Abstract
Significant delays in engraftment and lymphoid recovery are the 2 major challenges in cord blood transplantation. The cause for this delay is presumed to be the low numbers of hematopoietic precursors found in one unit of cord blood. One approach to increase the stem cell doses could be to combine cord blood units from different donors differing at the major histocompatibility complex (MHC). As a first step toward this goal, the kinetics of hematologic engraftment and immune reconstitution were compared between 1 unit (2.5 × 106 cells) of T-cell–depleted bone marrow cells from a single donor (C57BL/6 [H2b] or SJL/J [H2s]) and 2 units from different donors (C57BL/6 + SJL/J) after transplantation into lethally irradiated (8.5 Gy) BALB/c recipients (H2d). Addition of T-cell–depleted bone marrow from an MHC-mismatched allogeneic donor doubled the white blood counts compared with recipients of one single unit on days +10 and +14. Similar effects were also observed on platelets. The beneficial effect of additional cells on peripheral T-cell counts were first observed on day +14. Cells both from donors (C57BL/6 and/or SJL/J) and recipients (BALB/c) contributed to myeloid and lymphoid reconstitution. The chimeras containing cells from 3 strains of mice were able to mount a recall immune response. Our data suggest that combining stem cells from MHC-mismatched allogeneic donors is feasible, that it has beneficial effects on myeloid engraftment and T-cell phenotypic recovery, and that the long-term stable mixed chimeras are immunologically normal following T-cell–depleted bone marrow transplantation.
Introduction
Umbilical cord blood is a valuable source of hematopoietic stem cells for patients in need of stem cell transplantation when a matched-related or matched-unrelated donor is not available.1-4 Significant delays in engraftment and lymphoid recovery are the 2 major obstacles in cord blood transplantation, especially in adult recipients.2,3 Delay in myeloid engraftment increases the morbidity and mortality of this procedure. Moreover, delay in lymphoid engraftment renders the patients more susceptible to infections. Cord blood transplant recipients can succumb to infections even 1 or 2 years after transplantation.3 Mortality caused by infections can be as high as 50% in cord blood transplant recipients.2 3
Clinical studies suggest that total cell dose and CD34+cell dose correlate with the speed of engraftment and immune reconstitution as well as overall outcomes.2 There are data demonstrating that stem cell dose correlates with the speed of hematopoietic5 and lymphoid6 recovery after stem cell transplantation. However, very limited numbers of total nucleated cells and CD34+ cells are contained in one single cord blood unit.7 Several investigators have explored expansion of stem cells in cord blood ex vivo for clinical use.8-11 Significant expansion of hematopoietic progenitors ex vivo were observed by numerous investigators.8-12 However, it has been difficult to demonstrate that pluripotent stem cells can be expanded ex vivo. Moreover, the clinical benefit of ex vivo cord blood cells requires further clinical studies.13 Therefore, one alternative approach to increase the stem cell numbers in the graft may be to combine cord blood units from different donors, which may differ at the major histocompatibility complex (MHC).
This study explored the feasibility of combining stem cells from 2 different donors that differed at the MHC in a murine model of T-cell–depleted bone marrow transplantation. Stem cells from these 2 donors, which were MHC-incompatible, were transplanted together into a third incompatible recipient following total body irradiation. The effects of the cells from these 2 donors on the speed of engraftment and lymphoid recovery were analyzed in this murine model.
Materials and methods
Animals
BALB/c (H2d, CD45.2), C57BL/6 (H2b, CD45.2), SJL/J (H2s, CD45.1), and C3H/HeJ (H2k) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Animals were housed in a specific pathogen-free facility.
Antibodies
The antibodies used in this study included purified anti-CD3 (145-2C11), biotin-conjugated anti-CD8 (53-6.7), phycoerythrin (PE)–conjugated anti-CD45.1 (A20), Cy5PE-conjugated anti-CD3 (145-2C11), and their isotype controls from Pharmingen (San Diego, CA); biotin-conjugated H-2Db (CTDb), natural killer (NK) 1.1 (PK136), fluorescein isothiocyanate (FITC)–conjugated goat antihamster immunoglobulin, anti–H-2Db (CTDb), tricolor-conjugated strepavidin, anti-CD4 (CT-CD4), B220 (RA3-6B2), and their isotype controls from Caltag (South San Francisco, CA); FITC-conjugated anti-bromodeoxyuridine (anti-BrdU) (B44) and the isotype control from BDIS (San Jose, CA); Red 613–conjugated anti-CD4 (H129.19) from Life Technologies (Gaithersburg, MD); purified anti-CD11c (N418) from Harlan Bioproducts for Science (Indianapolis, IN); and purified antimouse immunoglobulin (Ig)–M from Jackson ImmunoResearch Laboratories (West Grove, PA).
T-cell depletion from bone marrow
Bone marrow cells flushed out from femurs, tibias, and humeri were strained through a 70-μm cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ). After washing, cells were resuspended in Cytotoxicity Medium (Cedarlane, Hornby, ON, Canada) at a concentration of 1 × 107/mL. Then, 0.1 μg purified anti-Thy1.2 monoclonal antibody per 1 × 107 cells (Pharmingen) was added and mixed. After 60 minutes of incubation on ice, the cells were washed once and then suspended in Cytotoxicity Medium containing 1:10 Low-Tox-M Rabbit Complement (Cedarlane). The cells were then incubated at 37°C for 60 minutes and washed twice before use. The final product contained fewer than 0.08% T cells. All bone marrow cells used in this study were T-cell–depleted.
T-cell–depleted bone marrow transplantation
BALB/c recipients, 9 to 12 weeks old at the time of transplantation, were lethally irradiated (8.5 Gy) and then injected intravenously via tail vein with various numbers of T-cell–depleted bone marrow cells. All experiments were performed according to Duke University guidelines. Mortality was recorded daily. Recipients were monitored for clinical signs of graft-versus-host disease (GVHD) by the following parameters: body weight and extent of skin changes (hair loss and erythema) as described.14-17 Skin biopsies (from the ear) for histologic evidence of GVHD were routinely taken on days +30, +70, and +100 after transplantation and when mice were moribund.18
Peripheral blood cell counts
Platelets were counted by means of a Sysmex F-820 semiautomated microcell counter (TOA Medical Electronics, Kobe, Japan). White blood cells were counted on a hemocytometer after being diluted in 1% acetic acid solution. The volume of the blood obtained was restricted to less than 50 μL per mouse per time point to minimize the negative impact on hematopoietic recovery. Preliminary experiments indicated that the cell counter is suitable for counting mouse blood cells.
Absolute counts of lymphocyte and lymphocyte subsets
First. 50 μL heparinized peripheral blood was stained with monoclonal antibodies for 15 minutes at room temperature. The stained whole-blood samples were then processed in Multi-Q-Prep (Coulter, Miami, FL) to lyse red blood cells. Before flow cytometric analysis, we added 50 μL Flow-Count fluorospheres (Coulter). The stained cells were analyzed by means of a Coulter EPICS XL flow cytometer equipped with System II software. The absolute counts were calculated automatically by the following formula: Absolute count = [total no. cells counted/total no. fluorospheres counted] × FC-assayed concentration. Absolute count is expressed as cells per microliter and FC indicates Flow-Count.
BrdU assay for detection of cellular proliferation on a single-cell basis
The assay was performed as described.19 First, 1.25 × 106 spleen cells per well were cultured with either T-cell mitogen (immobilized anti-CD3, 10 μg/mL) or B-cell mitogen (anti-IgM, 25 μg/mL) in flat-bottom 48-well culture plates at 37°C and 5% CO2 for 48 hours. A final concentration of 30 μM BrdU (Sigma Chemical, St Louis, MO) was added 24 hours before harvest. After culture, the cells were transferred into 6-mL tubes and washed once. The cells were stained with anti–surface marker antibodies and washed once. The cells were then resuspended in 0.5 mL FACS Permeabilizing Solution (BDIS) for fixation and permeabilization. After 3 hours' incubation at 4°C, cells were washed twice and stained with anti-BrdU antibody (1 μg/mL) (BDIS) in the presence of DNAse I (Sigma) at a final concentration of 4 mg/mL for 30 minutes at room temperature. The stained cells were analyzed by means of a Coulter EPICS XL flow cytometer equipped with System II software.
Immunization
Mice were immunized subcutaneously in the hind footpads with 3.5 nM whole ovalbumin (Sigma) emulsified in GERBU adjuvant (GERBU Biltechnid, Gaiberg, Germany).
Proliferation assay
At 9 days after immunization, the draining lymph node cells were harvested and cultured with various concentrations of whole ovalbumin at a concentration of 5 × 105 cells per well in a 96-well flat-bottom culture plate. After 64 hours' culture, the culture was pulsed with 1 μCi 3H-thymidine per well and cultured for a further 8 hours. The cultures were harvested and counted in a MicroBeta Trilux liquid scintillation counter (EG&G Wallac, Turku, Finland).
Cytotoxic T-lymphocyte assay
Graded numbers of responder cells were plated with 1 × 105 irradiated (20 Gy) spleen cells from C3H/HeJ mice per well in 96-well round-bottom plates. Plates were cultured at 37°C with 5% CO2 in an incubator for 112 hours. The cells were tested in situ for lysis of 51Cr-labeled 2-day concanavalin A (Con A) blast cells. After 4 hours of culture, supernatant was removed and counted in MicroBeta Trilux liquid scintillation counter after mixing with “SuperMix” scintillator (EG&G Wallac). In each experiment, cultures were tested in parallel for lysis of irrelevant targets to ensure antialloantigen-specific killing. Spontaneous release was obtained by incubating target cells with stimulator cells only. Maximal release was obtained after treatment with 1% Igepal CA-630 (Sigma). The percentage of specific release was calculated as follows:
Statistical analysis
Comparison among groups was done by unpaired t test or analysis of variance. All statistical analyses were done with Statview software (SAS Institute, Cary, NC).
Results
Addition of allogeneic T-cell–depleted bone marrow accelerates the engraftment of white blood cells and platelets
The speed of engraftment of white blood cells and platelets was monitored in the recipients of T-cell–depleted bone marrow cells from C57BL/6 mice (2.5 × 106), SJL/J mice (2.5 × 106), and both strains of mice (2.5 × 106 + 2.5 × 106) every 3 or 4 days after transplantation starting on day +7. In this model, 2.5 million T-cell–depleted bone marrow cells are the minimum for rescuing and engrafting the majority (more than 80%) of the lethally irradiated (8.5 Gy) BALB/c recipients. As shown in Table1, differences in the speed of reconstitution of white blood cells between the recipients of a single donor and combined donors were first observed on day +10. The differences were still significant on day +14. The peripheral blood counts in the recipients of a single donor started to catch up on day +18, and by day +21, the groups were similar. The similarity between the groups remained up to day +44, when the white counts had recovered to pretransplantation level (data not shown). The platelet engraftment paralleled that of the white blood cells and was significant at the earlier time points but not by day +21 (data not shown). White blood cell recovery in the recipients of combined allogeneic T-cell–depleted bone marrow cells (2.5 × 106 from C57BL/6 + 2.5 × 106 from SJL/J) was almost the average of that in the recipients of same number (5 × 106) of T-cell–depleted bone marrow cells from only C57BL/6 mice or SJL/J mice (Table 1).
Addition of allogeneic T-cell–depleted bone marrow accelerates the time to T-cell phenotypic recovery
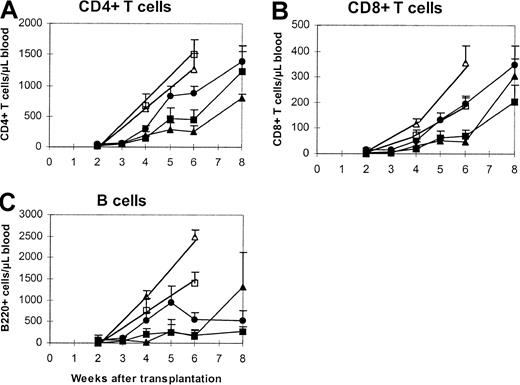
Preliminary data suggested that immune reconstitution was delayed after T-cell–depleted bone marrow transplantation at the cell dose of 2.5 × 106. Immune reconstitution by lymphocyte phenotype was followed every other week after stem cell transplantation (Figure1). Statistical differences in peripheral blood CD4+ T-cell counts between the recipients of a single donor and those of combined donors were first observed as early as on day +14 (Figure 1A), with the differences in favor of the combined-donor recipients. These differences persisted until day +42 and became insignificant on day +60 (when all groups were within the normal range). A similar pattern of reconstitution was also observed for CD8+ T cells (Figure 1B). B-cell recovery also demonstrated a trend toward a more rapid reconstitution, but owing to wider variability in the animals, it did not achieve statistical significance (Figure 1C). While the addition of a second unit accelerated hematopoietic recovery, compared with a single donor, this recovery was not as robust as the higher cell dose.
Phenotypic recovery of peripheral T and B cells.
Lethally irradiated (8.5 Gy) BALB/c mice were transplanted with one unit (2.5 × 106) of T-cell–depleted bone marrow cells from C57BL/6 (closed triangles) or SJL/J (closed squares) mice or the combination of both (closed circles). Peripheral blood was harvested in EDTA microtubes. Then, 50 μL blood was incubated with antibodies at room temperature for 15 minutes. The stained samples were then processed in Multi-Q-Prep to lyse the red cells. The samples were analyzed in a Coulter EPICS-XL flow cytometer equipped with System II software. The absolute counts were determined by using Flow-Count. The controls were mice that received a single-donor graft (open triangles indicate C57BL/6; open squares, SJL/J) at a dose equivalent to that of the combined group (5 × 106). The values represent the mean + SE of 3 to 15 animals per group. Similar experiments have been repeated twice. The pretransplantation levels (per microliter blood) are as follows: CD4+ T cells, 1912 ± 634; CD8+ T cells, 322 ± 246; B220+ cells, 1757 ± 422. P values as compared with the C57BL/6 + SJL/J group are shown in Table 2.
Phenotypic recovery of peripheral T and B cells.
Lethally irradiated (8.5 Gy) BALB/c mice were transplanted with one unit (2.5 × 106) of T-cell–depleted bone marrow cells from C57BL/6 (closed triangles) or SJL/J (closed squares) mice or the combination of both (closed circles). Peripheral blood was harvested in EDTA microtubes. Then, 50 μL blood was incubated with antibodies at room temperature for 15 minutes. The stained samples were then processed in Multi-Q-Prep to lyse the red cells. The samples were analyzed in a Coulter EPICS-XL flow cytometer equipped with System II software. The absolute counts were determined by using Flow-Count. The controls were mice that received a single-donor graft (open triangles indicate C57BL/6; open squares, SJL/J) at a dose equivalent to that of the combined group (5 × 106). The values represent the mean + SE of 3 to 15 animals per group. Similar experiments have been repeated twice. The pretransplantation levels (per microliter blood) are as follows: CD4+ T cells, 1912 ± 634; CD8+ T cells, 322 ± 246; B220+ cells, 1757 ± 422. P values as compared with the C57BL/6 + SJL/J group are shown in Table 2.
Stem cells from both allogeneic donors contribute to myeloid and lymphoid engraftment
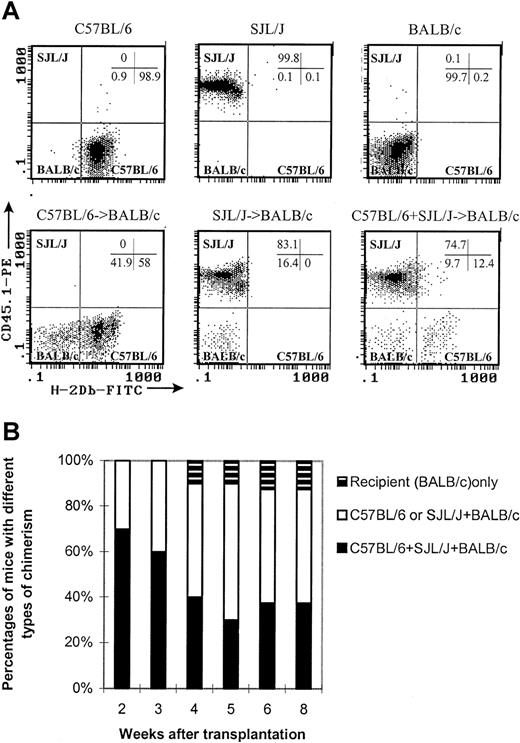
As seen in Figure 2A, cells from C57BL/6, SJL/J, and BALB/c mice could be distinguished by anti-CD45.1 and anti–H-2Db monoclonal antibodies (C57BL/6: H-2Db+CD45.1−; SJL/J: H-2Db−CD45.1+; BALB/c: H-2Db−CD45.1−). The chimerism on white blood cells, lymphocytes, lymphocyte subsets (CD4+ T cells, CD8+ T cells, B cells, and NK cells), and dendritic cells20 21(CD11c+CD3−CD4−B220−Gr-1−, not including CD4+CD8α+ dendritic cells) was followed over time after transplantation. As seen in Figure2B, 70% of the recipients of combined allogeneic donors (C57BL/6 + SJL/J) contained cells from both donors (C57BL/6 and SJL/J) plus the recipient (BALB/c), while the other 30% were the chimeras of one single donor (C57BL/6 or SJL/J) plus the recipient. The percentages of the recipients containing cells from 3 different strains of mice decreased over time. However, around 40% of recipients still maintained a mixed chimeric state 8 weeks after transplantation. Only 1 of 10 mice was not reconstituted by donor cells (Figure 2B). The mixed chimerism state was stable more than 200 days after transplantation (data not shown). Mixed chimerism was observed in all cell subsets tested: CD4+ T cells, CD8+ T cells, B cells, NK cells, dendritic cells, and myeloid cells (Table3). Interestingly, the recipients appeared to have a predominance of SJL/J as compared with C57BL/6 cells in the B-cell and myeloid lineages (Table 3).
Stable mixed chimerism in the recipients of cells from 2 different donors.
Peripheral blood was obtained at various time points after transplantation. First, 50 μL blood were incubated with antibodies at room temperature for 15 minutes. The stained samples were then processed in Multi-Q-Prep to lyse the red cells. The samples were analyzed in a Coulter EPICS-XL flow cytometer equipped with System II software. The absolute counts were determined by using Flow-Count. (A) Representative histograms show how to differentiate the cells of 3 different origins in different chimeric states. C57BL/6: H-2Db+CD45.1−. SJL/J: H-2Db−CD45.1+. BALB/c: H-2Db−CD45.1−. All histograms were CD45-gated. (B) Kinetics of chimerism.
Stable mixed chimerism in the recipients of cells from 2 different donors.
Peripheral blood was obtained at various time points after transplantation. First, 50 μL blood were incubated with antibodies at room temperature for 15 minutes. The stained samples were then processed in Multi-Q-Prep to lyse the red cells. The samples were analyzed in a Coulter EPICS-XL flow cytometer equipped with System II software. The absolute counts were determined by using Flow-Count. (A) Representative histograms show how to differentiate the cells of 3 different origins in different chimeric states. C57BL/6: H-2Db+CD45.1−. SJL/J: H-2Db−CD45.1+. BALB/c: H-2Db−CD45.1−. All histograms were CD45-gated. (B) Kinetics of chimerism.
Different donor-original T and B cells in the long-term stable mixed chimera proliferate normally when assessed on a single-cell basis
By means of BrdU, proliferation can be detected on a single-cell basis.19 Spleen cells, which were previously activated by T- or B-cell mitogens and labeled with BrdU, were harvested and stained with anti-BrdU, H-2Db, CD45.1 antibodies plus anti-CD4 and CD8 antibody for cells activated by T-cell mitogen or anti-B220 antibody for cells activated by B-cell mitogen antibodies simultaneously. The stained samples were then analyzed with a flow cytometer. As illustrated in Figure 3, CD4+ and CD8+ or B220+ cells were first gated (Figure 3A). These gated cells were further divided into C57BL/6- or SJL/J-derived cells on the basis of H-2Db and CD45.1 expression (Figure 3B). BrdU-incorporating cells could then be determined on these individual cell subsets (Figure 3C). Therefore, combining the BrdU assay with the chimerism assay, it was possible to determine whether the immune cells from different origins could proliferate upon response to mitogens. Table4 summarizes the data showing that T and B cells from different origin in a mixed chimera of the combined donor recipients could respond as well as those in the single-donor recipients and normal animals.
Flow cytometer–based proliferation assay with BrdU to detect proliferation of cells from different origins simultaneously.
More than 100 days after transplantation, 1.25 × 106spleen cells per well were cultured with immobilized anti-CD3 in a flat-bottom 48-well culture plate at 37°C and 5% CO2 for 48 hours. A final concentration of 30 μM BrdU was added 24 hours before harvest. After culture, the cells were transferred into 6-mL tubes and washed once. The cells were first stained with anti–surface marker antibodies, washed once, and then resuspended in 0.5 mL FACS Permeabilizing Solution for fixation and permeabilization. After 3 hours' incubation at 4°C, cells were washed twice and stained with anti-BrdU antibody (1 μg/mL) in the presence of DNAse I at a final concentration of 4 mg/mL for 30 minutes at room temperature. The stained cells were analyzed by means of a Coulter EPICS XL flow cytometer equipped with System II software.
Flow cytometer–based proliferation assay with BrdU to detect proliferation of cells from different origins simultaneously.
More than 100 days after transplantation, 1.25 × 106spleen cells per well were cultured with immobilized anti-CD3 in a flat-bottom 48-well culture plate at 37°C and 5% CO2 for 48 hours. A final concentration of 30 μM BrdU was added 24 hours before harvest. After culture, the cells were transferred into 6-mL tubes and washed once. The cells were first stained with anti–surface marker antibodies, washed once, and then resuspended in 0.5 mL FACS Permeabilizing Solution for fixation and permeabilization. After 3 hours' incubation at 4°C, cells were washed twice and stained with anti-BrdU antibody (1 μg/mL) in the presence of DNAse I at a final concentration of 4 mg/mL for 30 minutes at room temperature. The stained cells were analyzed by means of a Coulter EPICS XL flow cytometer equipped with System II software.
Long-term stable mixed chimera can mount a recall immune response upon the challenge of ovalbumin in vivo
Since both CD4+ and CD8+ T cells can recognize an antigen only when it is presented on the cell membrane by a self-MHC molecule,22 23 it raised the question of whether a stable mixed chimera containing MHC-mismatched cells can elicit a normal immune response. This potential for response was tested with the use of ovalbumin as the antigen challenge. As shown in Table5, the recipients of 2 MHC-mismatched donors could mount similar proliferation responses upon the challenge of ovalbumin in vivo.
Alloantigen-specific cytotoxic T lymphocytes can be induced in the long-term, stable mixed chimera
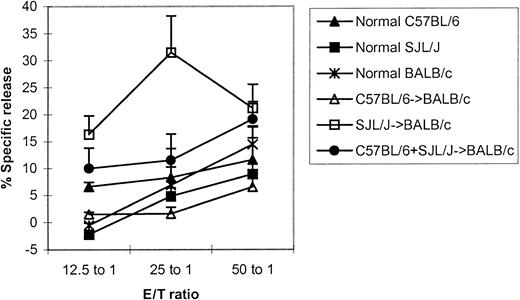
To further evaluate the immune function in the stable mixed chimera containing MHC-mismatched cells of 3 different origins, we tested the ability to produce alloantigen-specific cytotoxic T lymphocyte. As shown in Figure 4, the recipients of 2 MHC-mismatched donors were able to generate C3H/HeJ-specific cytotoxic T lymphocytes like the recipients of a single donor (not significant at the effector-target ratio of 50:1).
Alloantigen-specific cytotoxic T lymphocytes can be generated in stable mixed chimera containing cells of 3 different origins.
More than 100 days after transplantation, cells were activated with irradiated (20 Gy) spleen cells from C3H/HeJ mice in a 112-hour mixed lymphocyte reaction in 96-well round-bottom culture plates. The cells were tested in situ for lysis of 51Cr-labeled 2-day Con A blast cells from C3H/HeJ. The values represent the mean + SE of 3 values in each group. Not significant: C57BL/6+SJL/J→BALB/c versus normal mice or single-donor recipients at the effector-target ratio of 50:1.
Alloantigen-specific cytotoxic T lymphocytes can be generated in stable mixed chimera containing cells of 3 different origins.
More than 100 days after transplantation, cells were activated with irradiated (20 Gy) spleen cells from C3H/HeJ mice in a 112-hour mixed lymphocyte reaction in 96-well round-bottom culture plates. The cells were tested in situ for lysis of 51Cr-labeled 2-day Con A blast cells from C3H/HeJ. The values represent the mean + SE of 3 values in each group. Not significant: C57BL/6+SJL/J→BALB/c versus normal mice or single-donor recipients at the effector-target ratio of 50:1.
Discussion
The limited number of cells in a single umbilical cord blood unit restricts the use of umbilical cord blood in adult recipients.2 Delays in engraftment and lymphoid recovery are the 2 major clinical problems.2 These delays are likely to be the result of low numbers of hematopoietic progenitor cells, facilitator cells, or both that are found in umbilical cord blood.2 Several approaches to overcome these obstacles are possible. One approach has been to attempt to expand these cord blood cells ex vivo to increase the number of hematopoietic precursors.8 The results of expansion are encouraging in that CD34+ cells are increased several-fold,8but the clinical data demonstrating efficacy in cord blood patients is lacking.13 Another approach is to combine cord blood units, which may differ in MHC. The difficulty in such an approach would be whether one unit would reject the other in a graft-versus-graft reaction, and if not, whether the recipient would be immunologically normal.
To explore the potential of combining stem cells, we used T-cell–depleted bone marrow grafts in a murine transplantation model. Data from this study suggest that the addition of a second, different allogeneic donor (SJL/J) (allogeneic to the recipient [BALB/c] and the initial donor [C57BL/6]) accelerates the speed of myeloid engraftment (Table 1) and T-cell reconstitution (Figure 1 and Table 2). It is clear that hematopoietic stem cell dose correlates with the speed of myeloid5 engraftment. These data also demonstrate that increasing the stem cell dose, even from MHC-mismatched donors, accelerates T-cell recovery in cell numbers. As demonstrated in Tables 4 and 5 and Figure 4, the recovery in the combined units was at least equivalent to that found in the single units. Whether this equivalence is due to the lack of enhancement of immune function by the addition of other allogeneic cells or to a reflection of maximum stimulation from the antigen following transplantation is not clear.
The data from Table 3 suggest that stem cells from both allogeneic donor mice contribute to the hematopoietic and lymphoid engraftment. The resulting chimeric state (Figure 2) and the shortening of the time to myeloid engraftment and T-cell reconstitution most likely resulted from increased cell contents in the graft since both grafts were T-cell depleted. Thus, the more rapid recovery of myeloid cells is probably correlated with higher hematopoietic precursors. Whether the improved T-cell reconstitution is due to higher numbers of hematopoietic precursors exclusively or results from a few residual mature T cells carried with the graft is not clear. However, the T-cell–depletion method we have employed leads to an approximately 2 to 3 log T-cell depletion and results in the lack of cells responsive to interleukin 2 (data not shown).
T-cell depletion of both donors was performed since the murine model was one of major MHC mismatching. In this setting, T-cell depletion allows for engraftment without GVHD. Thus, the animals survive, allowing for the measurement of engraftment and lymphoid recovery. Moreover, besides the prevention of GVHD, T-cell depletion may also prevent graft-versus-graft interaction resulting in rejection of one or both grafts. It is interesting that, as demonstrated in Figure 2, the pattern of engraftment is mixed: some animals were mixed chimeras from both donors while others recovered from a single donor. However, the ability to respond to an immunogen did not differ between a single versus a double chimera (Table 5; Figure 4).
The beneficial effect of cell dose is seen only at the cell level, where engraftment is marginal. When the T-cell–depleted bone marrow cell doses were increased to the standard dose required for inducing full-donor chimerism (1 × 107 T-cell–depleted bone marrow cells), the shortening of the time to hematopoietic engraftment was not observed after a second allogeneic T-cell–depleted bone marrow graft was added, at least at the time points measured above (data not shown). These results support the notion that a threshold cell dose is needed for engraftment. Once that threshold is reached, the addition of more of the same type of hematopoietic precursors as found in the original graft does not result in a more rapid recovery. That is not to say that other, more differentiated precursors could not accelerate engraftment further, only that more standard, nonmobilized bone marrow cells did not result in a more rapid myeloid or lymphoid recovery.
Since self-MHC restriction strictly applies in T-cell response,22 23 one concern about this strategy is whether the 3-way mixed chimeras containing cells from MHC-mismatched allogeneic donors can mount an immune response. However, each individual T and B cell, regardless of origin, can respond normally to mitogens (Table 4) and the mixed chimeras contained both T cells and antigen-presenting cells (ie, dendritic cells) of several origins (Table 3). The presence of cells from several origins suggest that T cells may cooperate with the dendritic cells that share their MHC and mediate a normal immune response. As can be seen in Table 5, antiovalbumin proliferation responses in the recipients of 2 different donors were observed. While the responses in the mixed chimeras were weaker than those in normal animals (Table 5), no difference was observed between the recipients of a single donor and 2 donors. This weaker response could be secondary to the low donor cell doses infused. Similarly, like both normal mice and the recipients of single donor, the recipients of 2 donors can generate alloantigen-specific cytotoxic T lymphocytes (Figure 4).
In conclusion, addition of T-cell–depleted bone marrow allogeneic to the donor graft accelerated engraftment and T-cell reconstitution after allogeneic stem cell transplantation when a low cell dose of T-cell–depleted bone marrow was used. This more rapid recovery may result from increased hematopoietic stem/progenitor cell numbers. Since this study used the most severe cases of potential graft disparities (MHC-mismatched not only between donors but also between either donor and recipient), MHC-matched combinations may be likely to work in a similar pattern. These data suggest that combining several units of cord blood may be beneficial for larger patients with limited numbers of stem cells available, especially in the case of cord blood transplantation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Nelson J. Chao, Bone Marrow Transplantation Program, Duke University Medical Center, 2400 Pratt St, Suite 1100, Durham, NC 27705; e-mail: chao0002@mc.duke.edu.