Abstract
Malignant lymphocyte migration into lymph nodes is an important aspect of chronic lymphocytic leukemia (CLL), yet little is known about the processes involved. Here we demonstrate that CLL cells migrate across vascular endothelium in response to at least 3 chemokines, namely, CCL21, CCL19, and CXCL12. Moreover, transendothelial cell migration (TEM) in response to CCL21 and CCL19 was significantly higher for the malignant B cells of patients who had clinical lymph node involvement as compared with those of patients lacking such organomegaly. Furthermore, the expression of CCR7, the receptor for both CCL21 and CCL19, correlated with clinical lymphadenopathy, and blocking of CCR7 inhibited CLL cell TEM. By using immunohistochemistry we demonstrated that CCL21 and CCL19, but not CXCL12, are located in high endothelial venules and are, therefore, in an appropriate location to induce TEM. Regarding the adhesion receptors involved in TEM, α4 (most likely in association with β1) and αLβ2 were shown to be important in CLL cell TEM in vitro, but only the level of α4 expression correlated with the presence of clinical lymphadenopathy. The present studies are the first to shed light on the factors determining CLL cell entry into nodes and define the phenotype of circulating malignant cells likely to determine the pattern of lymph node enlargement in the disease.
Introduction
Malignant cell adhesion and migration are central aspects of the pathophysiology of chronic lymphoproliferative disorders.1-6 In chronic lymphocytic leukemia (CLL), these processes must determine the pattern and extent of organ involvement, including that of lymph nodes, but the receptors and ligands involved are still undefined.
The study is concerned with the mechanism of CLL cell entry into lymph nodes. This mechanism is important because substantial lymphadenopathy is a feature of more advanced disease that, in turn, is associated with a poor prognosis.7 In CLL, the normal architecture of lymph nodes is completely obliterated by infiltrating malignant cells, and this obliteration contributes to the immune defect commonly present in the disease.8 Furthermore, it is likely that the node provides an environment favoring the growth and survival of the malignant lymphocytes.9 10 There is, therefore, the prospect that interfering with CLL cell migration into nodes might have therapeutic potential.
Although little is known about the mechanisms determining lymph node enlargement in CLL, this process clearly involves a complex set of steps, including malignant cell movement into, and within, nodes and either their enhanced accumulation or reduced exit into lymphatic channels.11,12 In normal lymphoreticular tissues, these processes are controlled by lymphocyte responses to distinct chemokines, stromal cells, and extracellular matrix13-15and by maturation-induced changes in the cells' response to these microenvironmental signals.11,16,17 High endothelial venules (HEVs) are an important route of entry of lymphocytes into lymph nodes. Although HEVs are prominent in CLL nodes and malignant cells can be observed migrating through these vessels,18little is known about the chemokines, adhesion molecules, or their respective receptors involved in this migration process.
Regarding chemokines potentially relevant for CLL cell transendothelial migration (TEM), we examine the effects of 3 chemokines: CCL21, CCL19, and CXCL12. CCL21 (also known as secondary lymphoid tissue chemokine, 6Ckine, Exodus 2, and TCA-4) seemed relevant because it is a potent B-cell chemoattractant19 and is associated with HEV,20 and because knockout mice lacking receptor (CCR7) for this chemokine display defective B-cell entry into nodes.21 CCL19 (also known as Epstein-Barr virus–induced receptor ligand chemokine and macrophage inflammatory protein-3β [MIP-3β]) is also a B-cell chemoattractant, is produced by the stromal cells of extrafollicular zones of lymph nodes,22and binds to the same receptor (CCR7) as CCL21.23 CXCL12 (also known as stromal cell–derived factor-1α) and its receptor CXCR4 seemed relevant because this B-cell chemoattractant has been implicated in the homing of CLL cells to the bone marrow,24 is also found in lymphoreticular tissue,25-27 and has been shown to stimulate CLL cell TEM.28
Other chemokine receptors implicated in B-cell migration are CXCR5, CXCR3, and CCR6,29-31 but they did not seem relevant for the following reasons. Although CLL cells express CXCR5,32its ligand CXCL13 (also known as B-lymphocyte chemoattractant and BCA-1) was not investigated because the chemokine is involved in the formation of follicles rather than in entry of B cells into nodes.29,33 CXCR3 is also expressed by CLL cells.30,32 However, although CLL cells migrate in response to its ligands CXCL9 (also known as monokine induced by γ interferon) and CXCL10 (also known as interferon-inducible protein 10),30 these factors are inflammatory chemokines34 and are not associated with HEV.32 CCR6 and its ligand CCL20 (MIP-α; also known as MIP-3α) have been implicated in B-cell migration.31However, expression of this chemokine is associated with inflamed epithelium and inflammatory cells35 and was, therefore, thought unlikely to be involved in CLL cell TEM.
Here we present data suggesting that the CCR7 ligands CCL21 and CCL19 are likely to be important for CLL cell transmigration across HEVs into nodes. Furthermore, the expression levels of CCR7, and also those of the α4-integrin chain on CLL cells, were significantly higher in patients with clinical lymphadenopathy compared with those without lymph node disease. This study, therefore, identifies a chemokine receptor and an integrin chain associated with peripheral lymph node enlargement in CLL.
Patients, materials, and methods
Patients
Peripheral blood from 30 cases of CLL was studied with informed consent. The diagnosis was based on the presence in the blood of morphologically typical lymphocytes expressing low-density, light-chain restricted, surface immunoglobulin, together with CD5 and CD23.
Clinical details, including the presence or absence of lymph node enlargement (> 1cm at 2 or more sites as detected by clinical examination) and VH gene status, are listed in Table1. This table also lists the relevant phenotypic data obtained in the present study to allow assessment of their relationships to clinical disease.
Cell preparation and culture
CLL cells.
CLL cells were isolated from peripheral blood by Ficoll-Hypaque density gradient centrifugation and stored in liquid nitrogen before use. To ensure high CLL cell purity, only patients with counts of more than 50 × 109/L were studied. Frozen cells were rapidly thawed at 37°C and slowly reconstituted in RPMI containing 1% bovine serum albumin (BSA), 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Life Technologies, Paisley, United Kingdom). Cells were then allowed to recover for 60 minutes at 37°C in 5% CO2 in air (recovery times of 30, 60, and 120 minutes were tested and transmigration was similar at 60 and 120 minutes and greater than at 30 minutes). In selected patients (n = 3), both fresh and frozen cells were studied. Very similar transmigration was observed (data not shown).
Human umbilical vein endothelial cells.
Endothelial cells were stripped from the vein with trypsin and were cultured to confluence in Iscoves modified minimal essential medium containing 20% newborn calf serum, 2 mM L-glutamine, 100 μ/mL penicillin, 100 μg/mL streptomycin, and 15 μg/mL endothelial cell growth factor (Life Technologies). Human umbilical vein endothelial cells (HUVECs) that had been passaged up to 3 times were used in the transmigration assays.
Chemokines and antibodies
The following chemokines were used: CCL21, CCL19, CXCL12, and CXCL8 (interleukin-8) (all from R&D Systems, Oxford, United Kingdom).
Antibodies against CCL21 (goat polyclonal), CCL19, and CXCL12 (monoclonal antibody [mAb]; immunoglobulin G [IgG]2b and IgG1, respectively), and vascular cell adhesion molecule 1 (VCAM-1; goat polyclonal) (all from R&D Systems) were used for tissue staining. The following biotinylated second-layer antibodies were used: rabbit antigoat (Vector Laboratories, Peterborough, United Kingdom) and goat antimouse (Zymed, Cambridge, United Kingdom). Nonspecific goat immunoglobulin and mouse IgG1 and IgG2b were used as controls (all from R&D Systems).
A mAb (IgM; Pharmingen, Oxford, United Kingdom) was used to examine CLL cell expression of CCR7, the receptor for both CCL21 and CCL19; antibody staining was detected with fluorescein isothiocyanate (FITC)–conjugated goat antimouse immunoglobulin. An FITC-conjugated anti-CD19 (IgG1; Becton Dickinson, Oxford, United Kingdom) was used to determine both CLL cell purity and the nature of cells migrating in the TEM system (because only very high count cases were used, proportionally very few normal B cells were present in the cell preparations). For both mAbs, fluorescence was measured by fluorescence-activated cell sorter (FACS), with inclusion of nonspecific IgM and IgG1 (Becton Dickinson) as class-specific controls. Expression of αL (IgG2a), α4 (IgG2b), and β7 (IgG2a) integrin chains and of L-selectin (IgG2a) was measured with specific mAbs (all mAbs and isotypic controls from Becton Dickinson) and FACS analysis. Because we have previously shown that the level of expression of these integrins by CLL cells is low,5 a 3-layer technique using biotinylated horse antimouse antibody (Vector Labs) as second layer and streptavidin-phycoerythrin (Becton Dickinson) as the final layer were used. Nonspecific Ig2a and IgG2b (Becton Dickinson) were used as controls.
In addition, mAbs were used to determine which integrins and chemokine receptors might be involved in TEM. Blocking mAbs against the following were used: αL (IgG2a), α4 (IgG1) (both from, R&D Systems), α5 (IgG1; Pharmingen), β1 (IgG2b), β2 (IgG1) (both from R&D Systems), β7 (IgG1; Pharmingen), and CCR7 (rat IgG2a) (3D12, a kind gift from M. Lipp, MDC, Berlin). Nonspecific mouse IgG1, IgG2a, and IgG2b were used as controls (all from R&D Systems).
TEM assay
HUVECs were grown to confluence on the inserts of Transwell plates (5-μm pore size; Corning Costar, High Wycombe, United Kingdom). The HUVECs were washed in RPMI, and 5 × 105CLL cells (in RPMI containing 0.1% BSA, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin) were added to the inserts. Chemokines were then added to the bottom wells at the following concentrations: CCL21 and CCL19 at 10, 100, 1000, and 2000 ng/mL; CXCL12 at 10, 100, and 1000 ng/mL; and CXCL8 at 0.5, 5, and 50 ng/mL (all in RPMI containing 0.1% BSA, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin). It was shown in preliminary experiments that maximal migration indices (MIs; more to follow) were observed at CCL21 and CCL19 concentrations of 1000 ng/mL and at a CXCL12 concentration of 100 ng/mL. These concentrations of chemokine were, therefore, used in all subsequent experiments. No TEM was observed at any of the concentrations of CXCL8 (previous work from this department1 has shown that CXCL8 at 5 ng/mL induces maximal CLL cell movement on the extracellular matrix component hyaluronan). A range of incubation times was also tested (2, 4, 6, 8, and 24 hours); the MI was maximal at 6 hours and remained constant until 8 hours; therefore, an incubation time of 6 hours was routinely used. All assays were performed in triplicate.
After incubation, the undersides of the inserts were scraped to remove any cells that had recently transmigrated, and the cells were then harvested from the bottom wells. As some CLL cells had adhered to the bottom wells, EDTA (0.2%; Sigma, Poole, United Kingdom) was added for 5 minutes at 37°C before harvesting. These transmigrated cells were then counted in a hemocytometer. To determine the CLL cell content of the transmigrated cells, an aliquot was always stained for CD19 and analyzed by FACS. The MI (no. of CD19+ cells transmigrating with chemokine divided by no. of cells transmigrating in the absence of chemokine) was then calculated. Student t test was used to determine the statistical significance of the results.
Inhibition of TEM
CLL cells were incubated at 4°C for 30 minutes with blocking mAbs (concentrations) to the following integrin chains: β1 (1 and 10 μg/mL), β2 (1 and 10 μg/mL), β7 (1 and 10 μg/mL), α4 (0.5 and 5μg/mL), α5 (0.2 and 2 μg/mL), and αL (1 and 10 μg/mL). Also, in certain experiments a blocking mAb to CCR7 was used (50 and 100 μg/mL). These amounts of mAbs were chosen to cover the range of concentrations that have been reported to block adhesion in other systems (manufacturers' recommendations and M. Lipp, written personal communication, October 2001). CLL cells were then added to the Transwell inserts, and the TEM assay was performed as above.
In addition, CLL cells were incubated for 2 hours at 37°C with 1 μg/mL pertussis toxin (Sigma) to block chemokine receptor signaling and were washed. TEM measurements were performed as above.
Tissue staining
CLL nodes (n = 5) were diagnostic samples. Normal nodes (n = 5) were obtained from axillary clearance for breast cancer and were macroscopically and microscopically normal.
Formalin-fixed paraffin-embedded tissue was stained for chemokines as follows. After clearing and rehydration, slides were boiled in 10 mM sodium citrate buffer (pH 6) for 10 minutes and blocked with 10 mg/mL BSA before overnight incubation with antibodies to CCL21 (20 μg/mL) and VCAM-1 (1:200) at 4°C, or with mAbs to CCL19 (5 μg/mL) and CXCL12 (50 μg/mL) at 25°C. These amounts of the antibodies were chosen after titration over a range of concentrations. Sections were then incubated with biotinylated rabbit antigoat (CCL21 and VCAM-1) or with biotinylated goat antimouse (CCL19 and CXCL12) antibodies, followed by ExtraAvidin alkaline phosphatase (Sigma) and exposure to substrate (Fast Red/Naphthol AS MX phosphatase/levamisole; Sigma). Slides were counterstained with hematoxylin (Sigma).
VH hypermutation analysis
For each case, total RNA was extracted from CLL cells by using Trizol reagent (Life Technologies) and 1-μg aliquots were reverse transcribed with M-MuLV reverse transcriptase (Promega, Southampton, United Kingdom) and an oliog(dT)15 primer. Aliquots of the resulting complimentary DNAs were used to isolate clonally expressed VHDHJH sequences in 2 sets of VH gene family–specific polymerase chain reaction (PCR) reactions. In the first set of 7 reactions, the sense primers were consensus sequences derived from the framework region 1 (FWR1)36 of each of the 7 families. In the second set of 6 reactions, the sense primers were from the leader regions (families 1 and 7 with a common primer37). In all reactions, the same combination of 3 constant region-specific (α, γ, μ) antisense primers were used.36 The 50-μL reactions (20 pmol of each primer, 1.5 mM MgCl2, 100 μM of each dNTP, and 2.5 U Taq Polymerase [in supplied buffer; Promega]) were cycled 30 times by using a touch-down protocol,38 with the annealing temperature reducing from 63°C to 57°C over the first 12 cycles and then analyzed by electrophoresis. Products for a given family from 2 separate PCR reactions were cloned. Between 2 and 4 clones derived from each PCR (ie, a total of 4 to 8 cases from each patient) were sequenced commercially with a vector-specific primer. The sequences were accepted as representing the CLL clone if there was identity between and within FWR1 and the leader sequence-derived PCR clones.
The extent of VH gene hypermutation was determined by using VBASE.39 For the purpose of assigning prognostic significance to the extent of VH hypermutation, previous studies have used a threshold of either 2% or 5% divergence from the nearest germline IgVH gene to define hypermutation.36 40 Therefore, for our studies of the relationship between VH gene hypermutation and TEM/lymphadenopathy, we have used both the 2% and 5% hypermutation thresholds for our data analyses.
Results
CCL21, CCL19, and CXCL12 all induce CLL cell TEM
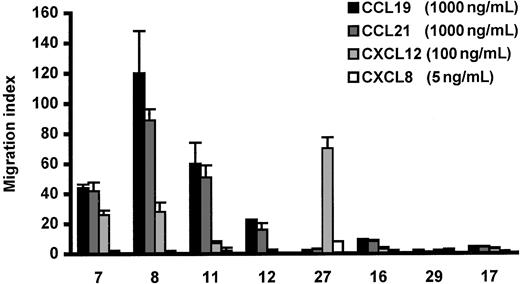
In the absence of chemokine, CLL cells showed little TEM (< 0.2%) in the Transwell migration assay. Variable CLL cell TEM (0%-12% of input cells) was seen in response to CCL21, CCL19, and CXCL12 (n = 8 cases; Figure 1). When CLL cell migration was observed, CCL21 and CCL19 produced comparable MIs, which were usually higher than those induced by CXCL12. As expected from our previous work showing that CXCL8 stimulates CLL cell movement within, but not into nodes,1 this chemokine failed to induce CLL cell TEM in any of the cases studied (Figure1).
Effect of chemokines on CLL cell TEM in a Transwell system.
Specific CLL cell TEM is expressed as a migration index as described in “Patients, materials, and methods.” The concentrations of chemokines were those that induced maximal TEM. The error bars represent SEM of triplicate measurements. The numbers on the X-axis indicate the patients referred to by number in Table 1.
Effect of chemokines on CLL cell TEM in a Transwell system.
Specific CLL cell TEM is expressed as a migration index as described in “Patients, materials, and methods.” The concentrations of chemokines were those that induced maximal TEM. The error bars represent SEM of triplicate measurements. The numbers on the X-axis indicate the patients referred to by number in Table 1.
Because the TEM induced by CCR7 stimulation seemed of potential in vivo relevance (more to follow), the reproducibility of cell migration in response to CCL21 was studied on 2 or more occasions in all 8 cases. When the MI was low (< 10; n = 4), it remained low on repeated testing. Similarly, when the MI was high (> 20; n = 4), it remained high but varied at different times of study (eg, in case 7 studied 5 times, the MI was between 48 and 120). CCL21-induced CLL cell TEM was completely abrogated by preincubation of CLL cells with pertussis toxin (MI = 0.7 ± 0.3; n = 3), an inhibitor of chemokine-receptor signaling.
Clinical analysis of the patients studied in Figure 1 indicated that the 4 patients whose cells repeatedly migrated in response to CCL21 and CCL19 all had lymph node enlargement, whereas those cases whose cells displayed little or no transmigration had no lymphadenopathy. Because at the onset of the present study CCL21, known to be concentrated in HEV,20 seemed to be the more likely candidate chemokine to induce CLL cell TEM in vivo, we examined the relationship between migration to CCL21 and clinical lymphadenopathy in a larger number of patients.
CCL21-mediated TEM of CLL cells is related to clinical lymphadenopathy
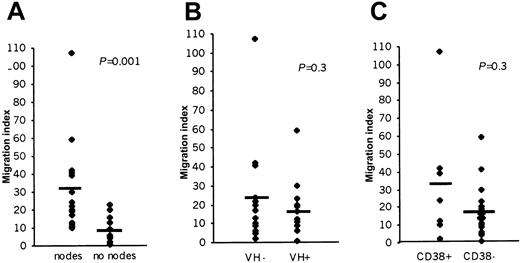
When an additional 22 CLL patients were studied, the mean MI was significantly higher in patients with nodal enlargement compared with those without lymphadenopathy (31 versus 10; P < .01; Figure 2A). This finding demonstrates that the ability of CLL cells to cross endothelium is related to the presence of clinical lymphadenopathy. As expected, because lymphadenopathy is one of the features used in clinical staging, there was also an association between high MI and later stages of the disease (stage 0 versus I-IV = 8.4 ± 0.2 versus 27 ± 6,P = .002).
The relationship between CCL21-induced CLL cell TEM and lymphadenopathy.
(A; n = 15 for each group), VH hypermutation status (B; n = 13 for each group) or CD38 expression (C; CD38+n = 7, CH38− n = 20). The bar represents the average MI of each patient group. VH− and VH+ = < 2% and > 2%, respectively. CD38+ = > 30% CLL cells expressing CD38.
The relationship between CCL21-induced CLL cell TEM and lymphadenopathy.
(A; n = 15 for each group), VH hypermutation status (B; n = 13 for each group) or CD38 expression (C; CD38+n = 7, CH38− n = 20). The bar represents the average MI of each patient group. VH− and VH+ = < 2% and > 2%, respectively. CD38+ = > 30% CLL cells expressing CD38.
In view of the recent interest in the fact that CLL can be divided into 2 very different prognostic groups according to the extent of VH gene hypermutation, we next examined the relationship between such hypermutation and TEM. In the patients (n = 26) for whom VH data were available, there was no significant difference (P > .05; Figure 2B) between CLL cell TEM in patients with or without hypermutation regardless of whether a threshold of 2% or 5% deviation from the germline was used in the analysis. However, more CLL patients with clinical lymphadenopathy had nonhypermutated VH genes (10 of 15 at < 2% VH mutation and 13 of 15 at < 5%VH mutation) than did those without nodes (3 of 11 at < 2% VH mutation and 4 of 11 at < 5% VH mutation). When the proportions were analyzed by Fisher exact test, the association between lymphadenopathy and VH nonhypermutation was significant when the 5% mutation threshold was used (P = .01) but not at the 2% threshold level (P = .11). We, therefore, concluded that the ability of CLL cells to migrate into, or accumulate in, nodes may be related to their VH hypermutational status.
Because expression of CD38 is a poor prognostic indicator in CLL40-42 and is often associated with the absence of VH hypermutation,40 we also examined the relationship between CD38 expression, TEM, and nodal disease. We found that, although there was a trend toward an association between high CD38 expression and lymphadenopathy (mean ± SE for patients with lymphadenopathy = 20.8% ± 6.3% CD38+ cells; for those without enlarged lymph nodes 6.8% ± 4.6%), it did not reach statistical significance (P = .08). There was no association between high CD38 expression (> 30% positive cells) and TEM (Figure 2C; n = 27; P = .29), reflecting that not all patients with lymphadenopathy displayed high CD38 expression, and yet in all cases the malignant cells migrated in response to CCL21.
In conclusion, the data show that clinical lymphadenopathy is clearly related to the ability of CCL21-stimulated CLL cells to undergo TEM. In addition, it seems that less mature non-VH hypermutated and CD38+ CLL cells have a greater propensity to accumulate in nodes than do more mature VH hypermutated and CD38 cells.
We next investigated the mechanism(s) responsible for the different CLL cell migratory responses to CCL21 observed in patients with lymphadenopathy, compared with those lacking lymph node enlargement. It has been shown in adult T-cell leukemia that CCL21 receptor (CCR7) expression is related both to the malignant cell chemotactic response to CCL21 and to organ involvement in the disease.43 We, therefore, examined the CCR7 expression of the CLL cells in our series of patients and related receptor levels to the presence or absence of lymphadenopathy.
CCR7 expression is higher in patients with lymphadenopathy
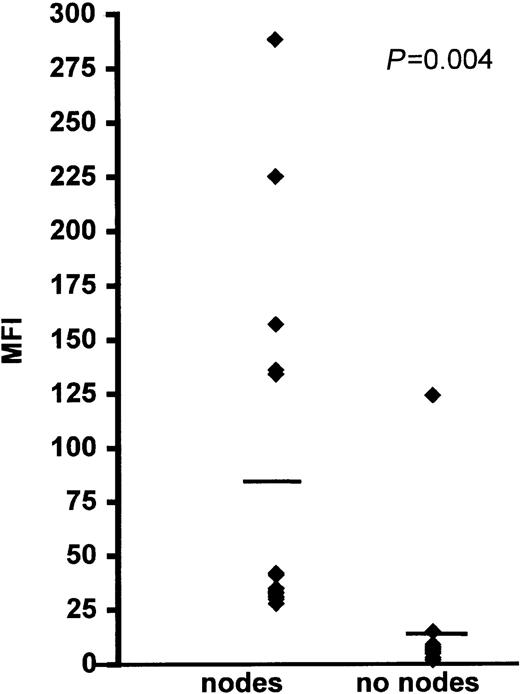
Variable CCR7 expression was detected on the CLL cells from all of the patients examined. In most cases, a distinct positive peak consisting of more than 95% of cells was observed. In 5 cases in which the cells had a mean fluorescence intensity (MFI) less than 20, a peak shift was seen, indicating that virtually all (> 95%) of the cells were weakly positive.
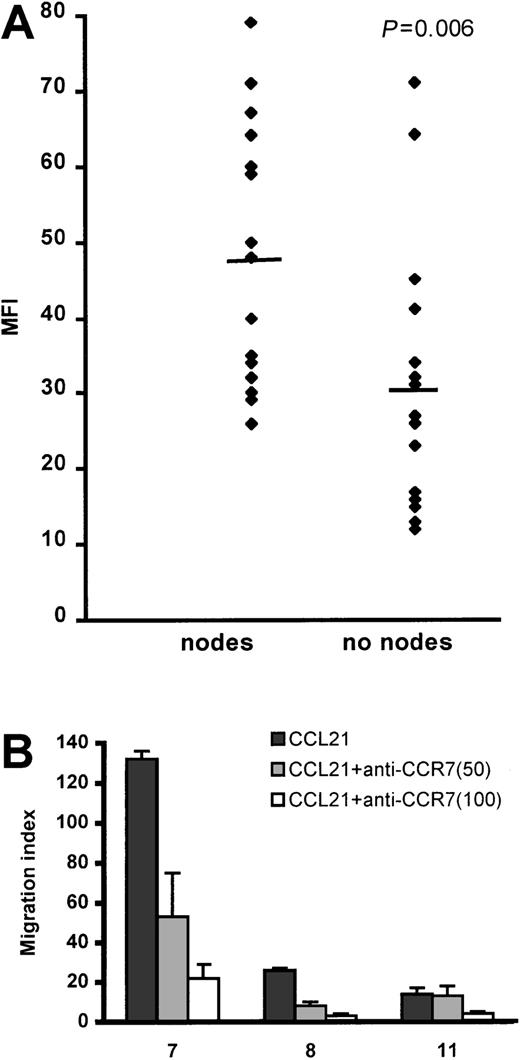
The intensity of CCR7 expression was significantly higher on the CLL cells of patients with lymphadenopathy compared with those without lymph node enlargement (Figure 3 and Table 1). This finding strongly suggests that transmigration to lymph nodes involves stimulation of CCR7. We, therefore, used an anti-CCR7 blocking mAb in TEM experiments and showed that this reagent strongly reduced CLL cell migration to CCL21 (Figure 3B).
CLL cell CCR7 expression and function in relation to lymph node enlargement.
(A) The bar represents the average mean fluorescent intensity (MFI) for each group. Receptor expression was measured by FACS analysis, using an indirect immunofluorescence technique. (B) This panel shows inhibition of CLL cell TEM migration by 2 concentrations (50 and 100 μg/mL) of blocking anti-CCR7 mAb. The numbers on the X-axis refer to the patients listed in Table 1. The error bars represent SEMs of triplicate measurements. Control rat IgG2a antibody had no inhibitory effect.
CLL cell CCR7 expression and function in relation to lymph node enlargement.
(A) The bar represents the average mean fluorescent intensity (MFI) for each group. Receptor expression was measured by FACS analysis, using an indirect immunofluorescence technique. (B) This panel shows inhibition of CLL cell TEM migration by 2 concentrations (50 and 100 μg/mL) of blocking anti-CCR7 mAb. The numbers on the X-axis refer to the patients listed in Table 1. The error bars represent SEMs of triplicate measurements. Control rat IgG2a antibody had no inhibitory effect.
Because both CCL21 and CCL19 are ligands of CCR7, we next examined the expression of these 2 chemokines in CLL and normal nodes. In addition, because CXCL12 also stimulated CLL cell TEM, we examined the distribution of this CXCR4- binding chemokine.
CCL21 and CCL19, but not CXCL12, are associated with HEVs
In CLL nodes, the normal architecture is completely replaced by infiltrating leukemic cells among which a variety of vascular and lymphatic channels are visible.8
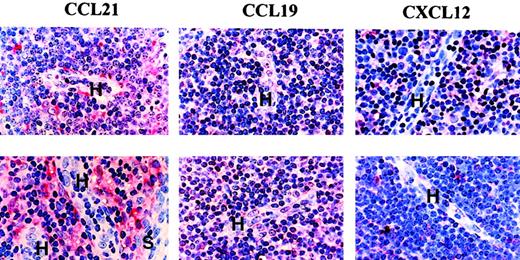
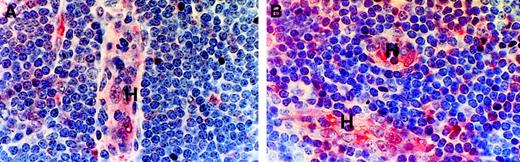
In both CLL and normal nodes (Figure 4), weak staining for both CCL21 and CCL19 was found in HEVs; in contrast, these vessels were negative for CXCL12. The stroma was reactive for all these chemokines with some stromal cells, including the peri-HEV fibroblasts, showing strong staining for CCL21 (Figure 4).
Chemokine expression in CLL and normal lymph node tissue.
Formalin-fixed, paraffin-embedded material was stained by a triple-layer technique involving antichemokine antibody, a biotinylated second layer, and ExtrAvidin-alkaline phosphatase as a third layer. H indicates HEV; S, sinus. Note that HEVs contain weak staining for both CCL21 and CLL19. Some stromal cells, including peri-HEV fibroblasts, showed particularly marked staining for SLC. Control staining with the relevant nonspecific antibodies was completely negative. In the top panels (n = 5); bottom, (n = 4).
Chemokine expression in CLL and normal lymph node tissue.
Formalin-fixed, paraffin-embedded material was stained by a triple-layer technique involving antichemokine antibody, a biotinylated second layer, and ExtrAvidin-alkaline phosphatase as a third layer. H indicates HEV; S, sinus. Note that HEVs contain weak staining for both CCL21 and CLL19. Some stromal cells, including peri-HEV fibroblasts, showed particularly marked staining for SLC. Control staining with the relevant nonspecific antibodies was completely negative. In the top panels (n = 5); bottom, (n = 4).
We, therefore, concluded that the location of CCL21 and CCL19, but not CXCL12, indicated that these chemokines can mediate CLL cell entry into lymph nodes. Thus, these histologic findings add relevance to our in vitro observations that migration to CCL21 and CCL19, and expression of the receptor (CCR7) for these chemokines, is related to the presence of lymphadenopathy in CLL. However, although the levels of CCR7 were significantly different between the patients with and without lymphadenopathy, there was some overlap in expression between the 2 groups. We, therefore, examined other factors that might be involved in determining the entry of CLL cells into lymph nodes in vivo.
We first examined the expression of L-selectin by CLL cells because this molecule is essential for the initial tethering of lymphocytes to HEVs before integrin-mediated arrest and subsequent diapedesis across endothelium.
L-selectin expression by CLL cells is not correlated with clinical lymphadenopathy
FACS analysis showed that L-selectin expression by CLL cells varies greatly from case to case. In all patients studied, at least a proportion (16%-82%) of cells expressed variable levels of L-selectin (MFI, 30-176). However, there was no correlation between the percentage positivity (P = .3), or MFI (P = .5), and the presence or absence of lymphadenopathy (data not shown).
As regards integrin involvement in lymphocyte TEM, the most important receptors are α4β1/β7 and αLβ2.13,14,44We5 and others45 have previously shown that CLL cells consistently express αLβ2, whereas expression of α4, in combination with either β1 or β7 chains, is variable. We, therefore, examined whether these integrins, as in normal lymphocytes, were involved in CLL cell TEM.
CLL cell TEM depends on αLβ2 and α4β1
CLL cell TEM was significantly reduced by blocking mAbs to either β1 or β2 integrins (data not shown). As expected, in all 3 cases, inhibition of CLL cell TEM was seen with anti-α4 or anti-αL mAbs, whereas anti-α5 mAb and isotypic control antibodies had no effect (Figure 5). Blocking with a combination of antibodies (anti-β1 + anti-β2 or anti-αL + anti-α4) was not more effective than blocking each integrin chain alone (data not shown; n = 3). Because a study46implicated α4β7, as well as α4β1, in normal lymphocyte migration into peripheral lymph nodes, we also tested the effect of a blocking anti-β7 mAb on CLL cell TEM. Inhibition was only seen in one of the 3 cases studied (data not shown).
Inhibition of CLL cell TEM with the use of blocking mAbs to αL, α4, and α5.
After preincubation for 30 minutes at 4°C with antibody (α4, 0.5 μg/mL; α5, 2 μg/mL; and αL, 1 μg/mL), CLL-cell migration toward CLL21 was tested in the TEM model. Isotypic control antibodies had no effect on migration. The numbers on the X-axis refer to the patients listed in Table 1. The error bars represent SEMs of triplicate measurements.
Inhibition of CLL cell TEM with the use of blocking mAbs to αL, α4, and α5.
After preincubation for 30 minutes at 4°C with antibody (α4, 0.5 μg/mL; α5, 2 μg/mL; and αL, 1 μg/mL), CLL-cell migration toward CLL21 was tested in the TEM model. Isotypic control antibodies had no effect on migration. The numbers on the X-axis refer to the patients listed in Table 1. The error bars represent SEMs of triplicate measurements.
Having shown that α4 and αL are involved in CLL cell TEM in vitro, we next examined the expression of these 2 integrins on CLL cells and related this expression to clinical lymph node involvement.
α4 expression is significantly higher on the CLL cells of patients with lymphadenopathy
CLL cells from all the patients who had lymphadenopathy expressed variable amounts of α4 as detected by FACS analysis with the use of a sensitive triple-layer technique (Figure6; Table 1). Whereas, in patients without lymphadenopathy, no α4 expression was observed in 14 of 15 patients studied (Figure 6; Table 1); in all 14 cases, the MFI of the test cells was identical to that of cells stained with isotypic control antibody. In the single patient without nodal enlargement whose cells expressed α4, CCR7 expression was low (MFI = 18 compared with a MFI of 7 for cells stained with isotypic control mAb).
α4 expression on CLL cells of patients with and without lymphadenopathy.
Cells were stained by a triple-layer avidin-biotin method and analyzed by FACS. The bar represents the MFI for each patient group. In patients without nodes, the MFI in all but one case was identical to that of the CLL cells stained with the isotypic control mAb.
α4 expression on CLL cells of patients with and without lymphadenopathy.
Cells were stained by a triple-layer avidin-biotin method and analyzed by FACS. The bar represents the MFI for each patient group. In patients without nodes, the MFI in all but one case was identical to that of the CLL cells stained with the isotypic control mAb.
The α4 on CLL cells is known to be associated with both β1 and β7 chains.5 Because CLL cell TEM was not only blocked by an anti-β1 mAb but, in one case, anti-β7 was also inhibitory, we also measured β7 expression. The β7 was variably expressed on the cells of all CLL patients. However, there was no correlation between levels of expression and lymphadenopathy (MFI, 28 ± 2 for patients with nodes; MFI, 32 ± 4 for patients lacking nodes; P = .3)
Regarding αL, this integrin chain was expressed by the malignant cells of all the CLL patients studied, and there were no differences in expression between the patients with and without lymphadenopathy (mean MFI, 56 ± 6 for patients with nodes; MFI, 56 ± 7 for patients without nodes; P = .6). Thus, although anti-αL inhibited TEM, this absence of correlation suggests that levels of expression are in all cases sufficient to support transmigration. It was, therefore, concluded that α4, most likely in combination with β1 is the key integrin in determining the entry of CLL cells into nodes after CCR7 stimulation. The endothelial ligand for α4 is VCAM-1.46It, therefore, seemed important to examine lymph node tissue for VCAM-1 expression.
Vascular endothelial cells in CLL node express VCAM-1
In CLL nodes, VCAM-1 staining was observed in HEVs and other vascular endothelial cells (Figure 7). Essentially similar staining was observed in normal lymph nodes (Figure 7).
VCAM-1 expression in CLL and normal lymph node tissue.
Formalin-fixed, paraffin-embedded material was stained by a triple-layer technique involving a mAb to VCAM-1, a biotinylated second layer, and ExtrAvidin-alkaline phosphatase as a third layer. H indicates HEV; (A), h=5; (B), n=4.
VCAM-1 expression in CLL and normal lymph node tissue.
Formalin-fixed, paraffin-embedded material was stained by a triple-layer technique involving a mAb to VCAM-1, a biotinylated second layer, and ExtrAvidin-alkaline phosphatase as a third layer. H indicates HEV; (A), h=5; (B), n=4.
Discussion
The present study is concerned with the process by which CLL cells enter lymph nodes. We demonstrated that a number of relevant chemokines (CCL21, CCL19, and CXCL12) can induce the malignant cells of a proportion of cases of CLL to migrate through vascular endothelium. However, we found that CCL21 and CCL19 induce more CLL cells to transmigrate than does CXCL12. Furthermore, we found that the ability of CLL cells to respond to CCL21 and CCL19 in vitro strongly correlates with the presence of clinical lymphadenopathy. We then went on to show that, although all CLL cells possess the receptor for these chemokines (CCR7), levels of expression are higher on the cells of patients with lymph node enlargement. These results are entirely compatible with a study in adult T-cell leukemia43 which found that the ability of the malignant cells to respond to CCL21 correlated with CCR7 expression and lymph node enlargement. Moreover, by using a blocking antibody, we now directly demonstrate involvement of this receptor in CLL cell TEM in response to CCL21.
In view of the current interest in the pathogenetic and prognostic significance of VH hypermutational status and CD38 expression in CLL,40,41,47,48 and because lymph node enlargement is a feature of more advanced disease and poor prognosis, we also examined whether these parameters are related to the degree of in vitro TEM and clinical lymphadenopathy. We found that there was no correlation between VH hypermutation and TEM, but more patients with lymphadenopathy had nonmutated VH genes than did patients without nodes. These findings are compatible with previous reports that lymphadenopathy can be found in patients with or without VH hypermutation.40,48 As for CD38 expression, the cells of patients with enlarged lymph nodes expressed more CD38 and had higher levels of TEM than did patients without lymphadenopathy. However, only the relationship between VH nonhypermutation (< 5%) and lymphadenopathy achieved statistical significance. With regard to CD38 expression and lymphadenopathy, it is interesting that a recent report of a very large number of CLL cases showed a significant positive correlation between these 2 parameters.42
By using immunohistochemistry, we next investigated the location of CCL21, CCL19, and CXCL12 in CLL and normal lymph node tissue. CCL21 and CCL19 were found in both HEVs and the surrounding stroma, whereas CXCL12 was found in stroma only. These findings, together with our demonstration of the correlation between CCR7 expression and lymphadenopathy, suggest that CCL21 and/or CCL19 are likely to be involved in the stimulation of CLL cell entry into lymph nodes. These conclusions are in accord with studies in knockout mice, suggesting that CCR7 is important for the migration of B cells into nodes.21 Furthermore, our immunohistochemical observations are compatible with previous studies in mice showing that, of the 2 chemokines, CCL21 is produced in HEV20 and that CCL19 produced by stromal cells is transcytosed through endothelium to the luminal surface of HEVs.49 Although our data suggest that CCR7 ligand(s) are important in CLL cell TEM, they do not rule out the possibility that other chemokines such as CXCL13 (for which CLL cells express receptor CXCR532) may be involved in the lymphadenopathy of the disease. However, this possibility seems unlikely because CLL nodes lack follicles, the formation of which critically depends on the presence of CXCL13.29 33
It has previously been reported that CXCL12 stimulates in the TEM of CLL cells, and it was proposed that this chemokine may be involved in the migration of CLL cells into the bone marrow.27 28 Our results confirm that CXCL12 is able to induce CLL cell TEM, but our immunohistochemical demonstration that the chemokine is not present in HEVs suggests that CXCL12 is not involved in CLL cell entry into nodes. It, therefore, seems likely that different chemokines direct CLL cells into distinct lymphoreticular tissues.
By showing that CCR7 stimulation is likely to be important for CLL cell entry into lymph nodes and in the generation of clinical lymphadenopathy, we next focused on the adhesive interactions involved. This focus seemed all the more important, given that there was some overlap in the levels of CCR7 expression in patients with and without lymphadenopathy. After demonstrating that L-selectin expression, despite being variable, did not correlate with clinical lymphadenopathy, we next concentrated on integrins. Both αLβ2 and α4β1/β7 integrins are known to be important for the TEM of normal lymphocytes.13,44,46,50 With the use of blocking mAbs, we demonstrated that these receptors are also involved in the TEM of CLL cells. Furthermore α4, but not αL or β7, expression, strongly correlated with the presence of lymphadenopathy. In addition, we demonstrated that the α4 ligand, VCAM-1, is expressed by the HEVs of CLL and normal nodes. Although earlier reports51,52 did not consider VCAM-1 as an important integrin ligand for lymphocyte migration into nodes, our findings support more recent animal studies that demonstrated that this molecule is the receptor on HEVs for α4-containing integrin ligands.13 46 We, therefore, conclude that α4 expression (most likely in association with β1), along with CCR7 receptor levels, play an important role in CLL cell entry into lymph nodes and in the generation of clinical lymphadenopathy.
There have been a number of previous studies of integrin expression on CLL cells.5,45 53 Most demonstrate α4 on the cells of approximately 40% of patients, a finding compatible with the present study (approximately 50% of our cases expressed α4), but they have not attempted to relate α4 expression to lymphadenopathy. We believe, therefore, that the present study is the first to demonstrate an association in CLL between malignant cell expression of a particular integrin chain and clinical lymph node enlargement.
In conclusion, then, the present study suggests that CCR7 engagement by CCL21 and/or CCL19 stimulates CLL cell entry into lymph nodes by HEV and that CCR7 and α4 expression are important for this process. Although we believe that the present findings are important for the entry of CLL cells into nodes, further studies are needed to determine why the malignant cells accumulate in, rather than exit from, lymph nodes in patients with clinically detectable lymphadenopathy.
We thank Dr M. Lipp of the Max Delbruck Centrum, Berlin, for his kind gift of a blocking CCR7 antibody (3D12).
Supported by the Leukaemia Research Fund (United Kingdom) (M.Z. and J.C.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kathleen J. Till, Department of Haematology, Royal Liverpool University Hospital, Daulby Street, Liverpool, L69 3GA, United Kingdom; e-mail: k.j.till@liv.ac.uk.