18R by IL-12, our observations suggest that other factors besides the induction of GADD45β by IL-12 and IL-18 may be the reason for the decrease in production of IFN-γ in T cells from tyk2-deficient mice. This might mean that the cross-talk between Jak-Stat and MAPK pathway was present in the cytokine signaling. We need further study to identify the factor that is involved in the synergy of IL-12 and IL-18 for IFN-γ production.
We thank A. Tomioka, A. Koutate, and M. Sato for their excellent technical assistance.
Supported by Grants-in-Aid for Scientific Research (nos. 11770577, 11307015, and 13218096) from the Ministry of Education, Science, Sports, and Culture in Japan, and by a Grant of Clinical Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kazuya Shimoda, First Department of Internal Medicine, Faculty of Medicine, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka, Fukuoka 812-8582, Japan; e-mail:kshimoda@intmed1.med.kyushu-u.ac.jp.