The idiopathic pneumonia syndrome (IPS) represents a common and often fatal complication of hematopoietic stem cell transplantation (HSCT). Gelsolin is a highly conserved actin-binding protein normally present in plasma that may serve a basic physiological role in limiting acute lung injury of diverse etiologies. We hypothesized that depletion of circulating gelsolin following HSCT might play a permissive role in the pathogenesis of IPS. Plasma gelsolin levels were measured by immunoblotting in frozen samples obtained weekly from 24 patients undergoing allogeneic HSCT. Patients with and without IPS were similar with respect to age, diagnosis, histocompatibility differences between donor and recipient, and conditioning regimen. Mean gelsolin levels in the 9 patients with rapidly fatal IPS were significantly lower than those in patients without this complication by week 3 after HSCT (101 ± 61 mg/L versus 221 ± 54 mg/L; P = .0002). Seven (88%) of the 8 patients with gelsolin levels of less than 100 mg/L in the first month after HSCT died from IPS within 3 months; conversely, gelsolin levels fell to less than 100 mg/L in 7 (78%) of the 9 patients who died from IPS within 3 months of HSCT (P = .0007). These findings suggest that gelsolin levels shortly after allogeneic HSCT can predict the later development of fatal IPS. Gelsolin replacement in selected transplant patients may offer a novel strategy to prevent or reverse IPS.
Introduction
Hematopoietic stem cell transplantation (HSCT) after high-dose chemotherapy has become standard treatment for many hematologic disorders.1,2 However, the procedure is associated with significant morbidity and mortality. Acute lung injuries of diverse causes are common and potentially lethal complications of transplantation.3 Idiopathic pneumonia syndrome (IPS) may be fatal in up to 80% of affected patients.3-7 While reduced pulmonary function prior to HSCT may predispose patients to the subsequent development of IPS, the pathophysiology of this disorder remains unclear.8-11
Cytoplasmic actin leaked from dying cells may be directly toxic to pulmonary endothelium12 and obstruct the microcirculation of the lungs.13 Actin-scavenging proteins appear to counteract the pathophysiological consequences of extracellular actin, but the capacity of this defense system is predictably overwhelmed by massive tissue injury.14-18 Gelsolin and Gc-globulin (vitamin D–binding protein) function as plasma actin sequestering proteins. After binding actin, the complexes are quickly cleared from the circulation, thus protecting the host from further damage. Gelsolin also affects the presentation of inflammatory phospholipids, such as lysophosphatidic acid (LPA), to vascular endothelial cell receptors.19-22
Experimental models and clinical observations have correlated acute lung injury and low gelsolin levels. Oleic acid–induced lung injury in rats is associated with detectable actin and decreased levels of gelsolin in the circulation.23 Rapid infusions of exogenous G-actin reduce the concentration of Gc-globulin and cause pulmonary endothelial and vascular injury in rat lungs.13Platelet thrombi enmeshed in a dense network of F-actin bundles are found in the pulmonary arterioles. These angiopathic changes can be averted if G-actin is incubated with Gc globulin before infusion. In patients with established acute respiratory distress syndrome (ARDS), plasma gelsolin concentrations are lower than those in healthy adults and patients with uncomplicated bacterial pneumonia.24 In a recent study of 65 patients who had sustained traumatic injuries, 10 (77%) of the 13 patients with gelsolin levels more than 2 SDs below the normal mean at the time of admission required prolonged mechanical ventilation, developed ARDS, and/or died during the ensuing hospitalization.25 Gelsolin levels fell dramatically as severe lung injury developed in mice exposed to high concentrations of inspired oxygen; infusion of recombinant human gelsolin diminished the acute inflammatory response.26
We hypothesized that cellular injury resulting from HSCT conditioning regimens could variably lower gelsolin levels.27-31Patients with markedly depleted plasma gelsolin might be at high risk for developing IPS. In this study, gelsolin levels were measured retrospectively in patients who had undergone HSCT, with identical conditioning and graft versus host disease (GVHD) prophylaxis, to determine whether the degree of reduction in plasma gelsolin levels could predict the later development of IPS and respiratory failure. Given the preliminary experimental evidence that gelsolin repletion can diminish the inflammatory response in acute lung injury, our findings could have important therapeutic implications.
Patients, materials, and methods
Patient selection and sample procurement
We performed an observational case-control study to investigate the relationship of gelsolin levels and pulmonary complications in patients following HSCT. We focused on patients who developed fatal IPS in the first 3 months after transplantation. The diagnoses were established by the attending oncologist for each patient and confirmed by one of the investigators. The 24 patients in this study represent a subset of a cohort of 185 patients enrolled in a randomized, placebo-controlled trial of interleukin-1 receptor antagonist (IL-1Ra) as prophylaxis for GVHD. All patients received cyclophosphamide (1800 mg/m2) on 2 consecutive days followed by 13.6 Gy total body irradiation in 8 fractions in the week prior to stem cell infusion. Cyclosporine was administered at a dose of 2.5 mg/kg intravenously every 12 hours beginning on day −3. Methotrexate was given at a dose of 15 mg/m2 on day 1, followed by 10 mg/m2 on days 3, 6, and 11. The study drug consisted of either recombinant human IL-1Ra (Amgen, Thousand Oaks, CA), given at a dose of 0.5 mg/kg/h by continuous intravenous administration from day −4 through day 10, or saline placebo. Blood was obtained from patients before HSCT for treatment of leukemia or myelodysplasia and at weekly intervals thereafter to measure cytokine levels.
For the present study, archived plasma specimens were selected from 13 patients who developed fatal respiratory failure at various times after HSCT and 11 patients with similar clinical characteristics but without serious pulmonary complications (although 1 patient had nonlethal IPS). These 11 control patients were matched to the study patients for diagnosis, stem cell donor, and treatment regimen. The primary protocol as well as ancillary analyses were approved by the institutional review boards of Dana-Farber Cancer Institute and Brigham and Women's Hospital.
Measurement of plasma protein concentrations
Albumin, gelsolin, and Gc-globulin concentrations were measured on archived plasma samples obtained between week −1 and week 11 after HSCT and stored for various lengths of time at −60°C by one of us (M.J.D.) who was blinded to the clinical course. The levels were later correlated with patient outcomes specifically related to the presence or absence of IPS. Albumin levels in the plasma samples were assayed by standard procedures in the clinical chemistry laboratory of Cooper Hospital, Camden, NJ.
Gelsolin levels were determined on thawed specimens diluted 1:100 with gel sample buffer by quantitative immunoblotting.25Different aliquots ranging from 2 to 8 μL were applied to 10% sodium dodecyl sulfate (SDS)–polyacrylamide minigels and transferred onto Immobilon-P membrane (Millipore, Bedford, MA). Human plasma gelsolin (Cytoskeleton, Denver, CO) was loaded as standards in at least 5 lanes of each gel in a concentration range comparable to the gelsolin concentrations in the samples. Rainbow-colored molecular weight markers (Amersham Pharmacia Biotech, Piscataway, NJ) ranging from 14 to 220 kDa were used to confirm the adequacy of the transfer from gel to blotting membrane. Membranes were incubated overnight at room temperature with monoclonal antibodies to human plasma gelsolin (GS-2C4; Sigma, St Louis, MO), followed by a peroxidase-conjugated sheep antimouse IgG antibody (Sigma) for approximately 4 hours. Quantitative immunoblotting for Gc-globulin was similarly performed on the same specimens, using rabbit polyclonal antibodies to human Gc-globulin (Dako, Carpinteria, CA); peroxidase-conjugated goat antirabbit IgG (Sigma) was used as the secondary antibody. Serial dilutions of Gc-globulin purified from human plasma were used as standards.
Enhanced chemiluminescence (Amersham, Arlington Heights, IL) was used to detect gelsolin and Gc-globulin bands. The integrated intensity of each band on the Western blot, minus the adjacent background signal, was quantified by the AMBIS Image Acquisition and Analysis system (San Diego, CA). Plots of band intensity versus known amounts of protein were fitted to lines drawn by Cricket Graph 1.3.2 (Cricket Software, Malvern, PA); lines with r greater than 0.94 were used to derive the gelsolin and Gc-globulin concentrations of the samples.
Statistical methods
All protein concentrations measured by immunoblotting were determined as the average of at least 2 duplicate independent blotting assays. Analyses of variance (ANOVAs) were used to determine whether a statistically significant difference existed between the different outcome groups over time. When significant differences (P ≤ .05) were found, pairwise comparisons were evaluated by t test, using the Bonferroni correction for multiple comparisons. Frequency distributions were compared by the Fisher exact test. Mixed-model repeated-measures analysis was performed to account for the covariance structure in the data.32
Results
Changes in gelsolin levels following HSCT
A total of 24 patients undergoing allogeneic peripheral HSCT were studied. The most common underlying diseases in this group were acute (n = 6; 25%) and chronic (n = 13; 54%) myeloid leukemia (Table1). Fourteen patients died within the first year following HSCT (median interval of 35 days [range, 20-239 days] after transplantation). Ten patients developed IPS and died less than 3 months after HSCT. Death was directly related to IPS in 9 of these cases (range, 20-50 days after transplantation) and to relapsed AML in 1 patient (73 days after transplantation). Four other patients died from treatment-associated complications culminating in respiratory failure between 134 and 239 days following transplantation. Ten patients survived at least one year after HSCT (range, 590-≥1462 days after transplantation), including 1 patient who had recovered from IPS but experienced a fatal relapse of underlying leukemia more than 2 years later. Patients with and without fatal IPS were similar with respect to age, diagnosis, histocompatibility differences between donor and recipient, and conditioning regimen.
Selected characteristics of 24 patients undergoing allogeneic peripheral HSCT
| . | Patients with early IPS-related death (n = 9) . | All others (n = 15) . |
|---|---|---|
| Age, median (range), y | 43 (19-48) | 40 (24-60) |
| Sex, no. | ||
| Male | 5 | 6 |
| Female | 4 | 9 |
| Stem cell source, no. | ||
| Histocompatible family member | 6 | 11 |
| Unrelated donor | 3 | 4 |
| Diagnosis, no. | ||
| CML (stable phase) | 5 | 8 |
| AML | ||
| In first remission | 2 | 1 |
| In subsequent remission | 1 | 2 |
| RA/RARS | 0 | 1 |
| RAEB, RAEB-T | 1 | 2 |
| CLL | 0 | 1 |
| Acute GVHD grade,33 no. (%) | 2 (22) | 11 (73) |
| 0-I | 7 | 13 |
| II-IV | 2 | 2 |
| 0-A | 7 | 8 |
| B-D | 2 | 7 |
| Veno-occlusive disease, no. (%) | 7 (78) | 5 (33) |
| Death within 1 y after transplantation, no. (%) | 9 (100) | 5 (33) |
| Survival after transplantation, median (range), d | 28 (20-50) | 1124 (73-≥1462) |
| . | Patients with early IPS-related death (n = 9) . | All others (n = 15) . |
|---|---|---|
| Age, median (range), y | 43 (19-48) | 40 (24-60) |
| Sex, no. | ||
| Male | 5 | 6 |
| Female | 4 | 9 |
| Stem cell source, no. | ||
| Histocompatible family member | 6 | 11 |
| Unrelated donor | 3 | 4 |
| Diagnosis, no. | ||
| CML (stable phase) | 5 | 8 |
| AML | ||
| In first remission | 2 | 1 |
| In subsequent remission | 1 | 2 |
| RA/RARS | 0 | 1 |
| RAEB, RAEB-T | 1 | 2 |
| CLL | 0 | 1 |
| Acute GVHD grade,33 no. (%) | 2 (22) | 11 (73) |
| 0-I | 7 | 13 |
| II-IV | 2 | 2 |
| 0-A | 7 | 8 |
| B-D | 2 | 7 |
| Veno-occlusive disease, no. (%) | 7 (78) | 5 (33) |
| Death within 1 y after transplantation, no. (%) | 9 (100) | 5 (33) |
| Survival after transplantation, median (range), d | 28 (20-50) | 1124 (73-≥1462) |
Patients with and without early fatal IPS did not differ significantly with respect to age, sex, stem cell source, underlying disease, or conditioning regimen.
CML indicates chronic myelogenous leukemia; AML, acute myelogenous leukemia; RA, refractory anemia; RARS, refractory anemia with ringed sideroblasts; RAEB, refractory anemia with excess blasts; RAEB-T, RAEB in transformation; CLL, chronic lymphocytic leukemia.
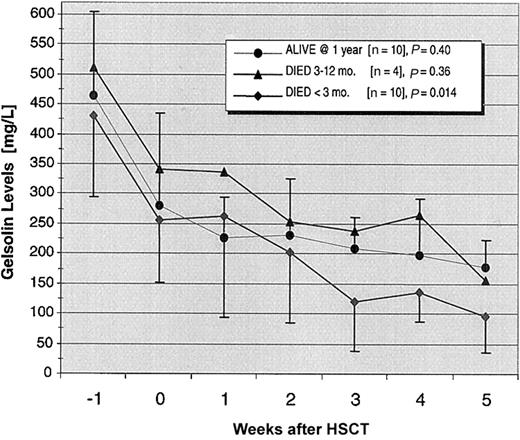
Gelsolin levels dropped precipitously in the week prior to HSCT in all patients while they were receiving their conditioning regimens and continued to fall during the subsequent 3 weeks (P = .0001; Figure 1). The decline in gelsolin levels from the time of HSCT through the first 5 weeks after transplantation reached statistical significance only in the 10 recipients who died within 3 months of transplantation (P = .014).
Declining gelsolin levels in patients undergoing allogeneic stem cell transplantation.
The 24 patients in the study were divided into 3 groups: 10 one-year survivors, 10 early deaths (< 3 months), and 4 late deaths (3-12 months) following transplantation. Mean gelsolin levels fell precipitously during the conditioning period in all groups. For the next 5 weeks, gelsolin levels declined significantly (P = .014) only in the patients who died within 3 months after transplantation. SDs are shown only for the 1-year survivors and patients experiencing early deaths.
Declining gelsolin levels in patients undergoing allogeneic stem cell transplantation.
The 24 patients in the study were divided into 3 groups: 10 one-year survivors, 10 early deaths (< 3 months), and 4 late deaths (3-12 months) following transplantation. Mean gelsolin levels fell precipitously during the conditioning period in all groups. For the next 5 weeks, gelsolin levels declined significantly (P = .014) only in the patients who died within 3 months after transplantation. SDs are shown only for the 1-year survivors and patients experiencing early deaths.
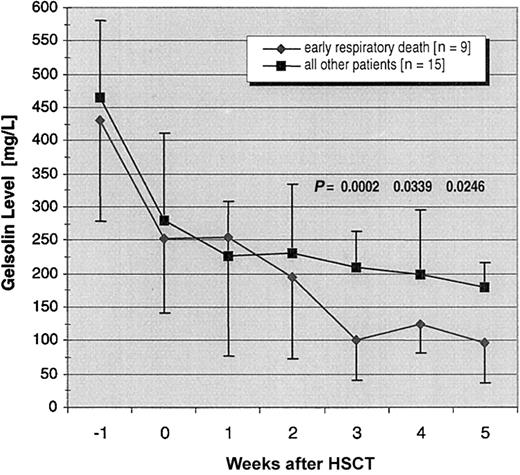
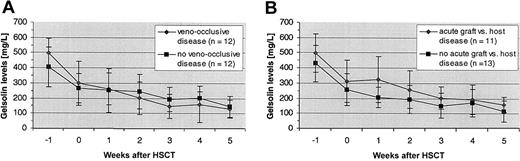
Seven (88%) of the 8 patients with gelsolin levels of less than 100 mg/L during weeks 1 through 5 after transplantation died of IPS within 2 months; conversely, 7 (78%) of 9 patients experiencing an early IPS-related death had gelsolin levels of less than 100 mg/L during this period (P = .0007). Nadir gelsolin levels in the 2 other patients who died of IPS in the 3 months after HSCT were 113 and 192 mg/L. Over time, gelsolin levels fell significantly more in the 9 patients who died of IPS-induced respiratory failure than in the remaining 15 patients (P = .009; Figure2). By 3 weeks after transplantation, mean gelsolin levels in the 9 patients with rapidly fatal IPS were significantly lower than levels in patients without this complication (101 ± 61 mg/L versus 221 ± 54 mg/L; P = .0002). Differences in baseline gelsolin levels were a covariate in the analysis, with lower baseline levels associated with lower follow-up levels (P = .001). Gelsolin levels were modestly lower in patients with veno-occlusive disease than in those without veno-occlusive disease by 2 weeks after transplantation, whereas the reverse trend was seen in patients with acute GVHD (Figure3); none of these differences approached statistical significance.
Declining gelsolin levels in patients dying of IPS within 3 months of transplantation contrasted with all other patients.
Mean gelsolin levels with SDs in the 9 patients who died from IPS in the early posttransplantation period are compared with those of the other 15 patients in the cohort (P < .01). The Pvalues shown in the figure are for pairwise comparisons between the 2 groups at 3, 4, and 5 weeks after transplantation, corrected for multiplicity. One patient, whose gelsolin level was 70 mg/L at week 2, died before the third week. Two other patients, with gelsolin levels of 70 mg/L and 72 mg/L at week 3, did not survive until week 4. A fourth patient, whose gelsolin levels were 94 mg/L at week 3 and 117 mg/L at week 4, died before week 5.
Declining gelsolin levels in patients dying of IPS within 3 months of transplantation contrasted with all other patients.
Mean gelsolin levels with SDs in the 9 patients who died from IPS in the early posttransplantation period are compared with those of the other 15 patients in the cohort (P < .01). The Pvalues shown in the figure are for pairwise comparisons between the 2 groups at 3, 4, and 5 weeks after transplantation, corrected for multiplicity. One patient, whose gelsolin level was 70 mg/L at week 2, died before the third week. Two other patients, with gelsolin levels of 70 mg/L and 72 mg/L at week 3, did not survive until week 4. A fourth patient, whose gelsolin levels were 94 mg/L at week 3 and 117 mg/L at week 4, died before week 5.
Gelsolin levels in patients with and without veno-occlusive disease and acute GVHD.
(A) Mean gelsolin levels were slightly lower in patients with veno-occlusive disease than without veno-occlusive disease after HSCT. (B) An opposite trend was seen in patients with acute GVHD.
Gelsolin levels in patients with and without veno-occlusive disease and acute GVHD.
(A) Mean gelsolin levels were slightly lower in patients with veno-occlusive disease than without veno-occlusive disease after HSCT. (B) An opposite trend was seen in patients with acute GVHD.
Last measured gelsolin level versus survival time
In the 9 patients with respiratory deaths due to IPS within 3 months of HSCT, there was a significant association between the patients' last gelsolin measurements and survival time following transplantation (r = 0.71; P = .02). When the group of patients in this analysis was expanded to include the other 4 patients who died between 3 and 12 months after transplantation, the last available gelsolin level remained significantly correlated with life expectancy for the total group of 13 patients who died of respiratory failure within the first year after HSCT (r = 0.81; P = .0005).
In the survival analysis of all patients, censoring at 35 weeks, lower gelsolin levels were associated with a higher chance of dying, with an estimated hazard ratio of 0.984 (95% confidence interval [CI], 0.974-0.994; P = .002). When the analysis was restricted to the 9 patients who died of IPS within 3 months of transplantation, the hazard ratio was 0.977 (95% CI, 0.963-0.991;P = .0013). Restated more intuitively, the likelihood of survival decreased by an estimated 2.3% for each 1-mg/L decrease in gelsolin levels.
Correlation between plasma levels of gelsolin, Gc-globulin, and albumin
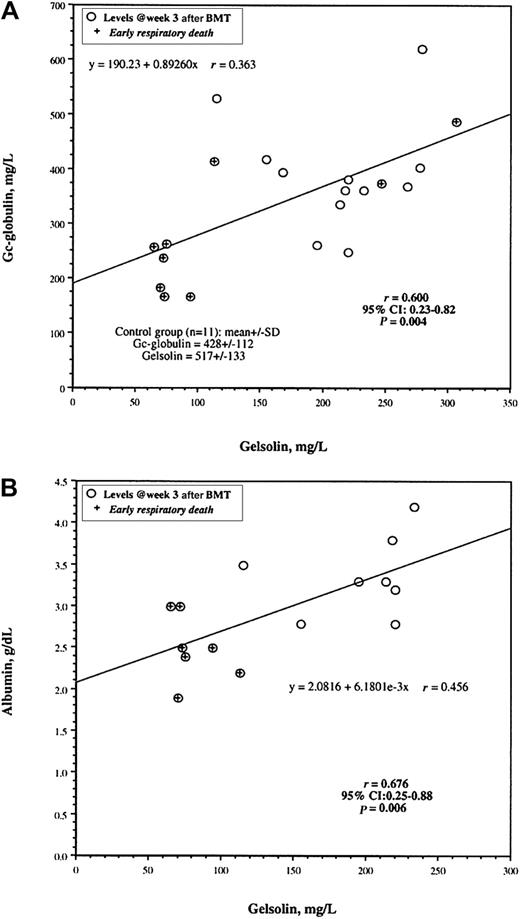
Gelsolin and Gc-globulin levels were measured on the same 21 patients, whereas albumin levels were determined on a subset of 15 patients. Gelsolin levels correlated with Gc-globulin and albumin levels on the samples taken at week 3 after transplantation (Figure 4). There was less overlap in gelsolin values than in albumin values at this time point for patients with and without early death from IPS.
Correlation between gelsolin levels and levels of Gc-globulin and albumin.
Levels of gelsolin and either Gc-globulin (n = 21; panel A) or albumin (n = 15; panel B) are plotted for individual patients. Crosses indicate patients dying of early IPS-induced respiratory failure. r values are the Pearson correlation coefficients. Regression lines were fitted by Cricket Graph version 1.3.2 (Cricket Software, Malvern, PA). Mean gelsolin and Gc-globulin levels with SDs assayed by immunoblotting are given for 11 healthy volunteers in the lower left corner of panel A.
Correlation between gelsolin levels and levels of Gc-globulin and albumin.
Levels of gelsolin and either Gc-globulin (n = 21; panel A) or albumin (n = 15; panel B) are plotted for individual patients. Crosses indicate patients dying of early IPS-induced respiratory failure. r values are the Pearson correlation coefficients. Regression lines were fitted by Cricket Graph version 1.3.2 (Cricket Software, Malvern, PA). Mean gelsolin and Gc-globulin levels with SDs assayed by immunoblotting are given for 11 healthy volunteers in the lower left corner of panel A.
Discussion
The development of IPS continues to be a frequent and life-threatening complication of HSCT.7 Pulmonary complications occur in more than half of HSCT recipients and are responsible for as much as 50% of transplant-related mortality.3,5-7 In cases where infection cannot be identified, widespread alveolar injury has been designated IPS.4 Potential causative factors include the direct toxic effects of conditioning regimens, unrecognized viral infections, inflammatory cytokines generated by tumor necrosis, and collateral injury from inflammatory cells attracted to injured epithelium.27-31 As with our patients, acute GVHD has not been consistently associated with the development of IPS.33 Preclinical data from murine HSCT models of IPS demonstrate that tumor necrosis factor α (TNFα) is a necessary but not sufficient contributor to IPS.31
Previous observations on the development of ARDS after trauma implicated depletion of circulating gelsolin in the pathogenesis of respiratory failure.25 The present data on patients undergoing HSCT indicate that reductions in plasma gelsolin levels begin at the time of transplant conditioning and progressively decline in patients who subsequently progress to develop respiratory failure. Presumably the conditioning regimen of radiation and chemotherapy produces tissue damage that depletes circulating gelsolin by exposing intracellular actin through cell damage. Patients whose gelsolin concentrations fall below 100 mg/L appear to be at a much higher risk of developing fatal IPS in the early posttransplantation period than patients in whom gelsolin levels are maintained at modestly higher levels. Marked depletion of plasma gelsolin after major trauma is likewise associated with secondary lung injury and a high mortality rate.25 Although albumin levels also decrease after trauma and HSCT, they are not as closely correlated with clinical outcomes as plasma gelsolin concentrations.
Although fatal respiratory failure follows the marked drop in gelsolin levels, we cannot prove causality simply from these observational data. Gelsolin depletion may simply reflect early lung injury. However, in both a murine model of hyperoxic lung injury26 and a rat model of burn-induced pulmonary capillary leak syndrome, gelsolin administration near the time of injury diminished the resultant lung damage. Serial measurements of plasma gelsolin levels in patients after HSCT indicate that gelsolin levels of 100 mg/L or less may predict the development and prognosis of IPS 1 to 2 weeks before respiratory failure ensues. Furthermore, after identification of patients at high risk of developing severe IPS, preemptive administration of recombinant human gelsolin may prevent this complication or abort its progression to acute respiratory failure.
We are indebted to Shelley Hurwitz, Division of Endocrinology, Departments of Medicine, Brigham and Women's Hospital, Harvard Medical School, for invaluable statistical advice.
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2002-06-1672.
Supported by the Edwin S. Webster Foundation and the Jock and Bunny Adams Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mark DiNubile, Merck Research Laboratories, PO Box 4, BL3-4, West Point, PA 19486; e-mail:mark_dinubile@merck.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal