The Philadelphia (Ph) translocation t(9;22)(q34;q11) is found in 15% to 25% of adults and 3% to 5% of children with acute lymphoblastic leukemia (ALL)1 and identifies patients with a particularly poor prognosis.2 The Ph translocation is also the hallmark of chronic myeloid leukemia (CML), a biphasic disease arising in the stem cell compartment, that inevitably progresses to a terminal blastic phase.3,4 The molecular consequence of the Ph translocation is the formation of a BCR-ABL fusion gene.5 In the majority of CML patients and approximately one third of patients with ALL the translocation breakpoint inBCR is found within a region spanning exons 12-16 termed the major breakpoint cluster region (M-bcr). This results in a p210BCR-ABL fusion protein.6 In about two thirds of patients with ALL and in rare cases of CML, theBCR breakpoint occurs further upstream between exons e2′ and e2, in a region termed the minor breakpoint cluster region (m-bcr) resulting in a p190BCR-ABLprotein.
We and others have recently reported deletions adjacent to the t(9;22) translocation junction on the derivative chromosome 9.7-11The deletions spanned several megabases, and usually resulted in loss of sequences from both chromosome 9 and 22 sides of the junction. Deletions were detected in 10% to 15% of CML patients and were associated with rapid onset of blast crisis and a significantly shorter survival,8,10 11 raising the possibility that similar deletions may contribute to the aggressive clinical course of Ph-positive ALL.
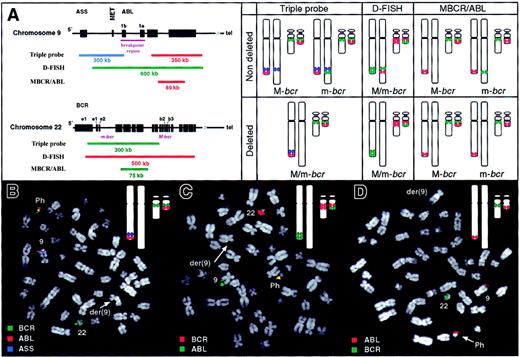
Here we present results from a study of 67 Ph-positive ALL patients (2 children and 65 adults). Bone marrow samples were screened for deletions of the derivative chromosome 9 by fluorescence in situ hybridization (FISH). The hybridization patterns expected in nondeleted and deleted Ph-positive metaphases with major or minor BCRbreakpoints for the 3 probe systems used are shown in Figure1A. Using the triple-probe system (Vysis, Downers Grove, IL, as described in Sinclair et al8 and Huntly et al11), loss of a blue signal on the derivative chromosome 9, indicating deletion of 9q sequences, was observed in 1 of 67 patients (Figure 1B). Forty-eight of 49 patients analyzed using the D-FISH system12 (QBiogene, Middlesex, United Kingdom) displayed both red and green signals on the derivative chromosome 9 indicating no overt loss of chromosome 9 or 22 material from the derivative chromosome 9 (data not shown). In the single patient in whom loss of 9q sequences had been observed using the triple-probe system, D-FISH demonstrated that the deletion involved loss of both chromosome 9 and 22 sequences (Figure 1C). The frequency of deletions in patients with Ph-positive ALL (1/67), was therefore significantly lower than the frequency of deletions in our study of CML11 (39/253, χ2: P = .002), suggesting that derivative chromosome 9 deletions are rare in Ph-positive ALL cases and do not account for the aggressive clinical course of this disease.
FISH analysis of bone marrow metaphases from Ph-positive ALL patients.
(A) Structure of ABL and BCR genes with composition of the indicated probe systems together with expected hybridization patterns using all 3 probe systems on Ph-positive metaphases. Chromosome 9 homologues are shown on left (normal 9 and derivative 9, respectively) while chromosome 22 homologues are shown on right (normal 22 and Ph, respectively). (B-D) FISH analysis of Ph-positive bone marrow metaphases from ALL patient with deletion of derivative chromosome 9. Each panel shows a metaphase together with an image of the expected hybridization signals (inset). (B) Triple-probe analysis. The blue ASS signal is absent from the derivative chromosome 9 indicating a deletion of chromosome 9 sequences from this chromosome. (C) D-FISH analysis with absence of colocalized signal on der(9) revealing deletion of 9q and 22q sequences from this chromosome. (D) MBCR/ABL analysis. Lack of a BCR signal on the Ph chromosome indicates an m-bcr breakpoint. This signal does not appear on the derivative chromosome 9 consistent with a deletion of this region. Images were captured using an epifluorescence microscope (Axioplan 2, Zeiss, United Kingdom), and SmartCapture 2001 software (Digital Scientific, United Kingdom).
FISH analysis of bone marrow metaphases from Ph-positive ALL patients.
(A) Structure of ABL and BCR genes with composition of the indicated probe systems together with expected hybridization patterns using all 3 probe systems on Ph-positive metaphases. Chromosome 9 homologues are shown on left (normal 9 and derivative 9, respectively) while chromosome 22 homologues are shown on right (normal 22 and Ph, respectively). (B-D) FISH analysis of Ph-positive bone marrow metaphases from ALL patient with deletion of derivative chromosome 9. Each panel shows a metaphase together with an image of the expected hybridization signals (inset). (B) Triple-probe analysis. The blue ASS signal is absent from the derivative chromosome 9 indicating a deletion of chromosome 9 sequences from this chromosome. (C) D-FISH analysis with absence of colocalized signal on der(9) revealing deletion of 9q and 22q sequences from this chromosome. (D) MBCR/ABL analysis. Lack of a BCR signal on the Ph chromosome indicates an m-bcr breakpoint. This signal does not appear on the derivative chromosome 9 consistent with a deletion of this region. Images were captured using an epifluorescence microscope (Axioplan 2, Zeiss, United Kingdom), and SmartCapture 2001 software (Digital Scientific, United Kingdom).
In patients without a deletion, the triple-probe system can distinguish M-bcr and m-bcr breakpoints by virtue of different signals on the derivative chromosome 9 (Figure 1A). In the current series 54 of 66 patients lacking a deletion had an m-bcr breakpoint while the remaining 12 patients had an M-bcr breakpoint. However, if a patient has a deletion of the derivative chromosome 9, then no hybridization signals will be present on this chromosome and so breakpoint position cannot be assessed using the triple-probe system. The MBCR/ABL system (QBiogene) was therefore used to study the single patient with a deletion. As shown in Figures 1A and D, the green BCR signal was absent from the Ph chromosome consistent with an m-bcr breakpoint. This result demonstrates that derivative chromosome 9 deletions are not restricted to patients with an M-bcr breakpoint.
Several mechanisms may account for the rarity of chromosome 9 deletions in Ph-positive ALL relative to CML. First, deletions are more common in patients with variant Ph translocations,8,10,11 and whereas variant translocations occur in 10% of patients with CML they are rare in ALL.13 None of the 67 patients presented here had a variant Ph translocation and this may account in part for the paucity of deletions.
Second, it is possible that a deletion is more likely to accompany a translocation with an M-bcr breakpoint. Since M-bcr breakpoints occur in the vast majority of patients with CML but in only a minority of patients with Ph-positive ALL, this could account for the rarity of deletions in the latter disease. Although the numbers are small our data are consistent with this idea since deletions were observed in only one of the 54 patients with an m-bcr breakpoint compared with 25 of 212 CML patients with a classical Ph translocation11 (P = .036).
Third, the rarity of deletions in Ph-positive ALL may reflect features of the target cell in which the translocation occurs. CML results from transformation of a multipotent stem cell,3 whereas ALL more often results from transformation of a committed B-cell progenitor.14 Lymphoid cells undergo antigen receptor rearrangements that require accurate joining of double stranded DNA breaks15 and may therefore employ mechanisms that minimize the concomitant occurrence of large deletions.
Supported by the Leukemia Research Fund and the Kay Kendall Leukaemia Fund.