Adult human bone marrow (ABM) is an important source of hematopoietic stem cells for transplantation in the treatment of malignant and nonmalignant diseases. However, in contrast to the recent progress that has been achieved with umbilical cord blood, methods to expand ABM stem cells for therapeutic applications have been disappointing. In this study, we describe a novel culture method that uses human brain endothelial cells (HUBECs) and that supports the quantitative expansion of the most primitive measurable cell within the adult bone marrow compartment, the nonobese diabetic/severe combined immunodeficient (NOD/SCID) repopulating cell (SRC). Coculture of human ABM CD34+ cells with brain endothelial cells for 7 days supported a 5.4-fold increase in CD34+ cells, induced more than 95% of the CD34+CD38− subset to enter cell division, and produced progeny that engrafted NOD/SCID mice at significantly higher rates than fresh ABM CD34+ cells. Using a limiting dilution analysis, we found the frequency of SRCs within fresh ABM CD34+ cells to be 1 in 9.9 × 105 cells. Following HUBEC culture, the estimated frequency of SRCs increased to 1 in 2.4 × 105cells. All mice that received transplants of HUBEC-cultured cells showed B-lymphoid and myeloid differentiation, indicating that a primitive hematopoietic cell was preserved during culture. Noncontact HUBEC cultures also maintained SRCs at a level comparable to contact HUBEC cultures, suggesting that cell-to-cell contact was not required. These data demonstrate that human brain endothelial cells possess a unique hematopoietic activity that increases the repopulating capacity of adult human bone marrow.
Introduction
The development of ex vivo culture methods that promote the expansion of adult human bone marrow (ABM) stem cells would have direct application in clinical gene therapy and stem cell transplantation. However, results obtained from stroma-based1 and stroma-free ex vivo culture systems2-5 have been disappointing, owing to insufficient activation of primitive CD34+CD38− cells, cell differentiation, and a loss of repopulating capacity following short-term culture.6 Moreover, increased CD34+ cell numbers, colony-forming cells (CFCs), and long-term culture initiating cells (LTC-ICs) are not quantitative indicators of in vivo repopulating potential.7-10Therefore, the importance of evaluating ex vivo cultured cells in an in vivo repopulation model has been emphasized.8
The nonobese diabetic/severe combined immunodeficient (NOD/SCID) model system has been used to measure the long-term reconstitution potential of ex vivo–expanded human lymphohematopoietic stem cells.7-10 SCID-repopulating cells (SRCs) are enriched in human cord blood (CB) as compared with adult ABM and mobilized peripheral blood11,12 and are most highly concentrated within the CD34+CD38−population.8 SRCs are considered to be biologically more primitive than assayable LTC-IC and CFC progenitors,1,9,10,13 which are found in both the CD34+CD38+ and the CD34+CD38− pools.8 As further evidence, gene transduction studies have shown that LTC-ICs and CFCs are readily transduced but contribute little to NOD/SCID engraftment.2 7
When human hematopoietic stem cells (HSCs) are cocultured in contact with bone marrow stroma or conditioned medium from stromal cultures, a percentage of LTC-ICs and CFCs can be expanded in vitro over several weeks.14-17 Similarly, bone marrow–, umbilical vein–, and yolk sac–derived endothelial cell cultures elaborate growth factors that regulate hematopoiesis18-20 and support the proliferation of myeloid, erythroid, and megakaryocytic progenitors.19,20 We have previously demonstrated that a porcine brain microvascular endothelial cell line (PMVEC) plus cytokines was capable of supporting a robust expansion of human CD34+CD38− progenitors21,22 while maintaining cells capable of repopulating SCID human (SCID-Hu) bone23 as well as cells capable of rescuing lethally irradiated baboons.24 In these studies, we did not quantify the frequency of repopulating cells in the transplanted grafts.
Recent studies using rigorous limiting dilution analyses have demonstrated that both stroma-containing and stroma-free culture conditions can support the quantitative expansion of SRCs within human CB.25-28 However, the ex vivo expansion of human ABM stem cells under similarly stringent conditions has not been demonstrated. In fact, a recent limiting dilution analysis demonstrated a 6-fold decline in SRCs within human ABM during short-term culture with ABM stroma.1 In this study, using quantitative limiting dilution analysis, we demonstrate that the number of engraftable SRCs within human ABM increases following coculture with primary human brain endothelial cells (HUBECs). The HUBEC ex vivo culture system has potential application in the expansion of ABM stem cells for clinical transplantation and will also be a new resource for the identification of molecules that affect stem-cell self-renewal.
Materials and methods
Isolation of primary HUBECs
Vessel segments (smaller than 10 cm) from the central nervous system (CNS) and outside the CNS (renal artery) were collected from cadavers within 12 hours after death under an approved tissue-procurement protocol. Vessel segments were placed in complete endothelial cell culture medium containing M199 (GIBCO/BRL, Gaithersburg, MD), 10% heat-inactivated fetal bovine serum (FBS) (Hyclone, Logan, UT), 100 μg/mLl-glutamine, 50 μg/mL heparin, 30 μg/mL endothelial cell growth supplement (Sigma, St Louis, MO), 100 U/mL penicillin, and 100 μg/mL streptomycin.
Vessels were incised longitudinally and oriented in such a fashion that the lumen side contacted the dish surface during in vitro culture. Well-developed endothelial cell colonies were evident by day 14, and confluent monolayers were achieved by day 30 of culture. Colonies were fed weekly with complete medium, and several passages of the primary cells were banked.
CD34+ cells plus HUBEC coculture
Purified human ABM CD34+ cells were obtained from Poietics Technologies (Gaithersburg, MD). HUBECs were subcultured at 1 × 105 cells per well in gelatin-coated 6-well plates (Costar, Cambridge, MA) as previously described.22After 72 hours, HUBEC monolayers were washed with phosphate-buffered saline (PBS), and the spent medium was replaced with ex vivo expansion culture medium (5 mL per well) consisting of Iscove modified Dulbecco medium (IMDM) (GIBCO/BRL) containing 10% FBS, 200 μMl-glutamine, 2 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF), 5 ng/mL interleukin 3 (IL-3), 5 ng/mL IL-6, 120 ng/mL stem cell factor (SCF), and 50 ng/mL flt-3 ligand (R & D Systems, Minneapolis, MN) to each well. Purified ABM CD34+ cells (1 × 105) were added to each well, and cultures were maintained at 37°C in 5% CO2 atmosphere. After 7 days of culture, nonadherent cells were harvested by washing the monolayers gently with warm complete culture medium. In the HUBEC noncontact cultures, ABM CD34+ cells were plated in the upper compartment of the culture well, separated from HUBEC monolayers by transwell inserts (0.4 μm) (Costar).
Immunofluorescence staining and cell cycle analysis
ABM CD34+ cells and cultured cells were stained with monoclonal antibodies against CD34–fluorescein isothiocyanate (FITC) and CD38–phycoerythrin (PE) (Becton Dickinson [BD], San Jose, CA) and analyzed by Epics Elite fluorescence-activated cell sorter (FACS) (Coulter, Hialeah, FL). Controls consisted of isotype-matched monoclonal antibodies (mAbs). We performed the surface, intracellular, DNA (SID) cell cycle analysis as previously described29 using anti-CD34–allophycocyanin (APC) (BD), CD38-PE (BD), Ki-67–FITC (Immunotech, Westbrook, ME), and 7-amino-actinomycin D (7-AAD) (Sigma). Isotype controls were performed in parallel for each sample.
In vitro methylcellulose colony forming assays
Purified ABM CD34+ cells and ex vivo cultured cells (5 to 500 × 102) were cultured in 35-mm culture dishes (Miles Laboratories, Naperville, IL) as previously described.22 Culture media consisted of 1 mL IMDM, 1% methylcellulose, 30% FBS, 10 U/mL erythropoietin, 2 ng/mL GM-CSF, 10 ng/mL IL-3, and 120 ng/mL SCF. At day 14, we evaluated triplicate cultures to determine the number of colonies (larger than 50 cells) per dish. NOD/SCID marrow cells were washed × 2 and placed (1 × 105) in methylcellulose containing culture media containing the above-noted human cytokines and analyzed at day 14 for evidence of human colonies.
Transplantation of fresh ABM CD34+ cells and HUBEC-cultured cells in NOD/SCID mice
NOD/SCID mice30 received transplants of either fresh purified ABM CD34+ cells or the progeny of ABM CD34+ cells cultured with HUBECs supplemented with GM-CSF plus IL-3 plus IL-6 plus SCF plus flt-3 ligand over a range of doses designed to achieve no engraftment in a significant fraction. To avoid donor variability, HUBEC cultures were established with the identical ABM CD34+ cells as used for transplantation into mice designated the “fresh ABM CD34+” group. Cells were transplanted via tail vein injection after irradiating the mice with 300 cGy by means of a 137Cs source as previously described.31 The mice received no CD34−accessory cells or exogenous cytokines to facilitate engraftment. Mice were killed at week 8, and marrow samples were obtained by flushing their femurs and tibias with IMDM at 4°C.
Flow cytometric analysis was performed as previously described with the use of commercially available monoclonal antibodies against human leukocyte differentiation antigens to identify engrafted human leukocytes and discriminate their hematopoietic lineages.31 Immunofluoresence staining of marrow cells was performed following our previously published procedures.31
Statistical analysis
For purposes of our limiting dilution assays, we scored a mouse that underwent transplantation as positive if at least 1% of the marrow cells expressed human CD45 via FACS analysis, based upon the engraftment criteria established by Ueda et al.27 We calculated the SRC frequency in each cell source using the maximum likelihood estimator as described previously by Taswell32 for the single-hit Poisson model.12,27 The χ2 provides a measure of the legitimacy of using pooled data and of the validity of applying the single-hit model.12 30 We calculated confidence intervals for the frequencies using the profile likelihood method. As a confirmation of the maximum likelihood estimator, we also applied a minimum χ2 estimator to the pooled data.
Results
HUBEC coculture supports ABM progenitor cell proliferation and expansion
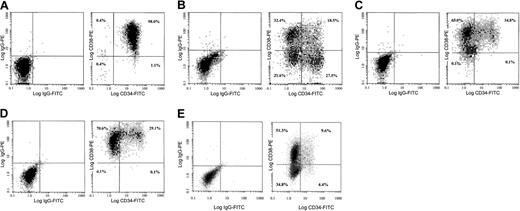
HUBECs displayed cobblestone morphology at confluence and more than 90% expressed von Willebrand factor, but we did not detect CD34 or CD38 expression by flow cytometry (data not shown). The effects of HUBEC contact and noncontact coculture, liquid suspension culture, and human nonbrain endothelial cell culture on CD34+ cell expansion and CFC generation were compared. All cultures were supplemented with GM-CSF plus IL-3 plus IL-6 plus SCF plus flt-3 ligand because our previous studies indicated that this combination optimized the expansion of ABM CD34+ cells.21 22HUBEC culture supported a 16.1-fold increase in total cells, a 5.4-fold increase in CD34+ cells, and a 212-fold increase in the CD34+CD38− subset (n = 8) (Table1). CD34+CD38−cells increased from 1.6% of the total population at day 0 to 21.6% at day 7 and constituted 64% of the day-7 CD34+ cell pool. HUBEC noncontact cultures supported a 16.4-fold expansion of total cells, a 2.8-fold increase in CD34+ cells, and a 35-fold increase in the CD34+CD38− population. In contrast, liquid suspension cultures and nonbrain endothelial cell cultures supported similar increases in total cells and CD34+ cells, but neither maintained CD34+CD38− cells at day 7 (Figure 1A-E) (Table 1).
HUBEC coculture promotes the expansion of human CD34+ subsets and CFCs compared with controls
| Conditions . | Cell yield . | No. cells procured × 105 . | No. CFCs × 104 . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| CD34+ . | CD34+CD38+(%) . | CD34+CD38−(%) . | CFU-GMs . | BFU-Es . | CFU-Mix's . | |||
| Input | 5.0 | 5.0 | 4.91 ± 0.07 (98.4) | 0.08 ± 0.04 (1.6) | 3.7 ± 0.9 | 0.6 ± 0.3 | 0.5 ± 0.2 | 4.7 ± 1.4 |
| HUBECs | 81.0 ± 3.4 (16.1-fold) | 27.0 ± 1.1 (5.4-fold) | 9.0 ± 0.4 (12.3) | 17.0 ± 0.5 (21.6) | 56.0 ± 2.2 | 4.8 ± 0.6 | 2.6 ± 0.3 | 63.4 ± 2.5 |
| HUBECs, noncontact | 82.0 ± 3.7 (16.4-fold) | 14.0 ± 0.1 (2.8-fold) | 10.4 ± 0.3 (13.0) | 2.8 ± 0.1 (3.4) | 30.0 ± 1.3 | 6.0 ± 0.5 | 4.3 ± 0.9 | 40.3 ± 0.5 |
| Stroma-free | 51.0 ± 1.3 (10.2-fold) | 15.1 ± 0.7 (3.0-fold) | 15.1 ± 0.6 (29.6) | 0.02 ± 0.02 (0.1) | 15.8 ± 4.5 | 0.7 ± 0.7 | 0.5 ± 0.9 | 17.1 ± 4.6 |
| Nonbrain ECs | 52.0 ± 2.8 (10.4-fold) | 18.1 ± 12.6 (3.6-fold) | 18.1 ± 12.6 (34.8) | 0 (0) | 22.2 ± 4.9 | 4.8 ± 0.3 | 0.6 ± 0.3 | 27.7 ± 6.2 |
| Conditions . | Cell yield . | No. cells procured × 105 . | No. CFCs × 104 . | Total . | ||||
|---|---|---|---|---|---|---|---|---|
| CD34+ . | CD34+CD38+(%) . | CD34+CD38−(%) . | CFU-GMs . | BFU-Es . | CFU-Mix's . | |||
| Input | 5.0 | 5.0 | 4.91 ± 0.07 (98.4) | 0.08 ± 0.04 (1.6) | 3.7 ± 0.9 | 0.6 ± 0.3 | 0.5 ± 0.2 | 4.7 ± 1.4 |
| HUBECs | 81.0 ± 3.4 (16.1-fold) | 27.0 ± 1.1 (5.4-fold) | 9.0 ± 0.4 (12.3) | 17.0 ± 0.5 (21.6) | 56.0 ± 2.2 | 4.8 ± 0.6 | 2.6 ± 0.3 | 63.4 ± 2.5 |
| HUBECs, noncontact | 82.0 ± 3.7 (16.4-fold) | 14.0 ± 0.1 (2.8-fold) | 10.4 ± 0.3 (13.0) | 2.8 ± 0.1 (3.4) | 30.0 ± 1.3 | 6.0 ± 0.5 | 4.3 ± 0.9 | 40.3 ± 0.5 |
| Stroma-free | 51.0 ± 1.3 (10.2-fold) | 15.1 ± 0.7 (3.0-fold) | 15.1 ± 0.6 (29.6) | 0.02 ± 0.02 (0.1) | 15.8 ± 4.5 | 0.7 ± 0.7 | 0.5 ± 0.9 | 17.1 ± 4.6 |
| Nonbrain ECs | 52.0 ± 2.8 (10.4-fold) | 18.1 ± 12.6 (3.6-fold) | 18.1 ± 12.6 (34.8) | 0 (0) | 22.2 ± 4.9 | 4.8 ± 0.3 | 0.6 ± 0.3 | 27.7 ± 6.2 |
Purified human ABM CD34+ cells (5 × 105) were plated per culture treatment (n = 8) as described in “Materials and methods.” Nonadherent cells were harvested on day 7 of culture and analyzed via flow cytometry. Numbers in parentheses in the cell-yield and CD34+ columns reflect the fold-increase in these populations following culture. Numbers in parentheses in the CD34+ CD38+ and CD34+CD38− columns indicate the percentage of each population as a subset of the total population. CFC values represent the total number of CFC per culture.
CFU-GMs indicates GM colony-forming units; BFU-Es, erythroid burst-forming units; CFU-Mix's, mixed CFUs.
HUBEC coculture supported a 15.1-fold increase in GM colony-forming units (CFU-GMs), a 5.2-fold increase in mixed CFUs (CFU-Mix's), and an 8.0-fold increase in erythroid burst-forming units (BFU-Es) compared with input values, and this CFC activity was significantly greater than that produced by liquid suspension cultures and nonbrain endothelial cell cultures (Table 1). Noncontact HUBEC cultures supported an expansion of total CFCs that was greater than either liquid suspension or nonbrain endothelial cell culture, but optimal CFC production occurred in the HUBEC contact cultures.
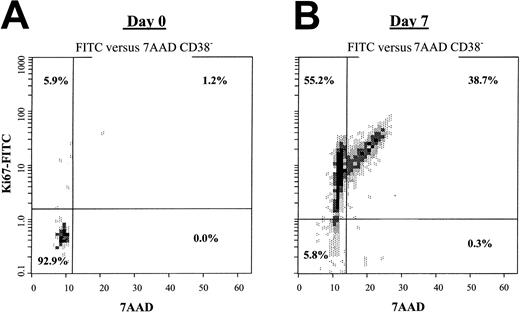
HUBEC contact and noncontact cultures induced a high percentage of quiescent ABM CD34+CD38− cells to enter cell division by day 7. At day 0, 95.7% ± 3.9% of the CD34+CD38− population resided in G0; 3.9% ± 3.2% in G1; and 0.7% ± 0.7% in G2/S/M phase. After 7 days of HUBEC coculture, 62.3% ± 9.9% of the CD34+CD38−cells had entered G1; 33.3% ± 7.6% were in G2/S/M phase; and only 3.9% ± 2.7% remained in G0 (Figure 2). Similarly, noncontact HUBEC cultures induced 49.1% ± 2.4% cells into G1, 25.3% ± 6.2% into G2/S/M phase, and only 25.1% ± 3.8% into G0. In contrast, we could not perform cell cycle analysis of CD34+CD38− cells from liquid suspension and nonbrain endothelial cell cultures owing to the undetectable frequency of CD34+CD38− cells at day 7.
Phenotypic analysis of bone marrow CD34+cells following ex vivo culture.
Purified human CD34+ cells were plated on confluent HUBEC monolayers, stroma-free liquid suspension cultures, nonbrain endothelial cell monolayers, and noncontact HUBEC cultures in the presence of optimal concentrations of GM-CSF plus IL-3 plus IL-6 plus SCF plus flt-3 ligand for 7 days. (A) The phenotype of untreated purified bone marrow CD34+ cells at day 0. (B) HUBEC-cultured bone marrow cells at day 7, demonstrating a high percentage of CD34+ and CD34+CD38−cells. (C-D) Stroma-free liquid suspension cultures (C) and nonbrain endothelial cell cocultures, demonstrating loss of CD34+CD38− phenotype cells (D). (E) HUBEC noncontact cultures, showing preservation of cells with CD34+CD38− phenotype. Harvested nonadherent cells were stained with FITC-conjugated CD34 mAb and PE-conjugated CD38 mAb and analyzed by FACS. Log fluorescence distribution of CD34 expression is shown along the x-axis, and CD38 expression along the y-axis. Cursor lines indicate the nonspecific staining levels of isotype-matched control mAbs.
Phenotypic analysis of bone marrow CD34+cells following ex vivo culture.
Purified human CD34+ cells were plated on confluent HUBEC monolayers, stroma-free liquid suspension cultures, nonbrain endothelial cell monolayers, and noncontact HUBEC cultures in the presence of optimal concentrations of GM-CSF plus IL-3 plus IL-6 plus SCF plus flt-3 ligand for 7 days. (A) The phenotype of untreated purified bone marrow CD34+ cells at day 0. (B) HUBEC-cultured bone marrow cells at day 7, demonstrating a high percentage of CD34+ and CD34+CD38−cells. (C-D) Stroma-free liquid suspension cultures (C) and nonbrain endothelial cell cocultures, demonstrating loss of CD34+CD38− phenotype cells (D). (E) HUBEC noncontact cultures, showing preservation of cells with CD34+CD38− phenotype. Harvested nonadherent cells were stained with FITC-conjugated CD34 mAb and PE-conjugated CD38 mAb and analyzed by FACS. Log fluorescence distribution of CD34 expression is shown along the x-axis, and CD38 expression along the y-axis. Cursor lines indicate the nonspecific staining levels of isotype-matched control mAbs.
HUBEC culture induces cell division within quiescent ABM CD34+CD38− cells.
(A) The dot plot shows the 7-AAD versus Ki-67 FITC profile for steady-state bone marrow CD34+CD38− cells, with the majority of the population residing in G0. (B) CD34+CD38− cells were FACS-sorted from 7-day HUBEC cocultures and stained for 7-AAD versus Ki-67 FITC expression, demonstrating that the majority of the CD34+CD38− population had entered cell cycle. Cell cycle analysis of CD34+ cells was performed by means of the SID method described in “Materials and methods.”
HUBEC culture induces cell division within quiescent ABM CD34+CD38− cells.
(A) The dot plot shows the 7-AAD versus Ki-67 FITC profile for steady-state bone marrow CD34+CD38− cells, with the majority of the population residing in G0. (B) CD34+CD38− cells were FACS-sorted from 7-day HUBEC cocultures and stained for 7-AAD versus Ki-67 FITC expression, demonstrating that the majority of the CD34+CD38− population had entered cell cycle. Cell cycle analysis of CD34+ cells was performed by means of the SID method described in “Materials and methods.”
HUBEC-cultured cells engraft NOD/SCID mice at a higher frequency than fresh ABM CD34+ cells
NOD/SCID mice received transplants of either fresh ABM CD34+ cells (n = 47) or the progeny of ABM CD34+ cells cultured with HUBEC × 7 days (n = 47) over a range of doses, which resulted in nonengraftment in a fraction of the mice.
Transplantation of 1 × 105 fresh ABM CD34+cells resulted in no engraftment in 10 mice, whereas transplantation of 5 × 105 to 1 × 106 ABM CD34+cells resulted in engraftment in only 10 of 22 recipients (45%) at low human CD45+ (huCD45+) cell levels (mean, 3.3%) (Figure 3A-C). At a dose of 1.5 × 106 ABM CD34+ cells, 7 of 7 mice that underwent transplantation showed human cell engraftment, suggesting that nonlimiting numbers of SRCs were present at that dose (Figure 3D). When the progeny of HUBEC cultures over the same dose range were transplanted, the rate of NOD/SCID engraftment increased (Figure 3A-C). The progeny of 1 × 105 ABM CD34+ cells cultured with HUBECs engrafted in 2 of 10 mice, and the progeny of 5 × 105 to 1 × 106 ABM CD34+cells engrafted in 21 of 22 mice (96%) at high levels (mean, 11.7% huCD45+ cells) (Figure 3A-C). Twelve mice receiving transplants of 5 × 105 to 1 × 106 fresh ABM CD34+ cells showed no human cell engraftment, but all 12 mice receiving transplants of the HUBEC-cultured progeny of these ABM CD34+ cells demonstrated engraftment of at least 1% huCD45+ cells. At a dose of 1.5 × 106ABM CD34+ cells, HUBEC-cultured progeny engrafted in 7 of 7 mice at levels of huCD45+ cell engraftment equivalent to fresh ABM CD34+ cells (Figure 3D).
Human cell engraftment in NOD/SCID mice receiving transplants of limiting doses of ABM CD34+ cells and HUBEC-cultured progeny.
(A) First, 1 × 105 ABM CD34+ cells (left) or their progeny following HUBEC culture (right) were transplanted into NOD/SCID mice. The level of human cells present in the murine bone marrow at 8 weeks was then determined by flow cytometric analysis of human CD45 expression. Panel B shows 5 × 105 ABM CD34+ cells or their HUBEC-cultured progeny; panel C, 1 × 106 ABM CD34+ cells versus HUBEC-cultured progeny; and panel D, 1.5 × 106 ABM CD34+ cells versus HUBEC-cultured progeny. Panel E shows the engraftment of progeny of 1.5 × 106 ABM CD34+ cells cultured with HUBEC noncontact cultures as well as the engraftment of the progeny of 3 × 106 ABM CD34+ cells cultured with GM-CSF plus IL-3 plus IL-6 plus SCF plus flt-3 ligand in the absence of HUBECs.
Human cell engraftment in NOD/SCID mice receiving transplants of limiting doses of ABM CD34+ cells and HUBEC-cultured progeny.
(A) First, 1 × 105 ABM CD34+ cells (left) or their progeny following HUBEC culture (right) were transplanted into NOD/SCID mice. The level of human cells present in the murine bone marrow at 8 weeks was then determined by flow cytometric analysis of human CD45 expression. Panel B shows 5 × 105 ABM CD34+ cells or their HUBEC-cultured progeny; panel C, 1 × 106 ABM CD34+ cells versus HUBEC-cultured progeny; and panel D, 1.5 × 106 ABM CD34+ cells versus HUBEC-cultured progeny. Panel E shows the engraftment of progeny of 1.5 × 106 ABM CD34+ cells cultured with HUBEC noncontact cultures as well as the engraftment of the progeny of 3 × 106 ABM CD34+ cells cultured with GM-CSF plus IL-3 plus IL-6 plus SCF plus flt-3 ligand in the absence of HUBECs.
To assess the capacity of noncontact HUBEC cultures to maintain SRCs, we also transplanted into NOD/SCID mice the progeny of ABM CD34+ cells that were cultured in this manner. The progeny of 1.5 × 106 ABM CD34+ cells plated in HUBEC noncontact cultures engrafted in 7 of 7 mice at high levels (mean, 62.8% huCD45+) comparable to the engraftment observed with the progeny of contact HUBEC-cultured cells transplanted at the same dose (Figure 3E). As a control, we also transplanted into mice the progeny of ABM CD34+ cells that were plated in liquid suspension cultures supplemented with the identical cytokines for 7 days. None of the 3 mice in this group showed human cell engraftment or human CFC activity (Figure 3E).
HUBEC-cultured cells engraft in NOD/SCID mice with multilineage differentiation
Figure 4A shows human CD45+ cell engraftment within a representative mouse that received a transplant of fresh ABM CD34+ cells (1 × 106) versus an animal receiving a transplant of the progeny of the same dose of ABM CD34+ cells following HUBEC culture. Detailed FACS analysis demonstrated lymphoid and myeloid differentiation in mice receiving transplants of limiting doses of HUBEC-cultured cells (Figure 4B). The proportion of CD34+ cells, CD19+ B cells, and CD13+ myeloid cells was highly similar within mice receiving transplants of limiting doses of HUBEC-cultured cells as compared with mice engrafted by means of fresh ABM CD34+cells, indicating that a highly primitive repopulating cell was sustained during HUBEC culture (Table2). Detection of human CFCs within NOD/SCID mice correlated closely with the huCD45+ cell engraftment that we observed. Mice receiving transplants of the progeny of 1 × 106 ABM CD34+ cells cultured with HUBECs demonstrated multilineage human CFC activity that was 41-fold greater than the human CFC activity within mice receiving transplants of the same dose of fresh ABM CD34+ cells (Table 2).
Phenotypic analysis of HUBEC-cultured cells engrafted in the bone marrow of NOD/SCID mice.
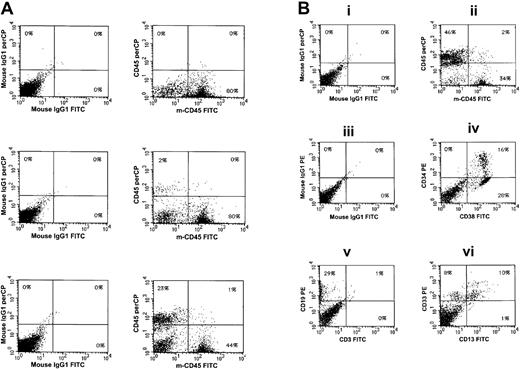
(A) Expression of human CD45+ cells within the bone marrow of a control NOD/SCID mouse that did not receive a transplant (top); expression of human CD45+ cells within the bone marrow of a NOD/SCID mouse that received a transplant of 1 × 106fresh ABM CD34+ cells (middle); and expression of human CD45+ cells within the bone marrow of a NOD/SCID mouse that received a transplant of the progeny of 1 × 106 ABM CD34+ following coculture with HUBECs (bottom). Isotype controls are shown at left. (B) Lineage distribution of engrafted human cells within a representative mouse that received a transplant of HUBEC-cultured cells. Panel Bi, murine marrow stained with isotype control IgG1-FITC and IgG1–peridinin chlorophyll A protein (IgG1-PerCP). Panel Bii, staining of murine marrow with anti-human CD45-PerCP and anti-murine CD45-FITC, showing both human and murine populations. Panel Biii, isotype staining with IgG1-FITC and IgG1-PE. Panel Biv, expression of human CD34-PE and CD38-FITC on engrafted cells within murine marrow. Panel Bv, staining with anti-CD19 and anti-CD3, demonstrating CD19 expression on engrafted human cells. Panel Bvi, expression of human CD33 and human CD13 on engrafted cells within the marrow.
Phenotypic analysis of HUBEC-cultured cells engrafted in the bone marrow of NOD/SCID mice.
(A) Expression of human CD45+ cells within the bone marrow of a control NOD/SCID mouse that did not receive a transplant (top); expression of human CD45+ cells within the bone marrow of a NOD/SCID mouse that received a transplant of 1 × 106fresh ABM CD34+ cells (middle); and expression of human CD45+ cells within the bone marrow of a NOD/SCID mouse that received a transplant of the progeny of 1 × 106 ABM CD34+ following coculture with HUBECs (bottom). Isotype controls are shown at left. (B) Lineage distribution of engrafted human cells within a representative mouse that received a transplant of HUBEC-cultured cells. Panel Bi, murine marrow stained with isotype control IgG1-FITC and IgG1–peridinin chlorophyll A protein (IgG1-PerCP). Panel Bii, staining of murine marrow with anti-human CD45-PerCP and anti-murine CD45-FITC, showing both human and murine populations. Panel Biii, isotype staining with IgG1-FITC and IgG1-PE. Panel Biv, expression of human CD34-PE and CD38-FITC on engrafted cells within murine marrow. Panel Bv, staining with anti-CD19 and anti-CD3, demonstrating CD19 expression on engrafted human cells. Panel Bvi, expression of human CD33 and human CD13 on engrafted cells within the marrow.
HUBEC-cultured cells generate multilineage human cell differentiation and human progenitor cell colonies within the marrow of NOD/SCID mice that underwent transplantation
| Culture conditions . | Human CFCs formed from ABM of NOD/SCID mice . | Percentage of engrafted CD45+ cells expressing specified CD cells . | |||||
|---|---|---|---|---|---|---|---|
| GM . | E . | Mix . | Total . | CD34 . | CD19 . | CD13 . | |
| Fresh ABM | |||||||
| CD34+cells | 0.8 ± 0.5 | 0.2 ± 0.2 | 0.2 ± 0.2 | 1.2 ± 0.8 | 28.2 ± 3.6 | 89.9 ± 4.1 | 9.5 ± 3.4 |
| HUBECs | 44.2 ± 15.9 | 3.4 ± 1.7 | 1.6 ± 1.5 | 49.0 ± 18.5 | 32.3 ± 9.4 | 77.7 ± 16.7 | 19.3 ± 7.7 |
| Culture conditions . | Human CFCs formed from ABM of NOD/SCID mice . | Percentage of engrafted CD45+ cells expressing specified CD cells . | |||||
|---|---|---|---|---|---|---|---|
| GM . | E . | Mix . | Total . | CD34 . | CD19 . | CD13 . | |
| Fresh ABM | |||||||
| CD34+cells | 0.8 ± 0.5 | 0.2 ± 0.2 | 0.2 ± 0.2 | 1.2 ± 0.8 | 28.2 ± 3.6 | 89.9 ± 4.1 | 9.5 ± 3.4 |
| HUBECs | 44.2 ± 15.9 | 3.4 ± 1.7 | 1.6 ± 1.5 | 49.0 ± 18.5 | 32.3 ± 9.4 | 77.7 ± 16.7 | 19.3 ± 7.7 |
NOD/SCID mice received transplants of either 1 × 106 ABM CD34+ cells or the progeny of this dose of ABM CD34+ cells following 7 days of HUBEC culture as described in “Materials and methods.” The CFC content in each group was determined from triplicate cultures of 1 × 105 NOD/SCID marrow cells per dish under procedures described in “Materials and methods” (n = 11). The numbers shown under the CD34, CD19, and CD13 columns indicate the percentage of engrafted human CD45+ cells that expressed the particular differentiation antigen. For animals receiving transplants of fresh ABM CD34+ cells, we were able to analyze only those mice with human cell engraftment ≥ 1%.
HUBEC coculture increases the frequency of SRCs within human bone marrow
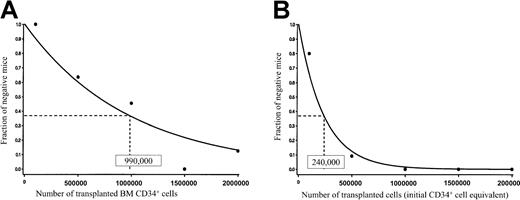
For statistical analysis, we pooled data from the limiting dilution assays of fresh ABM CD34+ cells and HUBEC-cultured cells, according to methods previously described.12,27 We calculated the frequency of SRCs using the maximum likelihood estimator.32 The value of χ2 in all cases was not statistically significant (P > .10), demonstrating internal consistency in our assays and allowing pooling of the data. The frequency of SRCs within fresh ABM CD34+ cells was 1 in 9.9 × 105cells (95% confidence interval [CI], 1/650 000 − 1/1 600 000) (Figure 5A). The SRC frequency within HUBEC-cultured cells was significantly higher, at 1 in 240 000 cells (CI, 1/140 000 − 1/410 000) (Figure 5B). Therefore, coculture of adult human ABM CD34+ cells with HUBEC monolayers supported a 4.1-fold increase in SRC. As further confirmation of the validity of applying the single-hit Poisson model to our limiting dilution assay, we also estimated the frequency of SRCs using the minimum χ2 estimation.12 Again, χ2 was not significant in all cases (P > .20).
HUBEC-culture increases the frequency of SRCs within adult human bone marrow
. (A) NOD/SCID mice (n = 47) received transplants of fresh ABM CD34+ cells over a range of doses, and the engraftment frequencies at each dose are plotted. The resultant curve indicates the estimated frequency of SRCs within this population. (B) NOD/SCID mice (n = 47) received transplants of the progeny of ABM CD34+cells cultured with HUBECs plus GM-CSF plus IL-3 plus IL-6 plus SCF plus flt-3 ligand. The engraftment frequencies are plotted at each dose, and the resultant curve indicates the frequency of SRCs within this population. The numbers shown within each box indicate the calculated frequency of SRC using the maximum likelihood estimator.
HUBEC-culture increases the frequency of SRCs within adult human bone marrow
. (A) NOD/SCID mice (n = 47) received transplants of fresh ABM CD34+ cells over a range of doses, and the engraftment frequencies at each dose are plotted. The resultant curve indicates the estimated frequency of SRCs within this population. (B) NOD/SCID mice (n = 47) received transplants of the progeny of ABM CD34+cells cultured with HUBECs plus GM-CSF plus IL-3 plus IL-6 plus SCF plus flt-3 ligand. The engraftment frequencies are plotted at each dose, and the resultant curve indicates the frequency of SRCs within this population. The numbers shown within each box indicate the calculated frequency of SRC using the maximum likelihood estimator.
Discussion
Bone marrow transplantation is a curative therapy for an increasing number of malignant and nonmalignant diseases.33 However, the ex vivo expansion of adult bone marrow for application in gene therapy, immune tolerance induction,34 and other purposes has been unsuccessful owing to the differentiation and cell death that occur when these cells are exposed to cytokines.1-5 In this study, using a limiting dilution analysis, we have demonstrated for the first time that the SCID-repopulating cell numbers within adult ABM can be quantitatively increased via ex vivo coculture with primary HUBECs. Coculture of ABM CD34+ cells with HUBECs supplemented with GM-CSF plus IL-3 plus IL-6 plus SCF plus flt-3 ligand induced greater than 95% of the CD34+CD38− population to enter cell division and supported a 4.1-fold increase in SRCs as compared with starting ABM CD34+ populations. Since stem cell division and maintenance of stem cell repopulating capacity are requirements for successful retroviral gene transfer, the HUBEC culture method may provide significant advantages for clinical gene therapy protocols. Although other factors, such as the expression of retroviral receptors on target stem cells,35 are also important predictors of the success of retroviral gene therapy, our previous investigations have shown that coculture of human ABM CD34+ cells with a PMVEC increased the gene transfer efficiency into the CD34+CD38− subset in the absence of measurable increases in the expression of retroviral receptors.36 We are currently examining the effect of HUBEC coculture on the gene transfer efficiency into BM CD34+CD38− cells and long-term repopulating cells in the NOD/SCID model.
In contrast to the results presented here, previous studies have indicated that the ex vivo culture of ABM stem cells results in a decline in repopulating capacity.1,37 Gan et al1 reported that 1-week culture of human ABM mononuclear cells (MNCs) with ABM stroma caused a 6-fold decline in SRCs as compared with unmanipulated ABM MNCs. Studies of mouse ABM cultures have demonstrated similar losses in the recovery of long-term repopulating cells after 3 to 4 weeks in culture.37 Of note, both the murine studies and the studies by Gan et al were performed in the absence of exogenous cytokines, which would have been expected to drive differentiation.4-6 In our studies, we supplemented HUBEC monolayers with a cytokine combination, which maximally induced progenitor cell division, and despite this, we observed a measurable increase in SRCs over time. Since exposure to GM-CSF plus IL-3 plus IL-6 plus SCF plus flt-3 ligand in the absence of HUBECs caused a decline in detectable SRCs over 7 days of culture, we postulate that HUBECs may have protected ABM SRCs from differentiation during exposure to cytokines while also supporting the self-renewal of this primitive population.
Although steady-state ABM CD34+CD38− cells are highly enriched for SRCs,8 we observed no correlation between the observed increase in SRCs (4.1-fold) and the larger increase in CD34+CD38− cells during culture (212-fold). This may be explained, in part, by a down-modulation of CD38 expression on committed progenitors that may have occurred during culture. Dorrell et al38 demonstrated that a significant percentage of cord blood CD34+CD38+ cells acquired a CD34+CD38− phenotype during 4-day culture with fibronectin plus IL-6 plus SCF plus G-CSF plus flt-3 ligand, and a depletion of retinoids within the culture may account for this result.39 In addition, apoptotic events associated with cytokine deprivation of transplanted CD34+CD38− cells40 may contribute to their loss of repopulating capacity. Similarly, Glimm et al41 and others3,42 43 have demonstrated that cell cycle–associated defects may adversely affect the ability of subpopulations of proliferating CD34+CD38−cells to contribute to in vivo engraftment. Our preliminary investigations indicate that coculture of sorted ABM CD34+CD38− cells with HUBECs results in a mean 6-fold increase in CD34+CD38− cells, an expansion that correlates closely with the 4.1-fold increase in SRCs that we have observed in this study (J.P.C. et al, manuscript in preparation). These results suggest that newly generated SRCs during 7-day HUBEC cultures are quite possibly CD34+CD38− cells. Cell-sorting studies of HUBEC-cultured populations should help determine which population is enriched for SRCs following culture and provide a more precise understanding of the effect of HUBEC culture on primitive ABM subsets.
There are several potential mechanisms through which HUBEC culture may have increased the SCID-repopulating capacity of adult ABM CD34+ cells. First, contact with HUBECs may have triggered self-renewal divisions within a subpopulation of primitive marrow cells, resulting in an absolute increase in SRCs. This result would be consistent with the single-hit Poisson model as it has been applied previously.12 Alternatively, exposure to HUBECs may have positively altered the engraftment capacity of the limited number of SCID-repopulating cells within the steady-state ABM CD34+ population. Alteration of adhesion receptor expression on primitive cells has been associated with enhanced engraftment in murine models,44,45 and the up-regulation of CXCR4 on CB cells during culture has been associated with increased engraftment in NOD/SCID mice.46 Engagement of the Jagged/Notch pathway also has been shown to promote the maintenance of primitive hematopoietic cells during culture.47 Finally, CD34− and CD34+CD38+ accessory cells contained within the HUBEC-cultured grafts may have facilitated the engraftment of SRCs within the recipient NOD/SCID marrow.48 49 However, since liquid suspension–cultured grafts contained equivalent numbers of CD34− and CD34+CD38+ accessory cells and failed to engraft NOD/SCID marrow, this explanation alone is incomplete. Whether HUBEC culture causes an absolute increase in ABM repopulating cells or augments the engraftment capacity of repopulating cells within adult bone marrow, the clinical impact of this method for stem cell expansion protocols would be the same: increased delivery of competent repopulating cells to the marrow.
The data from our noncontact HUBEC cultures with human ABM CD34+ cells suggest that cell-to-cell contact may not be required for the maintenance of marrow SRCs in this system. This result differs from previous studies, which have demonstrated a requirement for either stroma cell contact or adhesion via integrins to the fibronectin-COOH domain for the maintenance of adult-source stem cells during exposure to cytokines.50 51 In this study, noncontact HUBEC cultures maintained a percentage of CD34+CD38− cells at day 7, and more importantly, the progeny of these cultures maintained SCID-repopulating capacity. Although we did not perform a limiting dilution analysis to estimate the SRC frequency within noncontact HUBEC cultures, our results suggest a differential maintenance of SRCs within noncontact HUBEC cultures as compared with liquid suspension cultures. The lack of requirement for cell-to-cell contact may also be clinically advantageous since endothelial cell contamination of hematopoietic grafts would be eliminated.
Since the clinical transplantation of cord blood CD34+cells is limited by low cell numbers and delayed neutrophil/platelet engraftment,52 we are currently testing the capacity of HUBEC culture to expand repopulating cells within this population. Additionally, we will be performing serial transplantation studies to confirm that HUBEC coculture maintains cells with long-term repopulating capacity,53,54 and we plan to test HUBEC culture with other cytokine combinations, such as SCF plus flt-3 ligand plus thrombopoietin (TPO) plus IL-6/ soluble IL-6 receptor (sIL-6R),27 in order to further augment the expansion of SRCs presented here. Finally, the concentrations of IL-3 (5 ng/mL), IL-6 (5 ng/mL), and GM-CSF (2 ng/mL) that we used in this study were lower than other investigators have previously applied to induce stem cell proliferation in vitro.41 55 We will additionally test whether higher concentrations of these cytokines might further increase the SRC expansion observed here.
Although remarkable progress has been made recently in the ex vivo expansion of human cord blood SRCs, this progress has not translated into successful methods for the ex vivo expansion of either bone marrow– or peripheral blood–mobilized stem cells. The limiting dilution analysis presented here demonstrates that long-term repopulating cells within adult human bone marrow can be increased via exposure to human brain endothelial cells. This culture method may prove clinically useful for both the ex vivo expansion and genetic modification of adult human bone marrow stem cells.
The authors wish to acknowledge Dr David Venzon for providing the statistical analysis and for comments in the preparation of this manuscript.
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2002-04-1238.
Supported by a grant (PE 0603706N) from the US Office of Naval Research; also supported in part by research funding from Large Scale Biology Corp to J.P.C. via the Cooperative Research and Development Agreement (No. NCRADA-NMRDC/NMRI/Biosource-97-588) between the Naval Medical Research Center and Large Scale Biology Corporation and NIH grant P01 CA70970.
The Johns Hopkins University holds patents on CD34 monoclonal antibodies and related inventions. C.C. is entitled to a share of the sales royalty received by the University under licensing agreements between the University, Becton Dickinson Corp, and Baxter HealthCare Corp. The terms of these arrangements have been reviewed and approved by the University in accordance with its conflict of interest policies.
J.P.C. and T.A.D. are currently employed by Large Scale Biology Corp whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John P. Chute, Large Scale Biology Corporation, 3333 Vaca Valley Pkwy, Vacaville, CA 95688; e-mail:john.chute@lsbc.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal