Abstract
The platelet collagen receptor glycoprotein (GP) VI–Fc receptor γ-chain (FcRγ) complex transduces signals in an immunoreceptorlike manner. We examined a role for the Triton X-100–insoluble membrane rafts in GPVI–FcRγ complex signaling. Methyl-β-cyclodextrin (MβCD)-induced disruption of the membrane rafts inhibited not only platelet aggregation and secretion but also tyrosine phosphorylation of signaling molecules on stimulation through the GPVI–FcRγ complex. The GPVI–FcRγ complex was constitutively associated with membrane rafts wherein the Src family kinases and LAT were also present. Their association was not affected by the complex engagement but was highly sensitive to MβCD treatment. Thus, we provide the first evidence that the GPVI–FcRγ complex is constitutively and functionally associated with membrane rafts.
Introduction
Plasma membranes of many cell types contain microdomains commonly referred to as membrane or lipid rafts. They are enriched in sphingolipid and cholesterol that would preferentially self-associate to form lateral lipid assemblies in an unsaturated glycerophospholipid environment.1,2 Membrane rafts are resistant to solubilization in nonionic detergent but can be isolated from low-density fractions after flotation in a sucrose gradient. A variety of specific proteins are concentrated in raft domains, including many glycophosphatidylinositol-anchored proteins, Src family kinases, and linker for activation of T cells (LAT). Evidence has accumulated for membrane raft-dependent scaffolding of signaling complexes, mostly shown by studies on immunoreceptor signaling including T- and B-cell antigen receptors and Fcε receptor I.3,4 The functional significance of membrane rafts has been strengthened by the finding that receptor aggregates in membrane rafts can signal in a ligand-independent manner.5 Dorahy et al6 7 first identified the Triton X-100–insoluble membrane rafts of resting platelets that are enriched in Src, Lyn, and CD36. However, the exact role for membrane rafts of platelets remains to be clarified.
Platelets adhere to the extracellular matrix protein, collagen, at the site of vascular damage and become activated through specific membrane receptors, resulting in shape change, granule release, and aggregation. Previous studies have identified the integrin α2β1 and the glycoprotein (GP) VI–Fc receptor γ-chain (FcRγ) complex as 2 major receptors involved in platelet–collagen interaction.8 It has been well established that the GPVI–FcRγ complex signals in an immunoreceptorlike manner; the complex engagement causes activation of the Src family kinases, including Fyn, which then phosphorylate the immunoreceptor tyrosine-based activation motifs (ITAMs) of FcRγ. Syk kinase binds the tyrosine-phosphorylated ITAMs through its SH2 domain. It is then activated and phosphorylates phospholipase Cγ2 (PLCγ2), leading to its activation. The signals further expand through tyrosine phosphorylation of LAT, SLP-76, and others.8 Clemetson et al9 first reported the cloning of GPVI, a member of the immunoglobulin superfamily closely related to the Fc receptor for IgA and the natural killer cell receptors. The genomic structure of GPVI, mapped on chromosome 19q13.4, and alternative splicing forms of GPVI have been reported.10 Recent studies addressed the question of how α2β1 and the GPVI/FcRγ complex are involved in the hemostatic system on the exposed subendothelial collagens. For example, Zheng et al12 reconstituted the GPVI–FcRγ complex in RBL-2H3 cells and found that the complex-expressing cells had strong adhesive and signaling responses to convulxin (Cvx, a snake venom protein that is a GPVI-specific agonist11) and weak responsiveness to collagen-related peptide but that they had no responsiveness to collagen, suggesting that the direct binding of platelets to collagen should be mediated by α2β1 rather than by the GPVI–FcRγ complex. On the other hand, Nieswandt et al13performed the functional studies using β1-null or GPVI-deficient mouse platelets and indicated that GPVI–collagen interaction is an essential prerequisite for integrin-mediated firm adhesion followed by platelet thrombus formation. Thus, these contradictory reports propose unresolved problems of how the GPVI–FcRγ complex contributes to the activation of platelets interacting with subendothelial collagens. With these backgrounds in mind, we reasoned that membrane rafts might be another important aspect to understand how GPVI–FcRγ downstream signaling was initiated, and we have investigated the relationship between membrane rafts and the GPVI–FcRγ complex. We here report the constitutive and functional association of the GPVI–FcRγ complex with membrane rafts.
Materials and methods
Reagents
Methyl-β–cyclodextrin (MβCD) and cholesterol were obtained from Sigma (St Louis, MO). Polyclonal anti-LAT and anti–SLP-76 antibodies were from Upstate Biotechnology (Lake Placid, NY) and from Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Monoclonal anti–α-tubulin and antivinculin antibodies were from Neo Markers (Fremont, CA) and from ICN (Costa Mesa, CA), respectively. Preparation of human anti-GPVI immunoglobulin (Ig) G and its F(ab′)2fragments [F(ab′)2αGPVI] was performed as described previously,14,15 using the serum of a patient with GPVI deficiency14 who has been followed up as an outpatient in our department. For immunoblotting, anti-GPVI IgG was biotinylated as previously reported.16 All other reagents have been previously described.10 15-18
Preparation and stimulation of platelets
Washed platelets (109 cells/mL) were prepared as described previously19 and were extracted with 0 to 20 mM MβCD for 30 minutes in HEPES buffer at 37°C. In some experiments, 20 mM MβCD was replaced by the same concentration of MβCD complexed with cholesterol, which was prepared as Klein et al20reported. Platelets were pelleted by centrifugation, resuspended at a final concentration of 1 × 109 cells/mL in HEPES buffer, and stimulated by 50 μg/mL collagen or 50 ng/mL Cvx with gentle agitation for appropriate periods in HEPES buffer at 37°C.
Biotin labeling of platelet-membrane proteins
Membrane surface proteins of the washed platelets were labeled with biotin, using the enhanced chemiluminescence (ECL) Protein Biotinylation System according to protocols supplied by the manufacturer (Amersham Pharmacia Biotech, Piscataway, NJ). Biotin-labeled platelets were extracted with 0 or 20 mM MβCD and were resuspended in HEPES buffer as described above.
Immunoprecipitation and immunoblotting
Platelet aggregation and adenosine triphosphate release
For monitoring of platelet aggregation and adenosine triphosphate (ATP) release on a Lumi-aggregometer (Chrono-Log, Havertown, PA),15 the platelets were resuspended at a final concentration of 3 × 108 cells/mL in HEPES buffer and were stimulated with the indicated concentrations of collagen, Cvx, or phorbol 12-myristate 13-acetate (PMA).
Sucrose density ultracentrifugation
For separation of membrane rafts, platelets (109cells/mL × 0.6 mL), prepared and treated as described above, were lysed in ice-cold Triton X-100 lysis buffer (0.5% Triton X-100, 150 mM NaCl, 25 mM 2-morpholinoethanesulfonic acid, pH 6.5, 2 mM phenylmethylsulfonyl fluoride) and were subjected to sucrose density-gradient ultracentrifugation as Dorahy et al6reported. Gradients were fractionated into 12 equal (1 mL) portions and were analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), followed by immunoblotting as described previously.18
Flow cytometry
Using F(ab′)2αGPVI, anti-αIIbβ3 antibody (P2), or anti-α2 antibody (Gi9) (Immunotech, Marseilles, France) and each corresponding control F(ab′)2 or IgG, flow cytometric analysis was performed as previously reported.10
Results
Pretreatment with MβCD impairs collagen- or Cvx-induced platelet activation
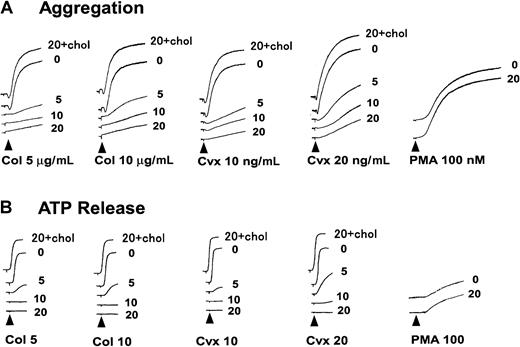
MβCD, which preferentially extracts plasma membrane cholesterol, has been widely used to disrupt membrane raft microdomains.21 To determine whether membrane rafts play an obligatory role in collagen-induced platelet activation, we studied the effects of MβCD on platelet aggregation and secretion on collagen stimulation. As shown in Figure 1A-B, pretreatment with MβCD (5-20 mM) yielded a dose-dependent inhibition in collagen (5 or 10 μg/mL)-induced platelet aggregation and ATP release. We also studied whether Cvx-induced platelet aggregation and ATP release were affected by MβCD pretreatment. The results showed its similar dose-dependent inhibitory effects on Cvx-induced aggregation and ATP release (Figure 1A-B). To rule out the possibility that the inhibitory effects of MβCD pretreatment on platelet function were caused by cell damage derived from its nonspecific action rather than by the disruption of membrane rafts, we took advantage of the MβCD–cholesterol inclusion complex and an intracellular stimulant, PMA. When platelets were incubated with 20 mM MβCD preloaded with cholesterol prepared as Klein et al reported,20 such pretreatment with the MβCD–cholesterol inclusion complex showed no inhibitory effects on platelet aggregation and ATP release induced by any of the agonists described above (Figure 1A-B). Furthermore, pretreatment with 20 mM MβCD, which almost completely abolished collagen- or Cvx-induced platelet aggregation, could not inhibit PMA-induced platelet aggregation or ATP release (Figure 1A-B). A concentration-response relationship was determined for the inhibition of collagen- or Cvx-induced platelet aggregation and ATP release by MβCD treatment and is shown in Figure2.
Collagen- or Cvx-induced platelet aggregation is inhibited by pretreatment with MβCD.
(A, B) Platelets were pre-incubated with the indicated concentration (0, 5, 10, or 20 mM) of MβCD or 20 mM MβCD preloaded with cholesterol (20 + chol) for 30 minutes, washed once, then resuspended in HEPES buffer. Aggregation and ATP release were monitored using lumi-aggregometer on the stimulation of collagen (Col; 5 or 10 μg/mL), Cvx (10 or 20 ng/mL), or PMA (100 nM).
Collagen- or Cvx-induced platelet aggregation is inhibited by pretreatment with MβCD.
(A, B) Platelets were pre-incubated with the indicated concentration (0, 5, 10, or 20 mM) of MβCD or 20 mM MβCD preloaded with cholesterol (20 + chol) for 30 minutes, washed once, then resuspended in HEPES buffer. Aggregation and ATP release were monitored using lumi-aggregometer on the stimulation of collagen (Col; 5 or 10 μg/mL), Cvx (10 or 20 ng/mL), or PMA (100 nM).
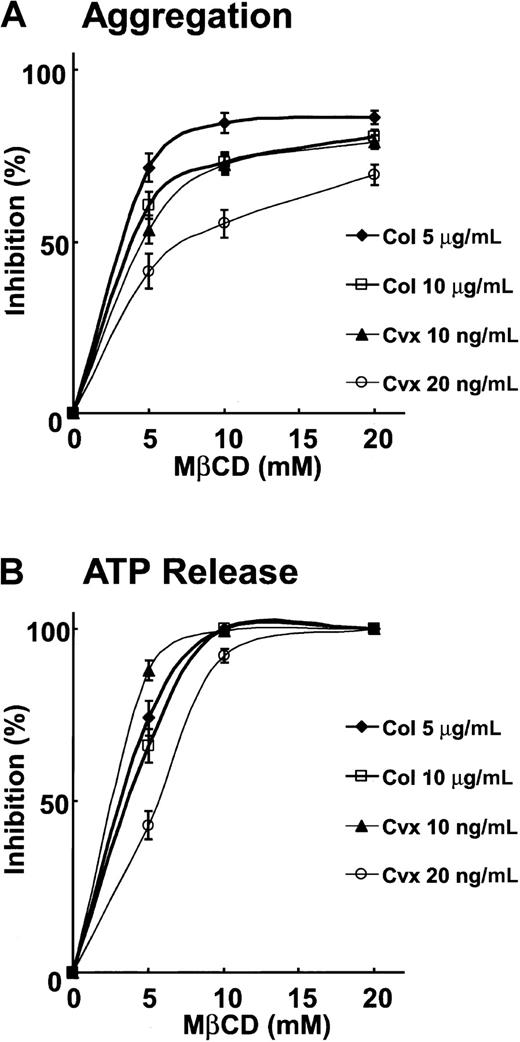
Pretreatment of platelets with MβCD dose-dependently inhibits collagen- or Cvx-induced platelet aggregation and ATP release.
Platelet aggregation and ATP release were studied as described in the legend to Figure 1. Inhibition of platelet aggregation and ATP release was calculated by comparing the maximal responses elicited by MβCD-treated platelets relative to those by untreated platelets. Data presented are mean ± SEM for 3 experiments.
Pretreatment of platelets with MβCD dose-dependently inhibits collagen- or Cvx-induced platelet aggregation and ATP release.
Platelet aggregation and ATP release were studied as described in the legend to Figure 1. Inhibition of platelet aggregation and ATP release was calculated by comparing the maximal responses elicited by MβCD-treated platelets relative to those by untreated platelets. Data presented are mean ± SEM for 3 experiments.
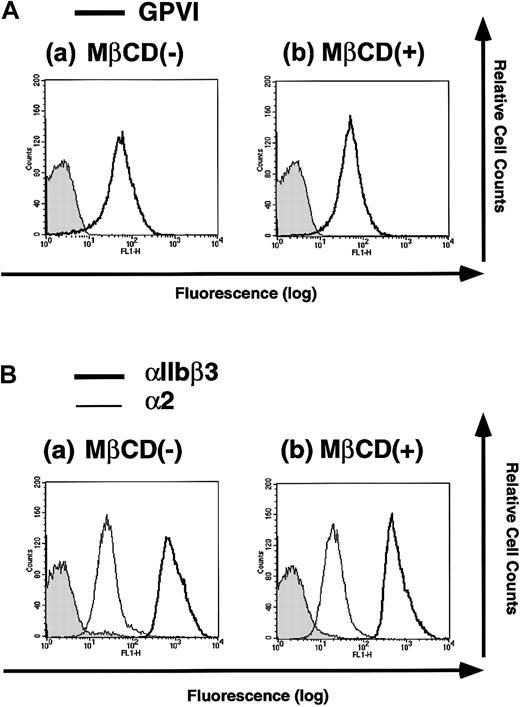
We next examined whether MβCD treatment diminishes the expression of membrane glycoproteins such as GPVI and the integrins αIIbβ3 and α2. Platelets were incubated with or without 20 mM MβCD for 30 minutes and were analyzed for the expressions of GPVI, αIIbβ3, and α2 with flow cytometry. MβCD treatment did not alter the expressions of those membrane glycoproteins (Figure3), eliminating the possibility that the inhibitory effects of MβCD pretreatment on collagen- or Cvx-induced platelet aggregation and ATP release are caused by the diminished expressions of GPVI, αIIbβ3, and α2, which are essential for such platelet functions.
Flow cytometry analysis of platelets treated with or without MβCD for surface GPVI and the integrins αIIbβ3 and α2.
Platelets were incubated with 0 (−) or 20 mM (+)MβCD for 30 minutes, washed once, and resuspended in HEPES buffer. (A) Cells were stained with F(ab′)2 fragments of human anti-GPVI IgG or those of control IgG followed by FITC-labeled antihuman IgG antibody and were analyzed by flow cytometry. (B) Cells were stained with anti-αIIbβ3 antibody (P2), anti-α2 antibody (Gi9), or mouse IgG1 as a negative control followed by FITC-labeled antimouse IgG antibody and were analyzed by flow cytometry.
Flow cytometry analysis of platelets treated with or without MβCD for surface GPVI and the integrins αIIbβ3 and α2.
Platelets were incubated with 0 (−) or 20 mM (+)MβCD for 30 minutes, washed once, and resuspended in HEPES buffer. (A) Cells were stained with F(ab′)2 fragments of human anti-GPVI IgG or those of control IgG followed by FITC-labeled antihuman IgG antibody and were analyzed by flow cytometry. (B) Cells were stained with anti-αIIbβ3 antibody (P2), anti-α2 antibody (Gi9), or mouse IgG1 as a negative control followed by FITC-labeled antimouse IgG antibody and were analyzed by flow cytometry.
Then we studied the effects of MβCD pretreatment on Cvx- or collagen-induced tyrosine phosphorylation of FcRγ, Syk, PLCγ2, LAT, and SLP-76. As shown in Figure 4, pretreatment of platelets with MβCD almost completely abolished Cvx- or collagen-induced tyrosine phosphorylation of those signaling molecules downstream of GPVI.
GPVI–FcRγ downstream signaling is inhibited by MβCD treatment.
Platelets pretreated with (+) or without (−) 20 mM MβCD were unstimulated or stimulated with 50 ng/mL Cvx or 50 μg/mL collagen (Col) for 30 seconds and were lysed in Triton X-100 lysis buffer. Immunoprecipitates (IP) were obtained from the lysates, resolved on SDS-PAGE, and immunoblotted (WB) with each specified antibody. Blots were also reprobed with antiphosphotyrosine antibody (αp-Tyr).
GPVI–FcRγ downstream signaling is inhibited by MβCD treatment.
Platelets pretreated with (+) or without (−) 20 mM MβCD were unstimulated or stimulated with 50 ng/mL Cvx or 50 μg/mL collagen (Col) for 30 seconds and were lysed in Triton X-100 lysis buffer. Immunoprecipitates (IP) were obtained from the lysates, resolved on SDS-PAGE, and immunoblotted (WB) with each specified antibody. Blots were also reprobed with antiphosphotyrosine antibody (αp-Tyr).
GPVI–FcRγ complex localizes in the low-density fractions of sucrose gradient in an MβCD-sensitive manner
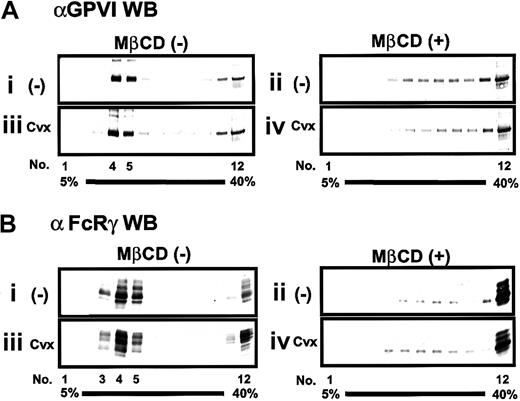
We next examined whether the GPVI–FcRγ complex was associated with membrane rafts. Using the method of Dorahy et al,6unstimulated or Cvx-stimulated platelets were lysed in Triton X-100–containing buffer, and the lysates were analyzed by 5% to 40% (wt/vol) sucrose gradient ultracentrifugation. Gradients were separated into 12 fractions from the top (5% sucrose) of the gradient. Those fractions were subjected to SDS-PAGE followed by immunoblotting with either anti-GPVI or anti-FcRγ antibody. Results revealed the following: GPVI and FcRγ were highly enriched in detergent-resistant fractions 3 to 5, whereas a very small part of these was recovered in detergent-soluble fractions 11 and 12; platelet activation through the GPVI–FcRγ complex with Cvx did not accompany further accumulation of the GPVI–FcRγ complex into fractions 3 to 5; MβCD-induced disruption of membrane rafts yielded an increase of GPVI and FcRγ in detergent-soluble fractions 11 and 12 (Figure5A-B).
Localization of the GPVI–FcRγ complex in the low-density sucrose-gradient fractions and effect of MβCD.
Platelets were preincubated without (i,iii) or with (ii, iv) 20 mM MβCD for 30 minutes, washed once, unstimulated (i, ii) or stimulated (iii, iv) with 20 ng/mL Cvx for 30 seconds, and lysed in Triton X-100–containing buffer. The whole lysate was separated into 12 fractions (1-12) by sucrose density (5%-40%) ultracentrifugation. An equal volume of gradient fractions was separated by SDS-PAGE and blotted (WB) with anti-GPVI or anti-FcRγ antibody.
Localization of the GPVI–FcRγ complex in the low-density sucrose-gradient fractions and effect of MβCD.
Platelets were preincubated without (i,iii) or with (ii, iv) 20 mM MβCD for 30 minutes, washed once, unstimulated (i, ii) or stimulated (iii, iv) with 20 ng/mL Cvx for 30 seconds, and lysed in Triton X-100–containing buffer. The whole lysate was separated into 12 fractions (1-12) by sucrose density (5%-40%) ultracentrifugation. An equal volume of gradient fractions was separated by SDS-PAGE and blotted (WB) with anti-GPVI or anti-FcRγ antibody.
We also tried to compare the localization of GPVI with that of other membrane proteins within the sucrose density gradients using resting platelets whose membrane surface proteins were biotinylated. Previous studies performed similar experiments and reported that the detection of biotinylated proteins in the low-density fractions revealed several major membrane protein bands at relatively higher molecular weights and faint bands at approximately 60 kd compared with a larger amount of biotinylated proteins in the high-density fractions.6,22Because this suggested to us that GPVI could be more sensitively detected by immunoblotting for its antigen rather than by its biotinylation probably because of its small amount, we first performed immunoblotting with biotinylated anti-GPVI IgG and followed this by staining with the streptavidin–horseradish peroxidase (HRP)/ECL system to detect GPVI and biotinylated membrane proteins. The result revealed that most biotinylated proteins were located in the detergent-soluble fractions rather than in the detergent-resistant fractions, where the 57-kd protein band (unreduced state) or the 78- to 168-kd protein bands were present (Figure 6A). When the blot was stained only with streptavidin–HRP/ECL, as in previous reports,6 22 the 57-kd protein band alone became faint and unclear, whereas the other membrane proteins were still clearly detected (data not shown), indicating that the 57-kd band is GPVI. Treatment of biotinylated platelets with MβCD shifted all the membrane proteins, including GPVI, into the detergent-soluble fractions (Figure 6B).
Comparison of the localization of GPVI with that of other membrane proteins within the sucrose density gradients.
Platelets, whose surface membrane proteins were biotinylated, were preincubated without (A) or with (B) 20 mM MβCD for 30 minutes, washed once, and lysed in Triton X-100–containing buffer. Whole lysate was separated into 12 fractions (1-12) by sucrose density (5%-40%) ultracentrifugation. An equal volume of gradient fractions was separated by SDS-PAGE and was blotted with biotinylated anti-GPVI antibody using the streptavidin–HRP/ECL system. Arrow indicates the position of GPVI.
Comparison of the localization of GPVI with that of other membrane proteins within the sucrose density gradients.
Platelets, whose surface membrane proteins were biotinylated, were preincubated without (A) or with (B) 20 mM MβCD for 30 minutes, washed once, and lysed in Triton X-100–containing buffer. Whole lysate was separated into 12 fractions (1-12) by sucrose density (5%-40%) ultracentrifugation. An equal volume of gradient fractions was separated by SDS-PAGE and was blotted with biotinylated anti-GPVI antibody using the streptavidin–HRP/ECL system. Arrow indicates the position of GPVI.
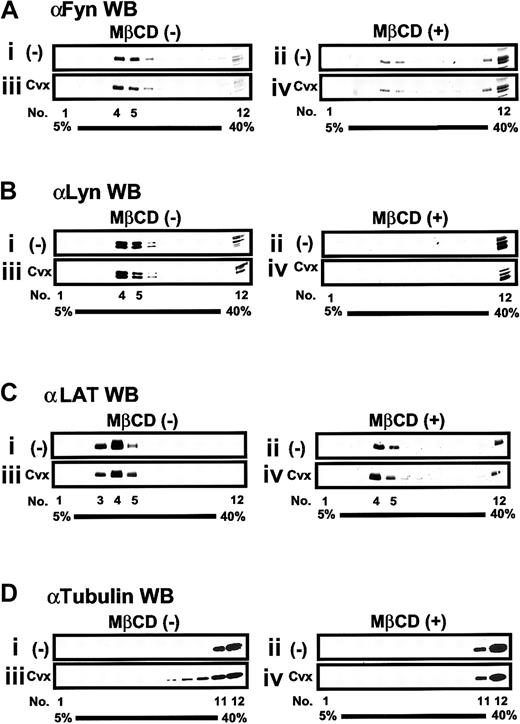
Distribution of raft- or nonraft-associated molecules on the sucrose gradient fractions
Previous studies established that Src family kinases and LAT, both of which require acylation for membrane raft targeting, are constitutively present in membrane rafts.4,21 Therefore, we studied the distribution of those signaling proteins on the sucrose gradient fractions obtained from unstimulated or Cvx-stimulated platelets. In accordance with the localization of GPVI and FcRγ, raft-associated proteins such as Fyn, Lyn, and LAT were detected almost exclusively in fractions 3 to 5 obtained from unstimulated platelets (Figure 7A-C). The amount of those proteins localized in fractions 3 to 5 showed little or no change after Cvx stimulation. Pretreatment of platelets with MβCD shifted Fyn and Lyn into the detergent-soluble fractions irrespective of stimulation with Cvx, whereas it did not lead to complete abolishment of LAT in fractions 4 and 5 (Figure 7A-C). This suggested that localization of Fyn and Lyn in membrane rafts was more sensitive to MβCD pretreatment than that of LAT. We could not detect any Syk, SLP-76, or PLCγ2 in fractions 3 to 5, but we did detect them in the detergent-soluble fractions, regardless of whether platelets were activated by Cvx (data not shown). We also monitored the location of nonraft-associated molecules such as cytoskeleton proteins α-tubulin and vinculin3,6 23 within sucrose gradient fractions. α-Tubulin and vinculin were excluded from fractions 3 to 5 irrespective of Cvx stimulation (Figure 7D and data not shown).
Sucrose density-gradient fractionation of raft- and nonraft-associated molecules in platelets.
Platelets preincubated without (i, iii) or with (ii, iv) 20 mM MβCD were unstimulated (i, ii) or stimulated (iii, iv) with 20 ng/mL Cvx for 30 seconds, lysed in Triton X-100–containing buffer, and separated into 12 sucrose density fractions (1-12) as described in the legend to Figure 5. An equal volume of gradient fractions was separated by SDS-PAGE and blotted (WB) with each specified antibody.
Sucrose density-gradient fractionation of raft- and nonraft-associated molecules in platelets.
Platelets preincubated without (i, iii) or with (ii, iv) 20 mM MβCD were unstimulated (i, ii) or stimulated (iii, iv) with 20 ng/mL Cvx for 30 seconds, lysed in Triton X-100–containing buffer, and separated into 12 sucrose density fractions (1-12) as described in the legend to Figure 5. An equal volume of gradient fractions was separated by SDS-PAGE and blotted (WB) with each specified antibody.
Discussion
A detergent-resistant membrane fractionated by isopycnic sucrose gradient ultracentrifugation has been widely used to define membrane rafts biochemically.1,2 Detergent-resistant fractions 3 to 5, isolated from platelets in our study, contained not only the GPVI–FcRγ complex but also well-known membrane raft-associated proteins, including the LAT and Src family kinases Fyn and Lyn.4,21 On the other hand, those fractions excluded proteins such as Syk, PLCγ2, α-tubulin, and vinculin, which are known to be associated with cytosol or cytoskeleton. Another important aspect for characterization of membrane rafts is the detergent sensitivity of membrane raft–associated proteins on cholesterol depletion with MβCD, which extracts cholesterol, the main constituent of membrane rafts, resulting in the disruption of membrane rafts.2 21 Treatment of platelets with MβCD shifted all the above-described membrane raft–associated proteins to the detergent-soluble fractions. Significantly, this was also true for the GPVI–FcRγ complex. Complete solubilization of nonraft membranes using our method was confirmed by evidence that most biotinylated membrane surface proteins were recovered in the detergent-soluble fractions, whereas several membrane proteins, including GPVI, were recovered in the detergent-resistant fractions. In addition, the disruption of membrane rafts with MβCD resulted in the detergent solubility of all membrane proteins. Thus, it can be concluded that the detergent-resistant membrane fractions isolated in our study shared properties attributed to authentic membrane rafts and that the GPVI–FcRγ complex is mostly associated with the Triton X-100–insoluble membrane rafts separated by sucrose gradient ultracentrifugation.
Data presented here provide evidence that the disruption of membrane rafts with MβCD impairs GPVI–FcRγ complex–mediated platelet activation. This report is the first study applying MβCD to platelet biology, and it demonstrates that MβCD can become a useful tool to investigate the functional role of membrane rafts for the platelet activation process for 3 reasons. First, nonspecific action of MβCD was denied because the pretreatment of platelets with MβCD plus cholesterol had no effect on platelet activation. Second, cholesterol depletion of platelet plasma membrane with MβCD did not diminish the expression of either αIIbβ3, α2β1, or GPVI. Third, PMA-induced platelet aggregation was not altered by MβCD pretreatment, implying that αIIbβ3 on MβCD-treated platelets can be activated by the inside-out signal and can bind fibrinogen to aggregate platelets. However, the question arises as to whether the pretreatment of platelets with MβCD specifically alters the function of the GPVI–FcRγ complex. It has been known that the membrane rafts of mammalian cells contain signaling proteins other than those studied here, such as heterotrimeric and Ras-like G-proteins.1 2Therefore, it is likely that the function of receptors using those G-proteins could be affected by the disruption of membrane rafts. In fact, we found that thrombin-induced platelet aggregation was also decreased by MβCD pretreatment (unpublished results, November, 2000). Although this suggested that the heterotrimeric G-protein–coupled receptor was functionally associated with membrane rafts, it was clearly beyond the scope of the current work to address this issue, and it merits future study.
The GPVI–FcRγ complex signals to the platelet interior by an immunoreceptorlike mechanism involving signaling molecules such as Fyn, Lyn, Syk, PLCγ2, LAT, and SLP-76.8 Considering these consecutive signaling events, one would expect that most signaling molecules involved in the GPVI–FcRγ complex engagement could be associated with membrane rafts. However, although well-known raft-associated proteins such as Fyn, Lyn, and LAT were present in membrane rafts, other signaling molecules—including Syk, PLCγ2, and SLP-76—were always located in the detergent-soluble fractions irrespective of the GPVI–FcRγ complex engagement. There have been contradictory results using detergent insolubility to determine whether various signaling proteins downstream of immunoreceptors, or even the T-cell antigen receptor, are associated with membrane rafts.23-25 As has been suggested by previous reports,5 26 this is probably because only proteins that are strongly associated with membrane rafts are Triton X-100 insoluble, whereas weakly associated proteins are sensitive to detergent extraction. Most tyrosine kinase substrates phosphorylated as the result of the GPVI–FcRγ engagement are localized primarily in the cytosol. GPVI–FcRγ engagement leads to transient association and activation of Syk, followed by Syk-mediated phosphorylation of downstream substrates, most of which are not stably associated with membrane rafts.
As with the T-cell antigen receptor, signal transduction through the GPVI–FcRγ complex can be initiated after antibody cross-linking,15,16 suggesting that the signaling is triggered by receptor oligomerization. Therefore, the most intriguing question is whether GPVI–FcRγ downstream signaling can be activated by a coalescence of membrane rafts because it has been shown that the aggregation of membrane rafts accompanies signaling by the T-cell antigen receptor in a ligand-independent manner.5 Cholera toxin B specifically binds ganglioside GM1 as a raft-associated molecule and has been widely used to cross-link GM1, resulting in the coalescence of membrane rafts.5,21 Washed platelets were incubated with biotinylated cholera toxin B, followed by the addition of streptavidin to cross-link GM1 under stirring conditions. Results showed that neither platelet aggregation nor ATP release was induced by GM1 cross-linking with rare exceptions, in which GM1 cross-linking was found to cause platelet aggregation and the activation of GPVI–FcRγ downstream signaling (data not shown). However, we did not succeed in reproducing steadily the data of platelet activation by GM1 cross-linking in those exceptional cases. It has been reported that GM1 is not only present in membrane rafts, but it is also present in nonraft membranes.3,5,27Furthermore, the number of GPVI expressed on human platelets has been estimated to be approximately 2000, much less than those of other receptors28 and possibly of GM1 itself or of GM1-associated membrane rafts. Therefore, it appears reasonable to assume that cholera toxin B–induced cross-linking of GM1 does not necessarily lead to specific coalescence of GPVI-associated membrane rafts that could cause activation of the GPVI–FcRγ downstream signaling pathway.
Most receptors on the surface of hematopoietic cells have modest raft affinity and are more recruited to membrane rafts when they are clustered after antigen binding.29 In contrast, the GPVI–FcRγ complex showed high enrichment in the raft-representing fractions and no further accumulation into them on complex engagement. Because the structural features of those receptors that confer an affinity for rafts have not been identified, there is no obvious explanation for the difference in raft affinity between the receptors. However, the constitutive raft affinity of the GPVI–FcRγ complex may be required for more efficient coalescence of small numbers of rafts containing the complex into larger rafts, the formation of which could cause platelet activation on complex engagement.
In conclusion, this is the first study to demonstrate an association between the GPVI–FcRγ complex and platelet membrane rafts, which play an essential role in downstream signaling through the complex. Future studies aimed at clarifying the relationship between the GPVI–FcRγ-associated membrane rafts and other receptors, such as α2β1, GPIb/IX, or αIIbβ3, should aid in our understanding of how platelet thrombi are formed on exposed subendothelial collagens in vivo.
We thank Ms. Ikuko Nakamura for her technical and secretarial assistance.
Supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan and Sankyo Foundation of Life Science.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hiroshi Takayama, Department of Hematology and Oncology, Clinical Sciences for Pathological Organs, Graduate School of Medicine, Kyoto University, 54 Shogoin-Kawaracho, Sakyo-ku, Kyoto 606-8507, Japan; e-mail: hiro@kuhp.kyoto-u.ac.jp.