Abstract
The β2 integrins leukocyte function antigen-1 (LFA-1, CD11a) and macrophage antigen-1 (Mac-1, CD11b) have been reported to play a role in the attachment of CD34+ cells to stromal cells in the bone marrow. When administered prior to interleukin-8 (IL-8), anti–LFA-1 antibodies completely prevent the IL-8–induced mobilization of hematopoietic stem cells in mice. Here, we studied the role of anti-β2 integrin antibodies in granulocyte colony-stimulating factor (G-CSF)–induced mobilization of hematopoietic progenitor cells. Administration of antibodies against the α chain of LFA-1 or against the α chain of Mac-1 followed by daily injections of G-CSF for more than 1 day resulted in a significant enhancement of mobilization of hematopoietic progenitor cells when compared with mobilization induced by G-CSF alone. Also, the number of late (day 28) cobblestone area–forming cells in vitro was significantly higher after mobilization with anti–LFA-1 antibodies followed by 5 μg G-CSF for 5 days than with G-CSF alone (119 ± 34 days vs 17 ± 14 days), indicating mobilization of repopulating stem cells. Pretreatment with blocking antibodies to intercellular adhesion molecule-1 (ICAM-1; CD54), a ligand of LFA-1 and Mac-1, did not result in an effect on G-CSF–induced mobilization, suggesting that the enhancing effect required an interaction of the β2 integrins and one of their other ligands. Enhancement of mobilization was not observed in LFA-1–deficient (CD11a) mice, indicating that activated cells expressing LFA-1 mediate the synergistic effect, rather than LFA-1–mediated adhesion.
Introduction
Mobilization of hematopoietic stem cells and progenitor cells is a property of a variety of cytokines. These include growth factors and chemokines such as granulocyte colony-stimulating factor (G-CSF),1-4 granulocyte-macrophage colony-stimulating factor (GM-CSF),5 stem cell factor,6 flt-3 ligand,7,8 interleukin-1 (IL-1),9 IL-3,10 IL-8,11,12 and thrombopoietin,13 either administered alone or in combination.14-16 The kinetics of stem cell mobilization induced by the various growth factors may vary substantially. For instance, flt-3 ligand induces mobilization in mice in 7 to 10 days,7 whereas the chemokine IL-8 induces mobilization within 30 minutes.11
Over the past 5 to 10 years G-CSF has emerged as the most widely used mobilizing agent in clinical studies employing stem cell mobilization.4 17 Despite its widespread clinical application, little is known about the mechanisms underlying G-CSF–induced stem cell mobilization.
Adhesion molecules play an important role in the interactions between hematopoietic progenitor cells (HPCs) and the bone marrow microenvironment.18-22 Among these, the β1 and β2 integrins are involved in the cellular interactions between HPCs, stromal cells, and the extracellular matrix.19 It has been shown that the β1 integrins very late antigen 4 (VLA-4; CD49d) and VLA-5 (CD49e) are expressed on colony-forming HPCs and stem cells.20-23 The ligands for these integrins, vascular cell adhesion molecule-1 (VCAM-1) and fibronectin, respectively, are expressed on cells of the bone marrow microenvironment.20,21,24 Their function in retaining the HPCs in the bone marrow is supported by the observation that in vivo administration of antibodies to VLA-4 and to its ligand VCAM-1 induces mobilization of HPCs in mice and primates.25 26
The β2 integrins leukocyte function antigen-1 (LFA-1) and macrophage antigen-1 (Mac-1) have been reported to play a role in the attachment of CD34+ cells to stromal cells through one of their ligands, intercellular adhesion molecule-1 (ICAM-1), and through heparan sulfate.18,26,27 Studies in our laboratory and by others have shown that the β2 integrin LFA-1 is not expressed on murine HPCs.28,29 In contrast to anti–β1 integrin antibodies, antibodies to the β2 integrins were unable to mobilize progenitor cells into the bloodstream in mice and primates.24 When administered prior to injection of IL-8, anti–LFA-1 antibodies completely prevented IL-8–induced mobilization of hematopoietic stem cells.30
Because of the role of β2 integrins in the adhesion of murine HPCs in the bone marrow and the inhibitory effect of anti–LFA-1 antibodies on IL-8–induced mobilization, we studied their role in G-CSF–induced stem cell mobilization. In this study we found that administration of antibodies to the β2 integrins LFA-1 and Mac-1 synergistically enhanced G-CSF–induced mobilization. In LFA-1–deficient mice, G-CSF–induced mobilization was not enhanced when compared with wild-type littermate controls. This suggests that the synergistic effect of administration of anti–LFA-1 antibodies on G-CSF–induced mobilization is not mediated by blocking of LFA-1–mediated adhesion but is due to activating cells expressing LFA-1.
Materials and methods
Animals
Male BALB/c mice with an age ranging between 8 and 12 weeks were purchased from Broekman BV (Someren, The Netherlands). Animals were fed commercial rodent chow and acidified water ad libitum. Heterozygous LFA-1–deficient (CD11a) C57Bl/6J mice were a kind gift from Dr T.W. Mak (Amgen Institute, Ontario Cancer Institute, Department of Immunology and Medical Biophysics, Toronto, Ontario, Canada). Homozygous CD11a-deficient mice were bred at the animal facility of TNO Laboratories (Leiden, The Netherlands).
Cytokines
Recombinant human G-CSF (filgrastim, Neupogen, Amgen, Thousand Oaks, CA; a kind gift from Amgen, Breda, The Netherlands) was diluted to the desired concentration in endotoxin-free phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin and administered as an intraperitoneal injection.
Monoclonal antibodies
For in vivo injection, rat antimurine monoclonal antibodies directed against the following adhesion molecules were used: H154.163 (neutralizing anti-CD11a, LFA-1, immunoglobulin G2a [IgG2a], kindly provided by Dr M. Pierres, Centre d'Immunologie, Marseille, France31), H155.78 (non-neutralizing anti-CD11a, LFA-1, IgG2a), 5C6 (neutralizing anti-CD11b, Mac-1, IgG2b, kindly provided by Dr M. Robinson),32 and YN1/1.7 (anti-CD54, ICAM-1, IgG2b).33 Endotoxin testing was done on the antibody to Mac-1. The endotoxin levels were 2.6 IU/mL as measured by the Limulus amoebocyte lysate assay. This concentration of endotoxin is not expected to have any effect on the mobilization of hematopoietic stem cells into the peripheral blood of mice.34
Preparation of cell suspensions
Mice were killed by CO2 asphyxiation. Peripheral blood was drawn by a cardiac puncture, and white blood cell counts were performed on a Sysmex F800 (TOA Medical Electronics, Kobe, Japan). Manual neutrophil counts were performed after May-Grünwald Giemsa (MGG) staining of the peripheral blood. Mononuclear cell suspensions were obtained after Ficoll separation as described.9 The bone marrow cells were removed from the femur by flushing the femur under sterile conditions with RPMI 1640 containing 500 μg/mL penicillin, 250 μg/mL streptomycin, and 2% fetal bovine serum (GIBCO, Grand Island, NY) and 6% heparin (400 IU/mL). All cells were washed twice in RPMI 1640 containing penicillin, streptomycin, and fetal bovine serum in the concentrations as described.
Progenitor cell assays
Granulocyte macrophage colony-forming units (CFU-GMs) were cultured as described previously.9 Briefly, peripheral blood mononuclear cells were cultured in 3.5-cm dishes containing 5 × 105 cells per milliliter in semisolid medium in the presence of recombinant murine GM-CSF (1.25 ng/mL; kindly provided by Dr E. Liehl, Novartis Forschungsinstitut, Vienna, Austria). Bone marrow cells were cultured in a concentration of 1 × 105 cells per milliliter. After 6 days of culture in a fully humidified atmosphere of 37°C containing 5% CO2, the number of colonies, defined as an aggregate of more than 20 cells, were scored using an inverted microscope.
FACS analysis
The number of mature neutrophils in blood and bone marrow was determined by flow cytometry. Peripheral blood cells were lysed using buffered ammonium chloride (NH4Cl 8.4 g/L, potassium bicarbonate [KHCO3] 1 g/L, pH 7.4) and washed with PBS containing 0.8 g/L albumin (CLB, Amsterdam, The Netherlands). The lysed peripheral blood cells and the unlysed bone marrow cells were then incubated for 30 minutes at 4°C with anti-CD3e and anti-CD45R/B220 antibodies—both phycoerythrin (PE)-conjugated—and anti–GR-1 antibody conjugated to fluorescein isothiocyanate. After washing the cells were incubated at 4°C with Cychrome-conjugated anti–Ly-5 antibody for 30 minutes (all rat antimouse monoclonal antibodies were purchased from PharMingen, San Diego, CA). Then the cells were washed again and then resuspended in PBS containing 0.8 g/L albumin. To determine the morphology of the Gr-1+ cells, cells were fluorescence-activated cell-sorter (FACS) sorted using a FACStar cytometer (Becton Dickinson, San Jose, CA). Within the total population of Ly-5 Cychrome+ cells, 2 populations were sorted: the Gr-1 strongly positive (CD3-PE− and B220-PE−cells) and the Gr-1 weakly positive cell fraction (CD3-PE−and B220-PE− cells). From both cell suspensions, cytospin preparations were made using a Cytofuge (Nordic Immunological Laboratories, Tilburg, The Netherlands). After drying, the cells were stained with MGG. Differential counts of the cell preparations were performed. In the peripheral blood samples, 99% of the Gr-1 strongly positive, CD3− and B220−, cells were mature neutrophils. In the bone marrow samples we found 93% of this population to be mature neutrophils. The Gr-1 weakly positive population contained mainly immature neutrophils. Both populations contained less than 1% monocytes. The same staining procedure as described above was used, but then fluorescence intensity was analyzed by flow cytometry (FACStar; Becton Dickinson). On the basis of morphology, Gr-1 strongly positive cells were regarded as mature neutrophils (Figure 1).
Neutrophils in the bone marrow were determined by FACS analysis.
Neutrophils represent the Gr-1 strongly positive population within the Ly5-Cy5+ cell fraction.
Neutrophils in the bone marrow were determined by FACS analysis.
Neutrophils represent the Gr-1 strongly positive population within the Ly5-Cy5+ cell fraction.
Genotyping
Progeny of CD11a+/− mice were genotyped at 6 weeks of age by polymerase chain reaction amplification of DNA samples from tail tissue. Presence of the targeted allele was detected by amplification using primers with sequence (5′ to 3′): ACCAGTCTCTGCTTCTTCTGCAC (forward) and sequence (5′ to 3′): TATCAGGACATAGCGTTGGCTACCC (reverse). Amplifications were performed on a GeneAmp PCR system 2400 (Applied Biosystems, Perkin Elmer, Nieuwerkerk a/d IJssel, The Netherlands) for 33 cycles with an oligonucleotide annealing temperature of 59°C.
Genotypes of animals were routinely confirmed by FACS analysis of the peripheral blood samples with an anti-CD11a antibody (clone I21/7; Caltag Laboratories, Burlingame, CA) as described above.
Detection of circulating and cellbound antibodies
To determine levels of free circulating antibody, plasma was obtained from mice at various time intervals after a single intraperitoneal injection of 100 μg anti–LFA-1 or anti–Mac-1 antibodies. A quantity of 2 × 105 peripheral blood leukocytes of untreated mice was then incubated with a volume of 50 μL plasma for 30 minutes at 4°C. Cells were washed once and subsequently labeled with PE-conjugated goat anti-rat IgG (Caltag Laboratories). After washing, the cells were resuspended in PBS containing 0.8 g/L albumin. To detect cellbound antibody, peripheral blood leukocytes and bone marrow cells were obtained from mice treated at various time intervals after a single injection of anti–LFA-1 or anti–Mac-1 antibody and labeled with PE-conjugated goat antirat IgG. The fluorescence intensity was analyzed using a flow cytometer.
CAFC assay
In vitro determination of HPC frequencies was performed by limited dilution analysis of cobblestone area–forming cells (CAFCs) in microcultures according to the method previously described.35,36 Briefly, cells were seeded on a preestablished stromal layer of the murine preadipocyte cell line (FBMD-1, kindly provided by Dr R. E. Ploemacher, Erasmus University, Rotterdam, The Netherlands). At weekly intervals until day 35 after initiation, cultures were scored using an inverted microscope for the presence of cobblestone areas, defined as colonies of immature hematopoietic cells (at least 6 cells per colony) residing within the preestablished stromal layer. The proportion of negative wells at each dilution was used in a Poisson-based limiting dilution analysis to calculate the CAFC frequency.35 37
Experimental design
In all experiments, mice were pretreated with either a single intraperitoneal injection of neutralizing anti–LFA-1 or anti–Mac-1 antibodies, control antibodies, or saline. After 24 hours the mice received intraperitoneal injections of G-CSF at different doses and schedules and during a variable number of days. LFA-1–deficient mice and wild-type littermate controls were treated with a daily injection of G-CSF for 5 days. Twenty-four hours after the last injection of G-CSF, the mice were killed by CO2asphyxiation and peripheral blood was obtained by cardiac puncture. The femurs were removed. Cell suspensions were prepared, and the numbers of HPCs were assessed according to the described procedures. An MGG staining of the peripheral blood cells was performed. The Leiden University Medical Center ethical committee on animal experiments approved of the experimental protocol.
Statistical analysis
Differences were evaluated using the Studentt test. P < .05 was considered statistically significant. To calculate the CAFC frequency, a Poisson-based limiting dilution analysis was used.
Results
Effect of anti–LFA-1 and anti–Mac-1 antibodies on mobilization of progenitor cells induced by G-CSF
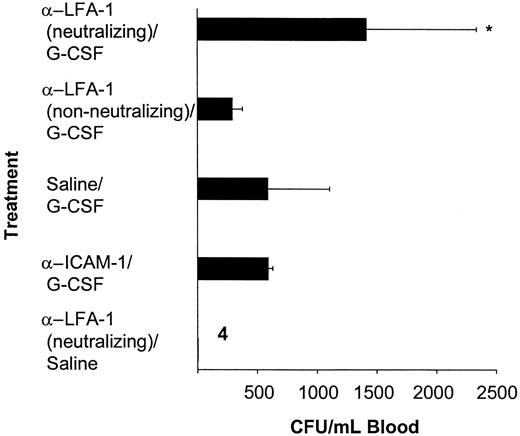
BALB/c mice were injected intraperitoneally with a single dose of 100 μg neutralizing anti–LFA-1 antibodies or saline followed after 24 hours by daily injections of 5 μg G-CSF or saline for 3 days. Treatment with neutralizing anti–LFA-1 antibody prior to G-CSF resulted in a significant increase in the number of circulating CFU-GMs compared with animals treated with G-CSF only (1417 ± 921 CFUs per milliliter vs 590 ± 513 CFUs per milliliter, P < .01, Figure 2). The antibody itself had no mobilizing capacity (Figure 2). Injection of a non-neutralizing anti–LFA-1 antibody or neutralizing antibody against ICAM-1 did not result in an increase of the G-CSF–induced mobilization (non-neutralizing anti–LFA-1 antibody plus G-CSF: 219 ± 84 CFUs per milliliter; anti–ICAM-1 antibody plus G-CSF: 262 ± 177 CFUs per milliliter; G-CSF: 590 ± 513 CFUs per milliliter; P not significant; Figure 2). The enhancement of G-CSF–induced mobilization observed after pretreatment with neutralizing anti–LFA-1 antibodies was independent of the dose and schedule of G-CSF used (Figure3). Only in the animals treated with G-CSF for 1 day after the pretreatment with the neutralizing anti–LFA-1 antibody, no significant increase in the number of circulating CFU-GMs was observed compared with mice treated with G-CSF only (anti–LFA-1 antibody plus G-CSF: 56 ± 36 CFUs per milliliter; G-CSF: 22 ± 20 CFUs per milliliter; P not significant; Figure 3).
The G-CSF–induced mobilization of progenitor cells is enhanced by pretreatment with anti–LFA-1 antibodies.
Mice were pretreated with a single injection of 100 μg neutralizing anti–LFA-1 (H154.163, n = 11) or non-neutralizing anti–LFA-1 (H155.78, n = 6), or anti–ICAM-1 (YN1/1.7, n = 3) or saline (n = 16). Twenty-four hours later, 5 μg G-CSF was started by daily intraperitoneal injections for 3 days. Twenty-four hours after the last injection, mice were killed and blood was harvested. Results are expressed as mean ± SD. *P <. 05 as compared with the saline-pretreated controls.
The G-CSF–induced mobilization of progenitor cells is enhanced by pretreatment with anti–LFA-1 antibodies.
Mice were pretreated with a single injection of 100 μg neutralizing anti–LFA-1 (H154.163, n = 11) or non-neutralizing anti–LFA-1 (H155.78, n = 6), or anti–ICAM-1 (YN1/1.7, n = 3) or saline (n = 16). Twenty-four hours later, 5 μg G-CSF was started by daily intraperitoneal injections for 3 days. Twenty-four hours after the last injection, mice were killed and blood was harvested. Results are expressed as mean ± SD. *P <. 05 as compared with the saline-pretreated controls.
Synergistic enhancement of G-CSF–induced mobilization of progenitor cells by anti–LFA-1 antibodies is independent of the dose and schedule of G-CSF.
Mice were pretreated with either a single injection of 100 μg anti–LFA-1 antibodies (n = 3-18) or saline intraperitoneally (n = 3-18). The following days, mice received a dose of 2.5 or 5 μg G-CSF daily intraperitoneally. Twenty-four hours after the last injection, mice were killed and peripheral blood was harvested. Results are expressed as mean ± SD. * P <. 05 as compared with the saline-pretreated controls.
Synergistic enhancement of G-CSF–induced mobilization of progenitor cells by anti–LFA-1 antibodies is independent of the dose and schedule of G-CSF.
Mice were pretreated with either a single injection of 100 μg anti–LFA-1 antibodies (n = 3-18) or saline intraperitoneally (n = 3-18). The following days, mice received a dose of 2.5 or 5 μg G-CSF daily intraperitoneally. Twenty-four hours after the last injection, mice were killed and peripheral blood was harvested. Results are expressed as mean ± SD. * P <. 05 as compared with the saline-pretreated controls.
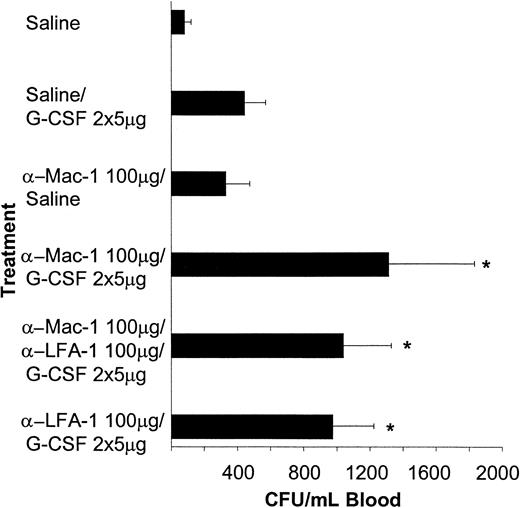
Pretreatment with different doses of neutralizing anti–Mac-1 antibody followed by G-CSF resulted in a similar increase in the number of peripheral blood progenitor cells as observed after treatment with anti–LFA-1 antibodies (Figure 4). In contrast to anti–LFA-1 antibodies, antibodies directed against Mac-1 exhibited modest mobilization when administered alone. Treatment with the combination of anti–Mac-1 and anti–LFA-1 antibodies prior to G-CSF did not result in a further enhancement of mobilization than obtained with either antibody alone (Figure 4).
The G-CSF–induced mobilization of progenitor cells is synergistically enhanced by pretreatment with anti–Mac-1 antibodies (5C6).
Mice were pretreated with a single injection of anti–Mac-1 antibodies intraperitoneally (n = 5-18) or saline (n = 5-18) followed by either 5 μg G-CSF per day or saline. The injections were given once daily for 2 days, starting 24 hours after antibody treatment. Twenty-four hours after the last injection, mice were killed and blood was harvested. Results are expressed as mean ± SD. *P < .05 compared with the saline plus G-CSF–treated controls.
The G-CSF–induced mobilization of progenitor cells is synergistically enhanced by pretreatment with anti–Mac-1 antibodies (5C6).
Mice were pretreated with a single injection of anti–Mac-1 antibodies intraperitoneally (n = 5-18) or saline (n = 5-18) followed by either 5 μg G-CSF per day or saline. The injections were given once daily for 2 days, starting 24 hours after antibody treatment. Twenty-four hours after the last injection, mice were killed and blood was harvested. Results are expressed as mean ± SD. *P < .05 compared with the saline plus G-CSF–treated controls.
Effect of anti–LFA-1 and anti–Mac-1 antibodies on cell counts in peripheral blood and bone marrow
In comparison with G-CSF–treated controls, mice treated with anti-β2 integrin antibodies prior to G-CSF administration exhibited a trend toward lower white blood cell and neutrophil counts in the peripheral blood as estimated by MGG staining or FACS analysis. As reported previously, mice treated with the neutralizing anti–LFA-1 antibody followed by G-CSF showed a significant decrease in platelet counts as compared with controls treated with G-CSF alone (Table1). This phenomenon was not observed in mice treated with anti–LFA-1 antibodies followed by G-CSF for 5 days or in mice treated with anti–Mac-1 antibodies only (data not shown).
Effect of treatment with anti–LFA-1 antibodies (α–LFA-1) and G-CSF on blood cell counts
| Treatment . | White blood cells, × 109/L . | Neutrophils, × 109/L . | Platelets, × 109/L . |
|---|---|---|---|
| Saline | 8.3 ± 1.7 | 2.0 ± 0.7 | 1068 ± 279 |
| Saline | |||
| G-CSF 2 d, 2.5 μg/d | 9.8 ± 2.5 | 2.8 ± 1.6 | 738 ± 84 |
| α–LFA-1 | |||
| G-CSF 2 d, 2.5 μg/d | 9.3 ± 1.7 | 2.6 ± 0.5 | 309 ± 58* |
| Saline | |||
| G-CSF 1 d, 5 μg/d | 13.3 ± 3.5 | 7.0 ± 2.0 | 610 ± 367 |
| α–LFA-1 | |||
| G-CSF 1 d, 5 μg/d | 8.2 ± 1.6 | 3.3 ± 0.4 | 281 ± 85* |
| Saline | |||
| G-CSF 2 d, 5 μg/d | 12.4 ± 3.0 | 4.6 ± 2.0 | 936 ± 244 |
| α–LFA-1 | |||
| G-CSF 2 d, 5 μg/d | 8.9 ± 1.6 | 3.2 ± 1.8 | 634 ± 111* |
| Saline | |||
| G-CSF 3 d, 5 μg/d | 10.5 ± 3.2 | 3.4 ± 1.1 | 709 ± 180 |
| α–LFA-1 | |||
| G-CSF 3 d, 5 μg/d | 8.4 ± 1.8 | 3.6 ± 1.1 | 351 ± 313* |
| Saline | |||
| G-CSF 5 d, 5 μg/d | 19.6 ± 2.8 | 12.5 ± 2.3 | 705 ± 89 |
| α–LFA-1 | |||
| G-CSF 5 d, 5 μg/d | 22.8 ± 6.3 | 16.3 ± 4.1 | 922 ± 313 |
| Treatment . | White blood cells, × 109/L . | Neutrophils, × 109/L . | Platelets, × 109/L . |
|---|---|---|---|
| Saline | 8.3 ± 1.7 | 2.0 ± 0.7 | 1068 ± 279 |
| Saline | |||
| G-CSF 2 d, 2.5 μg/d | 9.8 ± 2.5 | 2.8 ± 1.6 | 738 ± 84 |
| α–LFA-1 | |||
| G-CSF 2 d, 2.5 μg/d | 9.3 ± 1.7 | 2.6 ± 0.5 | 309 ± 58* |
| Saline | |||
| G-CSF 1 d, 5 μg/d | 13.3 ± 3.5 | 7.0 ± 2.0 | 610 ± 367 |
| α–LFA-1 | |||
| G-CSF 1 d, 5 μg/d | 8.2 ± 1.6 | 3.3 ± 0.4 | 281 ± 85* |
| Saline | |||
| G-CSF 2 d, 5 μg/d | 12.4 ± 3.0 | 4.6 ± 2.0 | 936 ± 244 |
| α–LFA-1 | |||
| G-CSF 2 d, 5 μg/d | 8.9 ± 1.6 | 3.2 ± 1.8 | 634 ± 111* |
| Saline | |||
| G-CSF 3 d, 5 μg/d | 10.5 ± 3.2 | 3.4 ± 1.1 | 709 ± 180 |
| α–LFA-1 | |||
| G-CSF 3 d, 5 μg/d | 8.4 ± 1.8 | 3.6 ± 1.1 | 351 ± 313* |
| Saline | |||
| G-CSF 5 d, 5 μg/d | 19.6 ± 2.8 | 12.5 ± 2.3 | 705 ± 89 |
| α–LFA-1 | |||
| G-CSF 5 d, 5 μg/d | 22.8 ± 6.3 | 16.3 ± 4.1 | 922 ± 313 |
On day 0, mice were treated with a single intraperitoneal injection of 100 μg antibody or saline. Twenty-four hours later, G-CSF was administered as a once-daily injection. Twenty-four hours after the last injection of G-CSF, mice were killed and blood was obtained by a cardiac puncture. Results are expressed as mean ± SD (n = 3-18, 2-3 experiments).
P < .05 compared with the saline-pretreated controls.
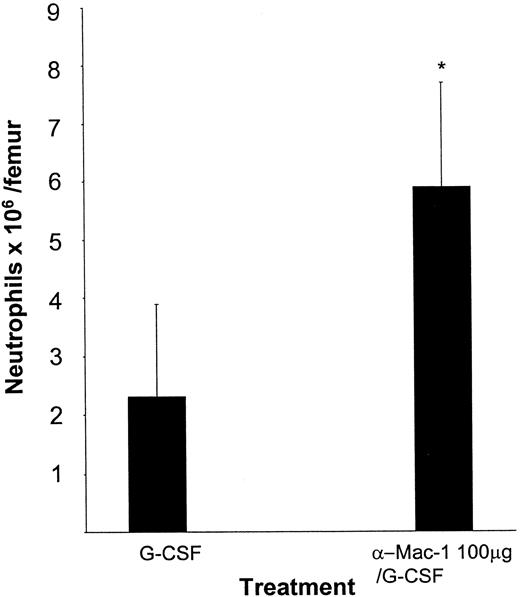
In the animals pretreated with the anti–Mac-1 antibody followed by injections of saline or G-CSF, the absolute neutrophil count in the bone marrow was significantly increased when compared with the G-CSF–treated control mice (anti–Mac-1 plus G-CSF: 5.9 × 106 ± 1.8 × 106 neutrophils per femur; G-CSF: 2.3 × 106 ± 1.6 × 106neutrophils per femur; P < .01; Figure5).
The number of neutrophils in the femur as assessed by FACS analysis is increased after pretreatment with anti–Mac-1 antibodies (5C6).
Mice were pretreated with a single injection of anti–Mac-1 antibodies intraperitoneally (n = 5-18) or saline (n = 5-18) followed by either 5 μg G-CSF per day or saline. The injections were given once daily for 2 days, starting 24 hours after antibody treatment. Twenty-four hours after the last injection, mice were killed and bone marrow was harvested. Results are expressed as mean ± SD. *P < .05 compared with the G-CSF–treated controls.
The number of neutrophils in the femur as assessed by FACS analysis is increased after pretreatment with anti–Mac-1 antibodies (5C6).
Mice were pretreated with a single injection of anti–Mac-1 antibodies intraperitoneally (n = 5-18) or saline (n = 5-18) followed by either 5 μg G-CSF per day or saline. The injections were given once daily for 2 days, starting 24 hours after antibody treatment. Twenty-four hours after the last injection, mice were killed and bone marrow was harvested. Results are expressed as mean ± SD. *P < .05 compared with the G-CSF–treated controls.
Effect of pretreatment with antibodies to β2 integrins on progenitor cells in the bone marrow
No significant differences in the number of CFU-GMs per femur of animals treated with either G-CSF alone or G-CSF in combination with antibodies to the β2 integrins were found (data not shown).
Estimation of circulating and cellbound antibodies
LFA-1 staining of bone marrow cells was observed for 96 hours after a single injection of 100 μg neutralizing anti–LFA-1 antibodies. In the peripheral blood, circulating and cellbound antibody could be detected up to 120 hours after a single injection. Free circulating antibody was measured up to 48 hours after a single injection of 100 μg anti–Mac-1 antibody. Cellbound antibodies on peripheral blood and bone marrow cells could be demonstrated for 72 hours after anti–Mac-1 antibody injection.
CAFC assay of peripheral blood and bone marrow
Committed progenitor cells and stem cells in blood and bone marrow of mice treated with a single injection of anti–LFA-1 antibody followed by G-CSF for 5 days were assessed in a CFU-GM and CAFC assay. A correlation was found between the CFU-GM assay and the number of CAFCs on day 7 (data not shown). The number of CAFCs–day 28 in the peripheral blood of mice treated with anti–LFA-1 antibody and G-CSF was significantly higher compared with mice treated with G-CSF only (119 ± 34 CAFCs per milliliter vs 17 ± 14 CAFCs per milliliter;P < .01; Figure 6). The number of CAFCs–day 28 in the bone marrow was not significantly different between the 3 groups of mice (saline: 655 ± 333 CFUs per milliliter; G-CSF: 374 ± 153 CFUs per milliliter; anti–LFA-1 antibody plus G-CSF: 472 ± 158 CFUs per milliliter).
The numbers of CAFCs in peripheral blood are increased after pretreatment with neutralizing anti–LFA-1 antibodies.
Mice were treated intraperitoneally with saline (n = 3), G-CSF 5 μg/d for 5 days (n = 9), or anti–LFA-1 antibodies followed by G-CSF during 5 days (n = 4). Twenty-four hours after the last injection, blood was obtained by cardiac puncture. Results are expressed as mean ± SD. *P < .05 compared with the G-CSF–treated controls.
The numbers of CAFCs in peripheral blood are increased after pretreatment with neutralizing anti–LFA-1 antibodies.
Mice were treated intraperitoneally with saline (n = 3), G-CSF 5 μg/d for 5 days (n = 9), or anti–LFA-1 antibodies followed by G-CSF during 5 days (n = 4). Twenty-four hours after the last injection, blood was obtained by cardiac puncture. Results are expressed as mean ± SD. *P < .05 compared with the G-CSF–treated controls.
Mobilization in LFA-1–deficient mice
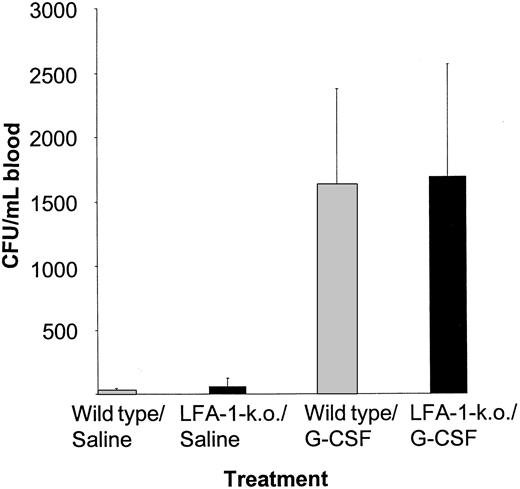
The number of circulating CFUs was not increased in LFA-1–deficient mice when compared with wild-type littermate controls (57 ± 66 vs 23 ± 21). Treatment with 5 μg G-CSF for 5 days did not reveal a difference in the number of CFU per milliliter of blood between both groups (LFA-1–deficient: 1694 ± 875 CFUs per milliliter; wild-type: 1629 ± 748 CFUs per milliliter; Figure7). Nor did the LFA-1–deficient mice show a difference in their white blood cell count, the number of platelets, or the number of progenitor cells in the bone marrow when compared with their wild-type littermate controls (data not shown).
The G-CSF–induced mobilization of peripheral blood progenitor cells is not enhanced in LFA-1 knock-out mice.
LFA-1 knock-out mice and their wild-type littermate controls were either treated with saline (n = 10 and n = 11, respectively) or with 5 μg G-CSF once daily for 5 days (n = 18 and n = 19, respectively). Twenty-four hours after the last injection, mice were killed and blood was obtained by cardiac puncture. Results are expressed as mean ± SD.
The G-CSF–induced mobilization of peripheral blood progenitor cells is not enhanced in LFA-1 knock-out mice.
LFA-1 knock-out mice and their wild-type littermate controls were either treated with saline (n = 10 and n = 11, respectively) or with 5 μg G-CSF once daily for 5 days (n = 18 and n = 19, respectively). Twenty-four hours after the last injection, mice were killed and blood was obtained by cardiac puncture. Results are expressed as mean ± SD.
Discussion
In this study we found that treatment with neutralizing antibodies to the β2 integrins LFA-1 and Mac-1 enhances stem cell mobilization induced by G-CSF administration. The increase in mobilization was independent of the dose and schedule of G-CSF used but was observed only under conditions when G-CSF alone induced mobilization. It was also observed at a maximal dose of G-CSF, suggesting the involvement of an additional pathway in the induction of mobilization. The combination of anti–LFA-1-and anti–Mac-1 antibodies did not result in further enhancement of mobilization, indicating that blocking either β2 integrin is sufficient for an optimal effect. The enhancing effect of anti–LFA-1 antibodies was specific, because a non-neutralizing, isotype-matched control antibody to LFA-1 did not affect mobilization. A low amount of endotoxin was detected in the anti–Mac-1 antibody. Although this was insufficient to induce stem cell mobilization, the possibility cannot be excluded that it contributed to the synergistic effect. The possibility was considered that the increased mobilization reflects a rebound phenomenon after administration of anti–LFA-1 antibodies. This was unlikely because free circulating antibody was present for the entire duration of the experiment.
LFA-1–deficient mice did not exhibit enhanced mobilization in response to G-CSF in comparison with wild-type littermate controls. This may be explained by the lack of activation of a subset of hematopoietic cells through the absence of the LFA-1 antigen and inability of antibody binding. Alternatively, the presence of a redundant pathway that compensates for the loss of the LFA-1 signal cannot be excluded. The pathway is not mediated through CD11b, because up-regulation of CD11b on the neutrophils of the LFA-1–deficient mice could not be detected by FACS analysis.
In recent years it has become evident that adhesion molecules play a role in retaining stem cells in the bone marrow microenvironment. Antibodies to the β1 integrin VLA-4 and its main ligand, VCAM-1, are capable of mobilizing HPCs in mice and in primates24,38whereas, consistent with previous reports, we found that anti–LFA-1 antibodies have no such capacity.24,30,39 The observed enhancement of G-CSF–induced mobilization by pretreatment with antibodies to these β2 integrins is not likely mediated through binding to ICAM-1, because administration of anti–ICAM-1 antibodies prior to G-CSF did not show an increase in mobilization. In previous studies we have shown that this anti–ICAM-1 antibody does inhibit IL-8–induced mobilization.30 This strongly suggests that the induction of mobilization by the anti–LFA-1 monoclonal antibodies is not due to inhibiting the adhesion of LFA-1 to ICAM-1, but it is more likely due to signaling processes induced by clustering LFA-1 receptors through antibodies.40 41The reason that a noninhibitory anti–LFA-1 antibody does not induce mobilization might be because it less strongly clusters and induces signaling as it binds to a distinct site on LFA-1 outside the ligand-binding pocket.
Previously it was found that neutrophils express LFA-1 and Mac-1 and release gelatinase-B upon activation by IL-8.42-44Therefore, it was hypothesized that neutrophils play a key role as accessory cells in mediating IL-8–induced mobilization.28,45 Indeed, IL-8–induced mobilization was absent in neutropenic mice and could be restored by administration of neutrophils.45 Neutrophils could also fulfill a crucial role in the enhancement of G-CSF–induced mobilization by anti–LFA-1 and anti–Mac-1 antibodies. Antibodies against the β2 integrins could inhibit adherence of neutrophils to platelet monolayers and P selectin.46 This may result in in vivo inhibition of migration through the endothelial wall into the vascular lumen. We observed an increase in the number of neutrophils in the bone marrow of mice treated with a combination of anti–Mac-1 antibodies and G-CSF (Figure 5), supporting a decreased migration of neutrophils from the bone marrow into the peripheral blood. Because administration of G-CSF results in neutrophil activation,47 48 we hypothesize that the increased mobilization observed after administration of antibodies to the β2 integrins prior to G-CSF is related to an increase of activated neutrophils in the bone marrow.
In accordance, recent studies in mice indicate that proteases released from neutrophils (ie, neutrophil elastase and cathepsin G) may be involved in G-CSF–induced stem cell mobilization. In the plasma of G-CSF–mobilized patients the concentration of VCAM-1 cleavage products was increased, concomitant with a decrease of VCAM-1 expression in the bone marrow. These results suggest that G-CSF–induced mobilization is mediated by interruption of the VCAM-1/VLA-4 pathway,49 through cleaving by neutrophil proteases.
Apart from its role in G-CSF–induced mobilization, the administration of antibodies to LFA-1 resulted in a significant thrombocytopenia. This phenomenon was also observed by Pruijt et al.30Thrombocytopenia was observed 2 hours after injection, suggesting a direct interaction of the antibody and platelets. In accordance, CD11a is expressed on the surface of murine platelets.50 In addition to this, the adhesion of megakaryocytes is an important process in platelet formation.51 Antibodies to CD18 inhibit the binding of megakaryocytes to cytokine-stimulated endothelial cells, thereby interfering with the platelet formation.52
In conclusion, G-CSF–induced stem cell mobilization is synergistically enhanced by a single injection of blocking antibodies to the β2-integrins LFA-1 and Mac-1. The enhancement is independent of the dose and schedule of G-CSF, suggesting the involvement of an additional pathway in mobilization induction. In LFA-1–deficient mice this phenomenon was not observed. This indicates that the synergistic effect observed is mediated by cells expressing LFA-1 that are activated by cross-linking LFA-1 receptors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Willem E. Fibbe, Dept of Hematology, Leiden University Medical Center, C2R, PO Box 9600, 2300 RC Leiden, The Netherlands; e-mail: w.e.fibbe@lumc.nl.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal