Abstract
Kaposi sarcoma–associated herpesvirus (KSHV) has been associated with several diseases, but the association between KSHV and multiple myeloma (MM) remains controversial. To address this issue, we studied patients with MM for the presence of viral RNA transcripts as well as KSHV-specific cellular immune responses. Highly sensitive reverse transcription–polymerase chain reaction assays for detection of viral transcripts of KSHV open reading frame (ORF) 26, ORF72, and ORF74 did not detect viral gene transcripts in long-term cultures of bone marrow stromal cells from 23 patients with MM. Moreover, sensitive assays for KSHV ORF65–specific and ORF73-specific cytotoxic T-lymphocyte (CTL) activity that readily and routinely detect CTLs specific for ORF65 and ORF73 in patients positive for human immunodeficiency virus and KSHV did not show any specific responses in 16 patients with MM, despite the presence of positive Epstein-Barr virus–specific CTLs in all cases. These data therefore do not show a biologically important association between ongoing KSHV infection and MM.
Introduction
Since the initial discovery of Kaposi sarcoma–associated herpesvirus (KSHV) in Kaposi sarcoma (KS) tissue, seroepidemiologic studies have associated this virus with KS, multicentric Castleman disease, and primary effusion lymphomas.1 In contrast, since the first detection of KSHV DNA in long-term cultured bone marrow stromal cells from patients with multiple myeloma (MM), the association between KSHV and MM has remained controversial.2-6 Different explanations have been offered to account for inconsistencies in seroepidemiologic and virologic studies searching for an association of KSHV with MM, including the presence of viral variants. Indeed, Ma et al7 reported unique KSHV open reading frame (ORF) 65 sequences that were detected exclusively in patients with MM but not in patients without MM who were seropositive for KSHV. Interestingly, in some MM patients, a mutation at the C-terminal end of ORF65 resulted in an extension of the ORF by 33 amino acids. These sequences were found only in samples from patients with MM and were suggested as a possible unique target of KSHV serologic immune responses in such patients, who lack seroreactivity against the “conventional” ORF65 protein.2-5
To investigate whether MM is associated with KSHV infection, we assayed 38 patients with MM for the presence of viral transcripts, virus-specific cellular immune responses, or both, including immune reactivity against this novel, MM-specific ORF65 protein sequence. Our findings do not support the hypothesis that an association of KSHV and MM has a biologic relevance.
Study design
Patients
Peripheral blood and bone marrow samples were obtained from 38 patients with MM at the Dana Farber Cancer Institute, as well as from human immunodeficiency virus (HIV)–seronegative, KSHV-seronegative and HIV-seropositive, KSHV-seropositive patients at the Massachusetts General Hospital's Partners AIDS Research Center under the auspices of institution review board–approved protocols. KSHV seropositivity was determined by testing for antibody specific for ORF73 (latency-associated nuclear antigen) as described previously.6 8
Detection of KSHV transcripts by reverse transcription– polymerase chain reaction (RT-PCR) assay
Total RNA was extracted from long-term bone marrow stromal cells (LT-BMSCs) from 23 patients with MM and 5 healthy donors by using an RNeasy Mini-kit (Qiagen, Valencia, CA). RT-PCR was performed in a thermal cycler (MJ Research, Watertown, MA) by using 5 μg total RNA and 25 pM each of forward and reverse primers. RNA was amplified by using a Superscript one-step RT-PCR with platinum Taq (Life Technologies, Rockville, MD). The thermal cycle profile consisted of denaturating at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 1 minute. The samples were amplified for 30 cycles. Viral transcripts of multiple nonoverlapping regions of the KSHV genome (ORF26, ORF72, and ORF74) were amplified by using previously described primers.6 12 All RNA was treated with amplification-grade deoxyribonuclease I (Gibco-BRL, Gaithersburg, MD) to eliminate DNA contamination. All PCR products were run on a 1.5% agarose gel. RNA from the BCBL-1 cell line served as a positive control, and β-actin served as an internal control to ensure adequacy of RNA.
Synthetic peptides
Overlapping peptide sets spanning the entire KSHV ORF 65 and ORF 73 amino acid sequence were synthesized as 22-mer peptides overlapping by 15 amino acids. The sequence of these peptides was based on the previously reported KSHV viral sequence from the KSHV-infected BC-1 cell line.9 In addition, 5 22-mer peptides were synthesized that correspond in sequence to the previously described, MM-unique 33-amino acid sequence at the C-terminal end of KSHV ORF65 (TLSSTTETAPPRWPTRGNPPLA, PRWPTRGNPPLAKRNRQRLDNR, LAKRNRQRLDNRGVTSTTSLFC, DNRGVTSTTSLFCQPSPPGTPIG, SLFCQPSPPGTPIGRPLFV).7 Control peptides derived from Epstein-Barr virus (EBV) were selected from previously described cytotoxic T-lymphocyte (CTL) epitopes.10 Up to 36 EBV control peptides were included per EBV control pool. All KSHV-encoded peptides were placed into pools containing 4 to 7 peptides and used at a final concentration of 10 μg/mL per individual peptide in enzyme-linked immunospot (ELISPOT) assays.
ELISPOT analysis
ELISPOT assays were performed by using frozen peripheral blood mononuclear cells (PBMCs) that were thawed overnight, counted, and plated at 1 × 105 to 2 × 105cells/well.11 The cells were incubated in duplicate wells for 16 hours with and without the peptide pools, and the plates were then washed and developed, as described previously.11Responses were considered positive when they were increased at least threefold above those of the no-antigen controls and when at least 3 spots/well were detectable. Thus, the lower cutoff level for specific responses was 15 antigen-specific cells/106 PBMCs. CD4 and CD8 T-cell separations were done to verify that all responses detected were mediated by CD8+ T cells (data not shown).
Results and discussion
Our previous studies showed that in 88% of cases, KSHV gene sequences can be amplified from DNA isolated from LT-BMSCs and peripheral blood dendritic cells obtained from patients with MM.5,6,12 However, positive PCR results were also obtained with DNA in up to 37% of our samples from healthy donors. These results suggested that KSHV could be involved in MM, but our incidence of KSHV detection exceeded current serologic estimates for the general population several-fold.13 Although these results were based on detection of DNA using a nested PCR approach and may represent nonspecific amplification products, detection of KSHV DNA in these samples in the absence of serologic evidence suggested 3 other possibilities: 1) the KSHV virus was present but in quantities too low to induce a serologic response; 2) a KSHV variant is associated with the pathogenesis of MM; or 3) KSHV is not associated with MM. Because our previous studies were designed to detect KSHV-specific sequences in as little as 0.01 pg total DNA (corresponding to 1 viral copy in 1 × 103 cells12), the very low copy number could explain the lack of serologic reactivity in patients with MM. Furthermore, sequence variability, such as the reported MM-specific ORF65 sequence, also could account for why KSHV was not detected in serum from MM patients.7 However, it seems unlikely that none of the serologic targets of KSHV would be conserved in patients with MM.
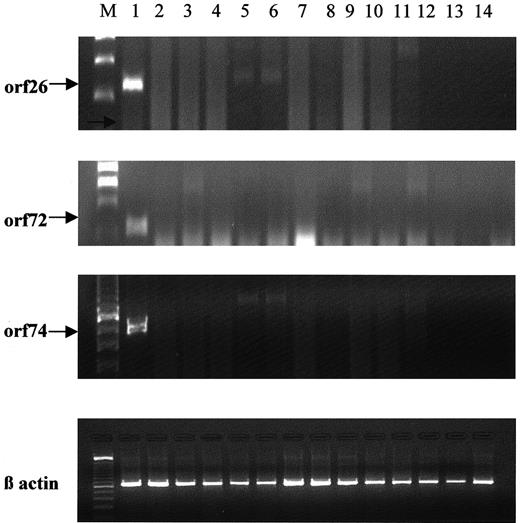
We therefore further analyzed patients with MM for the presence of viral transcripts to assess active viral replication by performing RT-PCR. Eighty percent of these patients had DNA amplified for KSHV-specific gene products in previous studies.5 KSHV gene sequences, however, were identified only on nested PCR and not on first-round PCR. RT-PCR for KSHV ORF26, ORF72, and ORF74 was performed with RNA samples isolated from LT-BMSCs from 23 patients with MM, as in previous studies.6 9 After RT and PCR amplification, none of these samples yielded any positive PCR product (Figure1). These data suggest that no active viral gene transcription had occurred in these patients, even in LT-BMSCs assayed after prolonged in vitro culture, as was necessary to obtain positive PCR products in all previous studies. These results support the hypothesis that active KSHV infection is not associated with MM.
RT-PCR analyses of KSHV gene expression.
RNA was extracted from LT-BMSCs from 23 patients with MM and 5 healthy donors, and ORF26, 72, and 74 regions of the KSHV genome were amplified. Representative results are shown. Lane 1, the positive control, included RNA from the KSHV-infected BCBL-1 cell line. Lanes 2 to 11 represent RNA from MM patient LT-BMSCs, and lanes 12 to 14 contained RNA from healthy donors.
RT-PCR analyses of KSHV gene expression.
RNA was extracted from LT-BMSCs from 23 patients with MM and 5 healthy donors, and ORF26, 72, and 74 regions of the KSHV genome were amplified. Representative results are shown. Lane 1, the positive control, included RNA from the KSHV-infected BCBL-1 cell line. Lanes 2 to 11 represent RNA from MM patient LT-BMSCs, and lanes 12 to 14 contained RNA from healthy donors.
In addition to these molecular analyses, we also conducted assays for virus-specific immune responses in the MM patients. Because our previous studies had shown that no serologic responses were detectable in MM patients,5,6 we next assayed cellular KSHV-specific immune responses. Thus, PBMCs from 16 patients with MM were tested in an ELISPOT assay for specific reactivity against a panel of overlapping peptides spanning the KSHV ORF73 amino acid sequence, as well as the ORF65 sequence with the 33 MM-KSHV unique residues.14 Of these 16 patients, 12 were included in our analysis of viral replication (Figure 1). No significant immune responses against these 2 proteins were detectable in these patients with MM, despite positive responses against EBV-derived control peptides (Table1). In contrast, strong ELISPOT responses against ORF73 and ORF65 were detected in the KSHV-positive controls. Interestingly, very weak responses against the reported MM-specific 33-amino acid–long extension of the ORF65 sequence were detected in 2 of 14 controls seropositive for KSHV, despite significant responses against the ORF65 sequence derived from the BC-1 cell line.
CTL activity against antigens derived from KSHV and EBV
| Antigen . | Cohort . | ||
|---|---|---|---|
| MM . | KSHV-positive* . | KSHV-negative . | |
| KSHV ORF73 | 0/16 | 10/141-153 | 0/10 |
| KSHV ORF65 | 0/16 | 8/14 | 0/10 |
| MM ORF65† | 0/16 | 2/14 | 0/10 |
| EBV‡ | 16/16 | 14/14 | 10/10 |
| Antigen . | Cohort . | ||
|---|---|---|---|
| MM . | KSHV-positive* . | KSHV-negative . | |
| KSHV ORF73 | 0/16 | 10/141-153 | 0/10 |
| KSHV ORF65 | 0/16 | 8/14 | 0/10 |
| MM ORF65† | 0/16 | 2/14 | 0/10 |
| EBV‡ | 16/16 | 14/14 | 10/10 |
Values are numbers of patients.
KSHV positivity was determined by latency-associated nuclear antigen-specific serologic assessment as described previously.2
The MM ORF65 antigen pool included the 4 peptides spanning the described MM-KSHV ORF65 sequence C-terminal to the stop codon in the previously reported ORF65.
Only those with a response to EBV are included in the table. However, 11 patients with MM who had no response to EBV were also negative for ORF65-specific and ORF73-specific responses on ELISPOT assays.
ELISPOT responses were considered positive if values were higher than 15 spot-forming cells (SFCs)/106 PBMCs. The range for positive ORF73-specific responses was 257 to 1280 SFCs/106 PBMCs, that for ORF65-specific responses was 40 to 120 SFCs/106 PBMCs, and that for positive EBV responses was 25 to 1500 SFCs/106 PBMCs.
EBV-specific CTL responses were also measured to provide a positive control in the ELISPOT assay to confirm immune competence in these patients. In the current study, only those with a positive EBV response were included; however, an additional 11 patients with MM who did not respond to the EBV-positive control were tested and excluded. The unresponsiveness to EBV does not necessarily reflect immune incompetence, because these patients represented a broad range of different HLA alleles examined by using one set of defined, HLA class I–restricted CTL epitopes.10 Nevertheless, the nonresponders to EBV analyses excluded from these analyses were also all negative for KSHV-specific CTL reactivity.
The lack of significant CTL responses specific for KSHV ORF65 and ORF73 in patients with MM, coupled with the inability to detect viral transcripts against ORF26, ORF72, and ORF74, suggest that KSHV does not play an important role in the pathogenesis of MM. However, our conclusions have some limitations. First, the level of viral replication may be very low and not detectable with the sensitive RT-PCR method used. Such low-level viral replication could also lead to an antigenic load insufficient for induction of humoral and cellular immune responses. Second, it has been suggested that a variant of KSHV that does not induce a humoral immune response detectable by available serologic tests is present in patients with MM.4,7However, our data suggest that these possibilities are unlikely, because very sensitive RT-PCR analyses, with a detection limit of 1 viral copy in 10 × 103 cells, was performed by using primers specific for 3 different KSHV genes.6,12 To avoid detection by this RT-PCR, the virus in patients with MM would have to be changed in all these locations and represent a very distant relative of KSHV. Moreover, if all those KSHV proteins that have been shown to be targeted by humoral or cellular immune responses were either altered or completely absent in the virus associated with MM, the elusive infectious agent would likely be unrelated to the KSHV described by Chang et al in 1994.15 Nevertheless, to examine such a possibility, we included the recently described ORF65 sequence unique to patients with MM7 in the ELISPOT analyses. No specific responses were detected in any of our patients with MM. Although we cannot rule out the possibility that ongoing viral replication was insufficient to maintain detectable memory CTL responses against the lytic ORF65 protein, one would expect to detect at least some responses against the latent gene product of ORF73, which is required for viral genome persistence.8 16
Although we cannot completely rule out the existence of a KSHV-related virus in patients with MM, the current findings constitute a strong indication that there is no ongoing KSHV viral replication in these patients at a level sufficient to induce humoral or cellular CTL responses. The apparent lack of viral transcripts in samples from MM patients, coupled with the absence of cellular immune responses readily and generally detectable in patients infected with KSHV, provide strong evidence against an association between KSHV and the pathogenesis of MM.
Supported by National Institutes of Health Grants RO-1 50947 and PO-1 78378, the Multiple Myeloma Research Foundation (N.R., D.H., and T.H.), and the Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Kenneth C. Anderson, Jerome Lipper Multiple Myeloma Center, Department of Adult Oncology, Dana Farber Cancer Institute, 44 Binney Street, Boston, MA 02115; e-mail:kenneth_anderson@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal