Recent studies have demonstrated that lineage marker–negative (Lin−) c-kitLo Flk-2/Flt3+IL-7R+ Sca-1Lo CD27+Ly-6C− Thy-1−CD43+CD16/32Lo/− terminal deoxynucleotidyl transferase (TdT)+ cells in murine bone marrow are functional lymphocyte precursors. However, it has not been clear if this is an obligate intermediate step for transit of multipotential hematopoietic stem cells to natural killer (NK) cells. We have now used serum-free, stromal cell–free cultures to determine that NK progenitors are enriched among an estrogen-regulated, c-kitLo subset of the Lin− fraction. However, several experimental approaches suggested that this population is heterogeneous and likely represents a stage where B and NK lineages diverge. Although most B-cell precursors were directly sensitive to estrogen in culture, much of the NK-cell precursor activity in that fraction was hormone resistant. B-lineage potential was largely associated with interleukin 7 receptor α (IL-7Rα) expression and was selectively driven in culture by IL-7. In contrast, many NK precursors did not display detectable amounts of this receptor and their maturation was selectively supported by IL-15. Finally, single-cell experiments showed that the Lin−c-kitLo fraction contains a mixture of B/NK, B-restricted, and NK-restricted progenitors. Two-step culture experiments revealed that NK precursors become hormone resistant on or before acquisition of CD122, signaling commitment to the NK lineage. CD45R is preferentially, but not exclusively, expressed on maturing B-lineage cells. Production of these 2 blood cell types is regulated in bone marrow by common and then independent mechanisms that can now be studied with greater precision.
Introduction
Natural killer (NK) cells have long been recognized for their importance in innate immunity, viral defense, and tumor surveillance.1 Additionally, NK cells represent barriers to bone marrow transplantation and produce cytokines that influence other cellular components of the immune system. Impressive progress has recently been made in understanding how NK cells recognize targets for cytolytic destruction while sparing normal cells.2 For example, it is now clear that self-tolerance of NK cells is maintained by inhibitory receptors, which are expressed on NK cells and recognize major histocompatibility complex (MHC) class I molecules. In mice, the Ly49 receptor family recognizes classical MHC I molecules, whereas the CD94/NKG2 receptors recognize the nonclassical Qa-1b MHC I molecule. Some additional NK molecules have been suggested to function as activating receptors.3 Recent reports suggest that the self-recognizing NK-cell receptor repertoire is built in successive fashion following contact of NK-cell precursors with environmental class I MHC molecules during development.3,4 However, many questions remain about the origin and lifespans of NK cells. Although still incomplete, a differentiation sequence is emerging that will eventually describe how hematopoietic stem cells generate NK cells in mice and humans.5 Although cells with NK-lineage potential can be found among undifferentiated thymocytes, and some properties are shared with T cells, the thymus is probably not the most important site for production of NK cells.6 7 The present study was designed to obtain more precise information about relationships between early NK-lineage precursors and those of other lymphoid lineages within bone marrow.
The NK-1.1 antigen encoded by the NKR P1C gene provides a useful NK-cell marker in selected strains of mice, but is also expressed on a subset of T cells (NK T cells). The DX5 antigen, recently shown to be identical to CD49, is somewhat less restricted to NK-lineage cells,8 and is probably acquired at a later stage. For example, there are NK-1.1+DX5− cells in bone marrow, whereas most NK-1.1+ spleen cells are DX5+.9 It is interesting that fetal and neonatal NK cells express high levels of CD94/NKG2A receptors, but not Ly49. The density of CD94/NKG2A gradually declines when Ly49 family receptors are acquired.10
Although interleukin 2 (IL-2) can be used to drive formation of NK cells in vitro, IL-2–deficient mice and IL-2 receptor α chain (IL-2Rα)–deficient mice have NK cells.11,12 In contrast, the IL-2Rβ, the common γ chain (γc) of the IL-2/IL-7 receptor and the tyrosine kinase JAK3 are all required for generation of NK cells.13-15 First cloned from a human bone marrow stromal cell line, IL-15 uses a receptor composed of an IL-15–specific α chain, the IL-2Rβ, and the γc.16-18 IL-15 induces NK-cell differentiation in vitro.19,20 Furthermore, NK cells are absent in either IL-15 or IL-15Rα chain knockout mice.21,22 Therefore, IL-15 is indispensable for NK-lineage development and lineage marker low/negative (LinLo/−) cells in marrow that display the CD122 (IL-2Rβ) receptor for this cytokine were recently determined to be committed NK-cell progenitors.9 Similar CD122+NK precursors can also be derived from multipotent Lin−Sca-2+ c-kit+ progenitors in vitro.23
Kondo and colleagues24 demonstrated that a category of Lin−, IL-7Rα+, Sca-1Lo bone marrow cells contained precursors of B, T, and NK cells. Single-cell assays revealed that at least some of these common lymphoid progenitors (CLPs) could give rise to B or T cells, but this point was not investigated with respect to NK-lineage differentiation potential. Subsequent studies from our laboratory described substantial, but incomplete, overlap between the CLP fraction and Lin−terminal deoxynucleotidyl transferase-positive (TdT+) cells previously designated early pro-B cells by Osmond and colleagues.25,26 Preferential sensitivity to sex steroids distinguishes these precursors from myeloerythroid-lineage cells and suggests one means of regulating rates of lymphocyte production.27 28
Serum-free, stromal cell–free cultures containing recombinant Flt3 ligand (FL), stem cell factor (SCF), and IL-7 have been described that efficiently support the differentiation of CLP/early pro-B cells to CD19-bearing B-lineage lymphocytes.26 29 We now show that addition of a fourth cytokine, IL-15, stimulates the formation of immature NK cells in these cultures. In addition to B and T lymphocytes, NK cells derive from rare hormone-regulated precursors in bone marrow. The acquired resistance of maturing NK precursors to estrogen provides a means of establishing when B and NK lineages diverge in bone marrow. This important event seems to occur within the early pro-B/CLP fraction coincident with, or just prior to, acquisition of the IL-15 receptor. These findings should be helpful for further analysis of mechanisms that coordinately and then independently regulate production of B and NK lymphocytes.
Materials and methods
Mice
BALB/c mice were obtained at 5 to 15 weeks of age from the Oklahoma Medical Research Foundation Laboratory Animal Resource Center (Oklahoma City) or Charles Rivers Breeding Laboratories (Wilmington, MA) and used for culture experiments. C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, ME).
Antibodies
The 1D3 anti-CD19 monoclonal antibody (mAb) was purified from the culture supernatant of hybridoma cells grown in our laboratory. Anti-CD45RA 14.8 mAb developed in our laboratory and the anti–Mac-1/CD11b (M1/70) mAb were used as culture supernatants of the respective hybridomas. The rat mAb to mouse IL-7Rα subunit (SB/199) was established in our laboratory, purified from hybridoma supernatant, and biotinylated.30 Purified anti-erythroid (TER-119), anti–Gr-1/Ly6-G (RB6-8C5), anti-CD2 (RM2-5), and anti-CD3 17A2 mAbs, fluorescein isothiocyanate (FITC)–conjugated anti-CD2 (RM2-5), anti-CD3 145-2C11, anti-CD8 53-6.7, anti-CD19 1D3, anti-CD45R/B220 (RA3/6B2), anti-CD122/IL-2Rβ (TM-β1), anti–Mac-1/CD11b (M1/70), and anti–Gr-1 /Ly-6G (RB6-8C5) antibodies, phycoerythrin (PE)–conjugated TER-119, anti–NK-1.1 (PK136), anti–pan NK cell (DX5), anti-CD45R/B220 (RA3/6B2), anti–Flk-2/Flt3 (A2F10.1), anti-CD19 1D3 antibodies, biotinylated anti-CD45RA 14.8, anti-CD94 18d3, and anti-CD19 antibodies and allophycocyanin (APC)–conjugated anti–c-kit, anti-CD45R/B220, anti–Mac-1, anti–pan NK cell (DX5/CD49), and anti-Ly49G2 4D11 antibodies were all purchased from BD Pharmingen (San Diego, CA).
Reagents
Recombinant mouse IL-7 was purchased from Endogen (Woburn, MA). Recombinant mouse IL-15 was purchased from Research Diagnosis (Flanders, NJ). Recombinant mouse SCF and mouse FL were purchased from R & D Systems (Minneapolis, MN). 1,3,5[10]-Estratriene-3, 17β-diol (β-estradiol, E2) was purchased from Sigma Chemical (St Louis, MO).
Isolation of Lin− early pro-B cells
Bone marrow cells were collected from 3 to 5 mice and suspended with phosphate-buffered saline (PBS) without Ca++ or Mg++ (PBS−) and supplemented with 3% fetal calf serum (FCS). Cells were incubated with antibodies to lineage markers (Gr-1 and Mac-1 for myeloid cells, anti-CD19 and anti-CD45RA for B-lineage cells, and TER-119 for erythroid cells) for 20 minutes. Then cells were washed and incubated with magnetic-activated cell-sorted (MACS) goat anti-rat IgG microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) for 15 minutes. Bead-labeled cells were depleted using MACS separation columns and a magnet following the manufacturer's instructions. These enriched lineage marker–negative bone marrow cells were then stained with a cocktail of labeled antibodies to the lineage markers (FITC-conjugated anti-CD2, anti-CD3, anti-CD8, anti–Gr-1, anti–Mac-1, PE-conjugated TER-119 anti-DX5, anti-CD19, and anti-CD45R/B220) and APC-conjugated anti–c-kit antibody. In some experiments, PE-labeled anti–Flk-2/Flt3 antibody and biotinylated anti–IL-7Rα antibodies were also used. In this case, PE-labeled TER-119 and DX5 antibodies were eliminated and FITC-conjugated anti-CD19 and anti-CD45R were used instead of PE-conjugated ones. Phycoerythrin–Texas red tandem-conjugated streptavidin (streptavidin-PE-TR; Caltag, Burlingame, CA) was used as the secondary reagent for biotinylated anti–IL-7Rα. Stained cells were sorted on a MoFlo (Cytomation, Ft Collins, CO). When reanalyzed after sorting, Lin− cells were generally more than 95% pure, that is, less than 5% had even slightly above background levels of staining. Subsets of these Lin− cells were usually more than 90% pure when sorted according to absence, low density, or high density of c-kit. The IL-7Rα+ cells were relatively homogenous, but the IL-7Rα− preparations included some cells with low, but above threshold, staining. In some experiments, LinLo/− cells were simply enriched by MACS separation. In those cases, antibodies to anti-CD2 for NK cells and anti-CD3 for T cells were added to the lineage marker cocktail.
Cell culture
Sorted cells were put into 24-well culture plates (Costar, Cambridge, MA) containing 1 mL X-VIVO15 medium (Biowhittaker, Walkersville, MD) containing 1% detoxified bovine serum albumin (Stem Cell Technologies, Vancouver, BC, Canada), 5 × 10− 5 M 2-mercaptoehtanol (2-ME), 2 mM l-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, and cytokines as indicated and cultured at 37°C and 5% CO2 in a humidified atmosphere. The concentrations of cytokines were IL-7, 1 ng/mL; FL, 100 ng/mL; SCF, 20 ng/mL; and IL-15, 50 ng/mL. Seven days later, cells were harvested; cell numbers and viability were determined with a trypan blue dye exclusion method and then subjected to flow cytometry analysis. For single-cell sorting experiments, cells were directly sorted into wells of 96-well U-bottom tissue culture plates (Costar) containing 100 μL of the same media. Individual wells were harvested 11 to 15 days later and analyzed by flow cytometry. For 2-step culture experiments, cells were first cultured with IL-7, FL, and SCF for 5 days and then washed and split into 2 cultures with either IL-7 alone or IL-15 alone for another 5 days. In 2-step sorting experiments, cells were first cultured with IL-7, FL, and SCF for 3 days before staining with anti-CD19, anti-CD11b/Mac-1, anti-CD45R, and anti-CD122. CD19− Mac-1− cells were sorted into 4 subsets according to the expression of CD45R and CD122 and cells in each subset were cultured with SCF, FL, IL-7, and IL-15 for another 5 days.
Immunofluorescence staining
For the analysis of surface antigens, cells were incubated with combinations of labeled antibodies in PBS supplemented with 3% FCS. They were then washed and incubated with PE–Texas red tandem-conjugated streptavidin to detect biotinylated primary antibodies. Stained cells were run on a FACScalibur flow cytometer (Becton Dickinson, San Diego, CA) and the data were analyzed with FlowJo software (Treestar, San Carlos, CA).
Hormone treatment in vivo
Time-release pellets of 17β-estradiol (0.1 mg/pellet, 21-day release; Innovative Research of America, Sarasota, FL) were implanted subcutaneously with a 10-gauge precision trochar. After 7 days, mice were killed and bone marrow progenitor cells were sorted.
Results
Formation of immature NK cells from lymphocyte precursors in culture
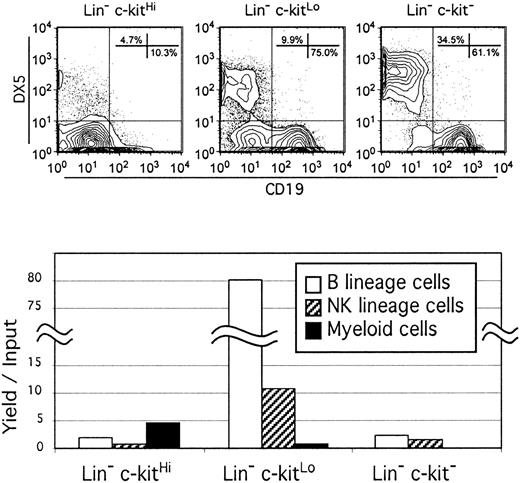
Previous studies have demonstrated that the c-kit tyrosine kinase receptor is progressively down-regulated as hematopoietic stem cells give rise to early lymphocyte precursors.31 Therefore, we used this characteristic to subdivide the Lin− fraction of bone marrow and assessed each for the potential to generate NK-lineage lymphocytes in culture. Preliminary experiments established that in addition to CD43+CD45R+CD19+B-lineage lymphoid cells, DX5-bearing cells efficiently emerged when IL-15 was added to serum-free medium that also contained SCF, FL, and IL-7.20,26 These DX5+ lymphocytes lacked CD3 and we examined a series of other markers associated with conventional NK cells. Although there are significant numbers of CD3−DX5+ NK-1.1− cells in spleens of C57BL/6 mice, most of the DX5+ cells that emerged in culture coexpressed NK-1.1 (Figure 1). The CD94 receptor was expressed on a subset of culture-generated NK cells but the Ly49G2 receptor typically expressed by mature NK cells was not detected (Figure 1). Although all Lin− fractions contained at least some precursors for these immature NK cells, careful analysis of the yield per input cell revealed enrichment among the c-kitLo subset (Figure 2). The c-kitLo cells represent 24% of the total Lin− fraction of bone marrow, whereas 62% are c-kitHi and 14% are c-kit−.31Even when adjusted on this basis, most of the precursors that can generate NK cells within 1 week of culture are Lin−c-kitLo. Longer intervals and addition of a fifth cytokine, either megakaryocyte growth and development factor (MDGF) or IL-3, increased the yield of NK cells from the c-kitHifraction (data not shown). Also, it is important to note that no NK cells emerged when IL-15 was the only cytokine and addition of DX5 to the cocktail of antibodies used for lineage depletion had no effect on the results (data not shown). This indicates that the cell suspensions used to initiate our cultures were not contaminated with NK cells whose receptors were modulated during handling. We conclude from these experiments that immature NK cells can be efficiently generated in stromal cell–free, serum-free cultures containing only recombinant cytokines. Most precursors that differentiate within 1 week of culture reside in the same Lin− c-kitLo fraction that contains CLP/early pro-B cells.
Generation of immature NK cells in one-step, serum-free, stromal cell–free cultures.
Lin− c-kitLo cells were isolated from bone marrow as described in “Materials and methods” and placed in serum-free, stromal cell–free cultures containing SCF, FL, IL-7, and IL-15. Seven days later, cells were harvested and compared with freshly isolated spleen cells by flow cytometry. The top 4 plots show expression of CD3, DX5, NK-1.1, and CD94 markers on cells derived from C57BL/6 mice, whereas the lower 2 histograms illustrate the Ly49G2 on NK-lineage cells generated from BALB/c mice.
Generation of immature NK cells in one-step, serum-free, stromal cell–free cultures.
Lin− c-kitLo cells were isolated from bone marrow as described in “Materials and methods” and placed in serum-free, stromal cell–free cultures containing SCF, FL, IL-7, and IL-15. Seven days later, cells were harvested and compared with freshly isolated spleen cells by flow cytometry. The top 4 plots show expression of CD3, DX5, NK-1.1, and CD94 markers on cells derived from C57BL/6 mice, whereas the lower 2 histograms illustrate the Ly49G2 on NK-lineage cells generated from BALB/c mice.
Most Lin− NK-cell progenitors are most abundant in the c-kitLo fraction of bone marrow cells.
Bone marrow cells bearing markers associated with various blood cell lineages were rigorously depleted with magnetic beads and high-speed cell sorting (as described in “Materials and methods”). The remaining Lin− cells were sorted into 3 fractions according to density of c-kit and cultured with SCF, FL, IL-7, and IL-15 for 7 days. Recovered cells were then counted and analyzed by flow cytometry for expression of B (CD19), NK (DX5), or myeloid (CD11b/Mac-1, not shown) lineage antigens (top panels). Absolute numbers of recovered cells of each type were then divided by the numbers of precursors used to initiate the cultures to obtain the yield per input values shown in the lower graph.
Most Lin− NK-cell progenitors are most abundant in the c-kitLo fraction of bone marrow cells.
Bone marrow cells bearing markers associated with various blood cell lineages were rigorously depleted with magnetic beads and high-speed cell sorting (as described in “Materials and methods”). The remaining Lin− cells were sorted into 3 fractions according to density of c-kit and cultured with SCF, FL, IL-7, and IL-15 for 7 days. Recovered cells were then counted and analyzed by flow cytometry for expression of B (CD19), NK (DX5), or myeloid (CD11b/Mac-1, not shown) lineage antigens (top panels). Absolute numbers of recovered cells of each type were then divided by the numbers of precursors used to initiate the cultures to obtain the yield per input values shown in the lower graph.
NK- and B-lineage lymphocytes both derive from estrogen-sensitive precursors
Our previous studies showed that functional B-lymphocyte precursors within the Lin− c-kitLo fraction are depleted when estrogen levels are high.26 28 There is a corresponding loss of phenotypically defined CLP/early pro-B cells, providing strong evidence that this is the major differentiation pathway for lymphocyte production. It was therefore important to learn if NK cells are made in similar fashion. The Lin−c-kitLo fraction was recovered from mice treated for 1 week with time-release estrogen pellets and used to initiate NK cultures. Elevated hormone levels only slightly reduced numbers of Lin− c-kitLo cells (data not shown), but functional precursors of both B- and NK-lineage cells were dramatically depleted (Figure 3). It is highly likely that both categories of lymphocytes derive from hormone-regulated Lin− c-kitLo precursors.
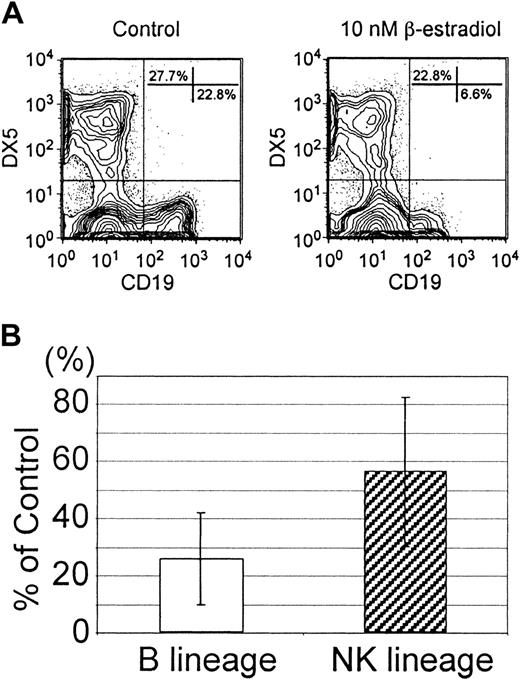
Both NK-and B-lineage lymphocytes derive from hormone-sensitive progenitors in the bone marrow.
Lin− c-kitLo bone marrow cells were prepared from mice implanted 7 days previously with estrogen pellets (β-estradiol–treated mice) or from control animals (control mice) and used to initiate cultures containing SCF, FL, IL-7, and IL-15. (A) The top panels show typical flow cytometry profiles for cells harvested 7 days later. (B) The data were also calculated according to absolute numbers of B-lineage (open bars) or NK-lineage (hatched bars) cells generated in culture per input cell.
Both NK-and B-lineage lymphocytes derive from hormone-sensitive progenitors in the bone marrow.
Lin− c-kitLo bone marrow cells were prepared from mice implanted 7 days previously with estrogen pellets (β-estradiol–treated mice) or from control animals (control mice) and used to initiate cultures containing SCF, FL, IL-7, and IL-15. (A) The top panels show typical flow cytometry profiles for cells harvested 7 days later. (B) The data were also calculated according to absolute numbers of B-lineage (open bars) or NK-lineage (hatched bars) cells generated in culture per input cell.
Identification of precursors with restricted NK-differentiation potential
The experiments described above revealed an important similarity between B- and NK-cell precursors but were not informative about whether they have a common origin within the Lin−c-kitLo fraction. We previously found that early pro-B cells are directly sensitive to estrogen and experiments were done to see if the same was true for NK progenitors.26 As expected, the hormone inhibited generation of B-lineage cells (Figure4). Absolute numbers of CD19+cells recovered from estrogen-containing cultures averaged 26% of those present in control cultures in 9 experiments. However, production of NK-lineage cells in the same cultures was more resistant to the hormone (averaged 56% of control values).
Most NK-cell precursors in the Lin−c-kitLo fraction are not directly sensitive to estrogen.
Lin− c-kitLo bone marrow cells were put into serum-free, stromal cell–free cultures along with vehicle (control) or estrogen (β-estradiol). One week later, the cultures were harvested and examined by flow cytometry for the production of B-lineage (CD19+) or NK-lineage (DX5+) lymphocytes. (A) Typical flow cytometry profiles are shown from one representative experiment. (B) Data from 9 independent experiments were normalized and expressed as average yields of B- and NK-lineage lymphocytes in estrogen-treated cultures relative to the untreated controls ± SE
Most NK-cell precursors in the Lin−c-kitLo fraction are not directly sensitive to estrogen.
Lin− c-kitLo bone marrow cells were put into serum-free, stromal cell–free cultures along with vehicle (control) or estrogen (β-estradiol). One week later, the cultures were harvested and examined by flow cytometry for the production of B-lineage (CD19+) or NK-lineage (DX5+) lymphocytes. (A) Typical flow cytometry profiles are shown from one representative experiment. (B) Data from 9 independent experiments were normalized and expressed as average yields of B- and NK-lineage lymphocytes in estrogen-treated cultures relative to the untreated controls ± SE
These findings could be most easily explained in terms of heterogeneity within the Lin− c-kitLo fraction and we conducted single-cell experiments to explore that possibility. The first 2 used Lin− c-kitLo bone marrow cells that were further enriched for B and NK precursors by sorting on the basis of Flk-2/Flt3 expression.26,32,33 These were directly sorted into individual wells of 96-well culture plates using pulse-width analysis to exclude doublets and direct inspection confirmed the effectiveness of this procedure. After 11 days of culture, an average of 23% of the wells contained proliferating clones and these were harvested for flow cytometry (Table1 and Figure5). The analysis revealed heterogeneity in the differentiation potential of precursors and individual wells also varied with respect to the degree of clonal expansion. Precursors with restricted B-lineage potential (Figure 5A,B) were most common and ranged in incidence between 1 of 6.3 to 1 of 10.7 cells. Next most abundant were progenitors that generated NK-lineage cells (1 of 19.2 to 1 of 24.9 incidence; Figure 5C). Only 6 of 566 tested clones were demonstrated to be common B/NK-cell progenitors (Figure 5D). Because CLPs had previously been sorted on the basis of IL-7Rα (CD127) expression,24 we added that as a sort criterion for a third experiment of this type. In addition, small clones were harvested and separately analyzed after a total of 15 days of culture to account for the possibility that NK precursors expanded at a slower rate (Table 1, experiment 3). Again, common B/NK progenitors were less abundant than NK-restricted and B-lineage–restricted cells. In all 3 experiments, some of the precursors differentiated in synchronous fashion, such that virtually all recovered cells expressed either CD19 or DX5 (Figure 5A,C). However, in other wells we found mixtures of single-positive and double-negative cells, suggesting there was an additional type of heterogeneity in the sorted fractions (Figure 5B). The sorting strategy and culture conditions were optimized for lymphoid progenitors and only 1 of the 950 wells with clonal proliferation contained CD11b/Mac-1+ myeloid cells.
Clonal analysis of B- and NK-differentiation potential
| Experiment . | Sorted cells* . | B-only clones† . | NK-only clones‡ . | B+NK clones1-153 . |
|---|---|---|---|---|
| 1 | Lin−c-kitLoFlk-2+ | 59/374 (1:6.3) | 15/374 (1:24.9) | 4/374 (1:93.9) |
| 2 | Lin− c-kitLoFlk-2+ | 18/192 (1:10.7) | 10/192 (1:19.2) | 2/192 (1:96.0) |
| 3 | Lin− c-kitLo Flk-2+IL-7Rα+ | 104/384 (1:3.7) | 13/384 (1:29.5) | 6/384 (1:64) |
| Experiment . | Sorted cells* . | B-only clones† . | NK-only clones‡ . | B+NK clones1-153 . |
|---|---|---|---|---|
| 1 | Lin−c-kitLoFlk-2+ | 59/374 (1:6.3) | 15/374 (1:24.9) | 4/374 (1:93.9) |
| 2 | Lin− c-kitLoFlk-2+ | 18/192 (1:10.7) | 10/192 (1:19.2) | 2/192 (1:96.0) |
| 3 | Lin− c-kitLo Flk-2+IL-7Rα+ | 104/384 (1:3.7) | 13/384 (1:29.5) | 6/384 (1:64) |
Each cell of the table shows the number of clones of a given type that were detected/total number of seeded wells, followed by the calculated frequency.
Single cells with the indicated characteristics were sorted and cultured as described in “Materials and methods.” Individual wells were harvested either 11 days (experiments 1 and 2) or 11 and 15 days later (experiment 3) and characterized by flow cytometry.
Cultures containing CD19+ cells but less than 5% DX5+ cells were counted as B-only clones.
Cultures containing DX5+ cells but less than 5% CD19+ cells were scored as NK-only clones.
Cultures containing at least 10% of both CD19+ and DX5+ cells were counted as B+NK clones.
The Lin− c-kitLoFlk-2+ fraction of bone marrow contains B/NK-bipotential progenitors as well as committed B- and NK-cell progenitors.
Single-cell cultures were initiated as described in “Materials and methods.” Typical examples are shown of individual culture wells that were harvested 11 to 15 days later and found by flow cytometry to contain (A) B cells, (B) B cells and undifferentiated cells, (C) NK cells, and (D) both B and NK cells. The results from 3 such experiments are summarized in Table 1.
The Lin− c-kitLoFlk-2+ fraction of bone marrow contains B/NK-bipotential progenitors as well as committed B- and NK-cell progenitors.
Single-cell cultures were initiated as described in “Materials and methods.” Typical examples are shown of individual culture wells that were harvested 11 to 15 days later and found by flow cytometry to contain (A) B cells, (B) B cells and undifferentiated cells, (C) NK cells, and (D) both B and NK cells. The results from 3 such experiments are summarized in Table 1.
In summary, the Lin− c-kitLo fraction of bone marrow contains estrogen-sensitive cells that are biased toward the B-lymphocyte lineage as well as estrogen-resistant NK progenitors. A surprisingly small number of precursors have the potential to generate both blood cell types.
Cytokine receptor expression and responsiveness mark NK-lineage progression
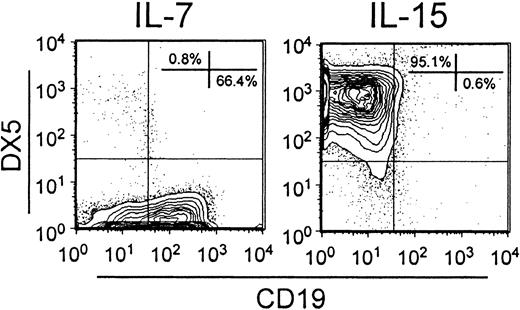
Four recombinant cytokines were used to support the expansion and maturation of cells in our cultures, and acquisition of functional receptors for those agents can represent useful milestones of differentiation.33 As noted above, IL-15 was required for efficient NK-lineage differentiation, but NK cells did not emerge when cultures of Lin− c-kitLo cells contained IL-15 alone. Further experiments were conducted to learn more about when precursors of the B and NK lineages become independently regulated by these positive stimuli. Lin− c-kitLo cells were sorted and placed in bulk cultures containing SCF, FL, and IL-7 for 5 days. The cells were then harvested, washed, and cultured for an additional 5 days in the presence of either IL-7 alone or IL-15 alone (Figure 6). It is clear that progression in the B and NK lineages was independently controlled by these 2 factors.
B and NK lineages diverge and become independently regulated by IL-7 or IL-15.
The Lin− c-kitLo fraction of bone marrow cells was placed in primary cultures containing SCF, FL, and IL-7 for 5 days. Harvested cells were then washed and cultured for an additional 5 days with either IL-7 alone or IL-15 alone. The recovered cells were then analyzed by flow cytometry as shown.
B and NK lineages diverge and become independently regulated by IL-7 or IL-15.
The Lin− c-kitLo fraction of bone marrow cells was placed in primary cultures containing SCF, FL, and IL-7 for 5 days. Harvested cells were then washed and cultured for an additional 5 days with either IL-7 alone or IL-15 alone. The recovered cells were then analyzed by flow cytometry as shown.
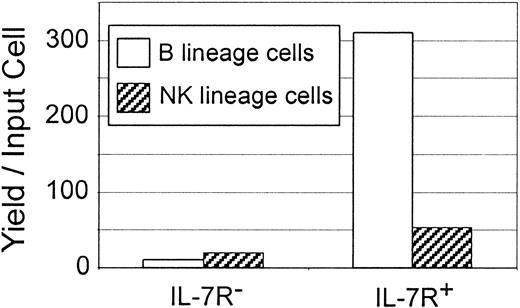
The Lin− c-kitLo Flk-2/Flt3+fraction was then sorted into subsets on the basis of IL-7Rα (CD127) expression and tested in cultures containing all 4 cytokines (Figure7). The B-lineage precursors were substantially enriched among the Lin− c-kitLocells that display this growth factor receptor. Given their relative abundance in the starting populations, we calculate that approximately 90% of the cells that can quickly generate CD19+ cells in culture are in this Lin− c-kitLoFlk-2/Flt3+ IL-7Rα+ subset of bone marrow. There was also some enrichment for NK-precursor activity in the IL-7Rα+ fraction, but we calculate that the Lin− c-kitLo Flk-2/Flt3+ category of bone marrow contains an equal number of IL-7Rα− NK precursors. Although B-lineage precursors likely traverse an IL-7Rα+ stage, the IL-7R is either never displayed or transiently expressed by many maturing NK-lineage cells.
B-cell precursors are more enriched than NK-cell precursors among the IL-7Rα+ subset of Lin−bone marrow cells.
The Lin− c-kitLo Flk-2/Flt3+fraction of bone marrow was isolated and further sorted into IL-7Rα+ and IL-7Rα− subsets before culture with SCF, FL, IL-7, and IL-15. Absolute numbers of recovered B (CD19+) and NK (DX5+) cells were determined by flow cytometry and calculated according to yield per input cell.
B-cell precursors are more enriched than NK-cell precursors among the IL-7Rα+ subset of Lin−bone marrow cells.
The Lin− c-kitLo Flk-2/Flt3+fraction of bone marrow was isolated and further sorted into IL-7Rα+ and IL-7Rα− subsets before culture with SCF, FL, IL-7, and IL-15. Absolute numbers of recovered B (CD19+) and NK (DX5+) cells were determined by flow cytometry and calculated according to yield per input cell.
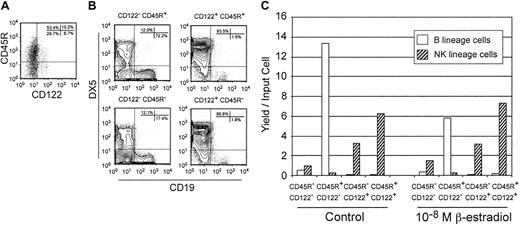
Similar experiments were done to explore the timing of acquisition of the IL-2Rβ (CD122) receptor used for responsiveness to IL-15. Expression of CD122 was beneath the level of detection in freshly sorted Lin− c-kitLo cells and we placed them in cultures containing SCF, FL, and IL-7 for 3 days. At that time, an average of 17.9% of recovered cells were CD122+ and a subset of those additionally expressed CD45R/B220 (Figure8A). All possible subsets of Mac-1/CD11b− CD19− cells were then sorted on the basis of CD122 and CD45R/B220 before being returned to cultures containing SCF, FL, IL-7, and IL-15 for an additional 5 days (Figure8B). Restriction to the NK-cell lineage was almost complete in cells expressing CD122, regardless of CD45R/B220 status. In contrast, cells with B potential were almost exclusively present in the CD122− fractions and a majority had acquired CD45R/B220. The relative expansion and differentiation potential of maturing subsets of the Lin− c-kitLo category is considered for a representative experiment in Table2. It can be seen that proliferation was modest in the 3-day primary cultures, when some cells were acquiring either or both markers. Each input Lin−c-kitLo cell could generate 10 CD19+lymphocytes and did so primarily via a CD122−CD45R/B220+ intermediate. In contrast, the greatest yield of NK-lineage cells took place via CD122+ CD45R/B220+ precursors, followed closely by the CD122+ CD45R/B220− pathway.
Restriction to the NK lineage occurs as Lin− c-kitLo cells acquire the CD122 receptor for IL-15.
Lin− c-kitLo cells were first cultured for 3 days with SCF, FL, and IL-7. They were then stained, analyzed, and sorted into 4 categories based on the acquisition of CD45R/B220 or CD122 (A). Each subset was then returned to culture along with SCF, FL, IL-7, and IL-15 for an additional 5 days. Finally, the recovered cells were analyzed by flow cytometry to assess the generation of B-lineage (CD19+) or NK-lineage (DX5+) lymphocytes (B). Absolute yields per input cells were calculated following primary and secondary cultures and are presented in Table 2. A similar experimental design was used to determine which of the 4 subsets present at the end of the first culture interval were sensitive to estrogen. The results are presented as yields of NK- or B-lineage cells per precursor placed in the secondary cultures (C).
Restriction to the NK lineage occurs as Lin− c-kitLo cells acquire the CD122 receptor for IL-15.
Lin− c-kitLo cells were first cultured for 3 days with SCF, FL, and IL-7. They were then stained, analyzed, and sorted into 4 categories based on the acquisition of CD45R/B220 or CD122 (A). Each subset was then returned to culture along with SCF, FL, IL-7, and IL-15 for an additional 5 days. Finally, the recovered cells were analyzed by flow cytometry to assess the generation of B-lineage (CD19+) or NK-lineage (DX5+) lymphocytes (B). Absolute yields per input cells were calculated following primary and secondary cultures and are presented in Table 2. A similar experimental design was used to determine which of the 4 subsets present at the end of the first culture interval were sensitive to estrogen. The results are presented as yields of NK- or B-lineage cells per precursor placed in the secondary cultures (C).
Divergence of B- and NK-cell lineages in 2-step cultures
| Sorted cells* . | CD122−CD45R− . | CD122−CD45R+ . | CD122+CD45R− . | CD122+ CD45R+ . | ||||
|---|---|---|---|---|---|---|---|---|
| Yield in the 1st culture† . | 0.92 . | 1.66 . | 0.21 . | 0.32 . | ||||
| After 2nd culture‡ . | B . | NK . | B . | NK . | B . | NK . | B . | NK . |
| Yield/input (2nd culture)2-153 | 1.22 | 0.85 | 6.04 | 1.00 | 0.41 | 19.91 | 0.29 | 17.58 |
| Yield/1 Lin− c-kitLocell2-154 | 1.13 | 0.79 | 10.03 | 1.67 | 0.09 | 4.15 | 0.09 | 5.58 |
| Sorted cells* . | CD122−CD45R− . | CD122−CD45R+ . | CD122+CD45R− . | CD122+ CD45R+ . | ||||
|---|---|---|---|---|---|---|---|---|
| Yield in the 1st culture† . | 0.92 . | 1.66 . | 0.21 . | 0.32 . | ||||
| After 2nd culture‡ . | B . | NK . | B . | NK . | B . | NK . | B . | NK . |
| Yield/input (2nd culture)2-153 | 1.22 | 0.85 | 6.04 | 1.00 | 0.41 | 19.91 | 0.29 | 17.58 |
| Yield/1 Lin− c-kitLocell2-154 | 1.13 | 0.79 | 10.03 | 1.67 | 0.09 | 4.15 | 0.09 | 5.58 |
Lin− c-kitLo bone marrow cells were cultured with IL-7, FL, and SCF for 3 days and sorted by surface markers as shown.
These values represent numbers of cells of the indicated types harvested from primary cultures divided by the input cell numbers.
Each subset of cells that was harvested from the primary cultures was recultured with IL-7, FL, SCF, and IL-15 for 5 days. B-lineage (CD19+) and NK-lineage (DX5+) cells were then determined by flow cytometry.
The yields of B- and NK-lineage cells in secondary cultures were determined by dividing harvested cells by the input cell numbers.
These values provide an index of how effective each type of progenitor was in producing lymphocytes. They were calculated by multiplying final cell numbers harvested from the secondary culture by numbers of cells used to start the primary cultures.
Additional experiments were done with the 2-step culture system to learn when NK-lineage cells become hormone insensitive. Cultures were initiated with an enriched fraction of LinLo/− bone marrow and β-estradiol was added to subgroups of sorted cells that were present 3 days later. As shown in Figure 8C, NK-cell production from either the CD122+ CD45R− or CD122+ CD45R+ subsets was unaffected by estrogen, whereas B-cell generation from the CD122−CD45R+ fraction was inhibited. Therefore, hormone insensitivity by maturing NK-lineage precursors corresponded to acquisition of CD122.
The IL-7 knockout mice have reduced numbers of NK cells34and the cytokine enhanced production of DX5+ cells in our cultures (data not shown). On the other hand, IL-7 is not thought to be essential for generation of NK cells in vivo.35 36 This accords with our finding that a substantial number of NK precursors do not display the IL-7R. Furthermore, B and NK lineages diverge at some point and can be independently driven by IL-7 and IL-15, respectively. Our results are compatible with a differentiation sequence where acquisition of the IL-15R corresponds with loss of B-lineage potential. Given the heterogeneity of precursors within the Lin−c-kitLo fraction discussed above, it was not surprising to see segregation of cell fates and marker expression during only 3 days of culture.
Discussion
Although substantial progress has been made in identifying early lymphocyte precursors, important questions remain about when the NK lineage diverges from those corresponding to other blood cell types. Our findings support and extend previous studies in showing that the largest population of NK-cell precursors is contained within an estrogen-sensitive, Lin− c-kitLoFlk-2/Flt3+ subset of bone marrow. Approximately half of the functional precursors display the CD127 receptor for IL-7, and the Lin− c-kitLo Flk-2/Flt3+ fraction gives rise to precursors expressing the CD122/IL-15R. At or prior to that event, NK-lineage cells become hormone insensitive and lose the potential for B-lineage differentiation. Clonal assays also showed that B and NK lineages are diverging at the Lin−c-kitLo stage. Acquisition of the CD45R/B220 marker was not indicative of differentiation fate. Whereas sex steroids may negatively control the abundance of precursors for all lymphocytes, IL-7 and IL-15 independently drive their differentiation in B- and NK-lymphocyte lineages.
Similarities with respect to cytotoxic capability and sharing of some surface markers suggest that T and NK cells may be closely related.37 Early precursors for NK and other lymphoid lineages can be found in the double-negative 1 fraction of the adult thymus.38 Furthermore, clonal assays were used to detect bipotential T/NK progenitors in the fetal thymus.6,39However, NK cells develop normally in athymic mice and other evidence suggests that most NK cells are normally produced in the bone marrow.7 The selective dependence of NK cells on the Ets-1 transcription factor and the Id-2 transcriptional repressor, as well as their relative independence from PU.1, are additional indications of their separation from the T-lymphocyte lineage.40-42 For these reasons, the focus of our study was on NK-cell precursors in bone marrow.
A series of previous studies suggested that an intact bone marrow microenvironment is required for differentiation of marrow precursors to functional NK cells.43 Some of those experiments used osteopetrotic mice that were treated for prolonged periods with high doses of estrogen. The hormone sensitivity of early lymphocyte precursors we have now found must be a consideration in the interpretation of such experiments. However, the new results do not detract from the overall conclusion that bone marrow is a principal site for NK-cell generation. The immature NK cells previously discovered in the spleens of osteopetrotic mice are likely similar to the estrogen-resistant CD122+ NK precursors described here.20 However, it is remarkable that functional maturation did not occur until the cells were isolated from that environment and stimulated with IL-15, an event that normally occurs within bone marrow. Furthermore, marrow destruction by the marrow-seeking isotope strontium 89 (89Sr) was also accompanied by functional NK deficiency.43 We conclude from these and other studies that the marrow environment is particularly well suited to the production and maturation of NK-cell precursors. Early stages in that process may be regulated by hormones.
Although acquisition of the full repertoire of NK-cell receptors requires interaction in trans with stromal cells,4,33 we found it possible to generate immature CD3−DX5+ NK-1.1+CD94+/− Ly49G2− NK cells under defined conditions. Serum-free, stromal cell–free methods originally developed for efficient propagation of early B-lymphocyte precursors were simply modified by addition of recombinant IL-15.26 29 Cloning efficiencies of up to 1 of 3.1 were obtained with Lin−c-KitLo bone marrow cells.
Because of their low abundance, early lymphohematopoietic precursors are usually studied in suspensions that have been depleted of lineage marker–bearing cells. We used CD3, CD8, Mac-1/CD11b, Gr-1/Ly-6G, TER-119, CD45R/B220, CD19, DX5, and CD2 antigens for this purpose and then further separated marrow cells according to the density of c-kit. Previous studies have shown that long-term repopulating stem cells and very early precursors are present in the c-kitHifraction.28,31,44 As just one indication of their position in the lineage, substantial time is required for Lin−c-KitHi to generate CD19+ lymphocytes in culture.31 Most Lin− TdT+ early pro-B cells and similar precursors that have been designated CLPs can be found in the Lin− c-KitLo fraction of marrow.24,26,31 The results of estrogen treatment experiments suggested that this represents a major pathway for B- and T-lineage lymphocyte differentiation.28,45 Some dendritic cells also likely derive from the Lin− c-kitLofraction.46 47 We now show that the same category, representing 0.15% of marrow nucleated cells, contained most of the functional precursors for NK cells.
Magnetic bead separation and high-speed sorting now make it possible to isolate many subsets of blood cell precursors and test their differentiation potential. However, it can be difficult to arrange them into a sequence corresponding to normal bone marrow. We used 2 hormone treatment strategies to determine that the Lin−c-KitLo fraction is an important intermediate in NK-cell production. Functional NK precursors, as well as B-cell precursors in the Lin− c-KitLo fraction were selectively depleted in estrogen-treated mice.
One previous study demonstrated that at least 1 of 20 Lin−c-KitLo IL-7Rα+ CLPs in bone marrow have the potential to generate B and T cells.24 Some NK-precursor activity was present in the same fraction, but single-cell analyses were not used to assess how they related to B/T-committed cells and our hormone treatment experiments suggested they might be heterogeneous. That is, Lin− c-KitLo cells with NK potential were more resistant than B-cell precursors when directly exposed to the hormone in culture. Indeed, the Lin− c-KitLoflk-2/flt3+ fraction was found by clonal analysis to contain B-restricted, NK-restricted, and common B/NK precursors. This was the case regardless of whether IL-7Rα was used as an additional sort criterion. We included Flk-2/Flt3 in all of those experiments because it excludes long-term repopulating stem cells and enriches for functional lymphocyte precursors.28,32,33,48,49 These results complement another study, where limiting-dilution analysis of a Lin− c-Kit+ Sca-2+ fraction of bone marrow showed that it contained the same 3 categories of precursors.4 Therefore, the NK and B lineages must diverge within or before a Lin− c-KitLoFlk-2/Flt3+ IL-7Rα+/− stage.
Environmental cues required for generation of early lymphocyte precursors remain poorly understood. They are present in IL-7Rα−/− mice,28 and NK-cell production in vivo appears to be IL-7 independent.35,36 On the other hand, addition of IL-7 to cultures of Lin−c-KitLo precursors enhanced generation of B- and NK-lineage cells (see Veiby et al50 and this report). Our analysis of the Lin− c-KitLo fraction revealed substantial differences in B- and NK-lineage precursors at that stage. Whereas nearly all of the former expressed CD127/IL-7Rα, this receptor was undetectable on half of the NK precursors. This result raises questions about whether it is appropriate to designate common lymphoid progenitors on the basis of CD127.24 Large numbers of NK cells may be generated without ever acquiring that particular receptor.
Progression of cells in the 2 lineages is independently driven by IL-7 and IL-15, respectively. Both cytokines use receptors containing γc, but responses to IL-15 require an IL-15–specific α chain, as well as the CD122 (IL-2Rβ) chain shared with IL-2R. Consistent with previous studies, we found that expression of CD122 signals NK-lineage commitment.6,9,23 In contrast to CD122, acquisition of CD45R/B220 by maturing precursors in our 2-step cultures did not correspond with gain or loss of NK-lineage potential. It has long been known that CD45R/B220 is not restricted to B cells and NK-lineage cells variably express this marker.51 52 We conclude that common positive signals support early stages of lymphohematopoiesis, whereas gradual segregation of lymphocyte lineages is accompanied by expression of functional receptors for different cytokines.
Our findings suggest that sex steroids negatively control production of early precursors corresponding to all lymphoid lineages. We previously found that estrogen and androgen receptors are down-regulated as cells progress from Lin− c-kitHi through Lin− c-kitLo and subsequent stages of B-lineage differentiation.53 Consequently, estrogen has no direct influence on CD19+ precursors in bone marrow.54 It now appears that maturing NK-lineage precursors also become hormone insensitive, beginning at the Lin− c-kitLo stage. Drawing on experience with B-lineage lymphocytes, a number of predictions can be made and addressed in future studies. For example, production of NK cells in marrow is likely to be suppressed during pregnancy and elevated in hypogonadal and castrated animals.55 56
It must be stressed that our experiments were conducted with adult marrow and NK-lineage differentiation pathways may be different during embryonic life. As in the case of our adult marrow experiments, acquisition of CD122 signals restriction of NK-precursor fate in fetal thymus.6 However, fetal and neonatal NK cells express high levels of CD94/NKG2A receptors, but not Ly49. Subsequently, CD94/NKG2A gradually declines as the Ly49 family of receptors are acquired.10 Although single cells with restricted lymphoid potential are present in adult marrow 9,24,26,46 (and this report), B/T-cell precursors have not been detected in fetal tissues.57 Furthermore, we have recently found that early lymphocyte precursors do not acquire functional receptors for sex steroids until after birth.53 It will be extremely important to learn if fetal and adult lymphocytes derive from separate pools of stem cells or pathways of differentiation.
Many details remain about when and how early precursors of NK cells become distinct from those corresponding to other blood cells. However, the present findings provide a better framework for understanding this process. Gene-targeting experiments should reveal precisely when particular factors such as Ets-1 and Id-2 are required, whereas studies on cell labeling should now be performed to learn about population dynamics, turnover rates, and NK-cell lifespans.
The authors thank Dr Lisa Borghesi for critical reading of the manuscript, Ms Viji Dandapani for cell sorting, and Dr Takafumi Yokota for experimental help and suggestions.
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2002-02-0653.
Supported by grant AI20069 and AI33085 from the National Institutes of Health. P.W.K. holds the William H. and Rita Bell Chair in biomedical research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Paul W. Kincade, Immunobiology and Cancer Program, Oklahoma Medical Research Foundation, 825 NE 13th St, Oklahoma City, OK 73104; e-mail:kincade@omrf.ouhsc.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal