Tumor necrosis factor-α (TNF-α), a cytokine possessing pleiotropic biological activities, is produced by leukemic lymphocytes in patients with chronic lymphocytic leukemia (CLL) and acts as an autocrine and paracrine growth factor in this disease. In this study, TNF-α levels were determined in 150 patients with CLL and correlated with disease characteristics, prognostic factors, and survival. The mean TNF-α plasma concentration in the patients with CLL was significantly higher than in the healthy control population (16.4 versus 8.7 pg/mL; P < .0001). Patients having an elevated TNF-α level had more advanced Rai and Binet stage disease, higher serum β2-microglobulin (β2M) levels, a greater percentage of cells expressing CD38, and lower hemoglobin and platelet levels. Patients having chromosomal abnormalities such as 11q deletion, trisomy 12, and chromosome 17 aberrations had a higher mean TNF-α level (27.5 pg/mL) than patients having a diploid karyotype or other miscellaneous cytogenetic abnormalities (14.2 pg/mL;P < .001). The TNF-α level was a predictor of survival when the Cox proportional hazards model was used with TNF-α entered as a continuous variable (P = .0001). Also, patients having a TNF-α level above the mean value of 14 pg/mL had significantly shorter survival duration (P = .00001). The TNF-α level remained predictive of survival in Cox multivariate analysis independent of Rai staging and β2M, hemoglobin, prior therapy, white cell count, and platelet level (P = .005). We conclude that the TNF-α level serves as a prognostic factor in patients with CLL and that inhibition of TNF-α in these patients could have therapeutic importance.
Introduction
Tumor necrosis factor-α (TNF-α) is a cytokine having a peptide structure that possesses pleiotropic biological activities. The active form of TNF-α is a homotrimer having a molecular mass of 53 kd.1 The gene for TNF-α has been localized to the short arm of chromosome 6.2TNF-α plays a physiological role in host defense, inflammation, and cell differentiation and a pathological role in diverse conditions such as fever, cachexia, septic shock, rheumatoid arthritis, and inflammatory bowel disease.3,4 Monocytes and macrophages are the main source of TNF-α, although other cell types, in particular, T and B lymphocytes and large granular lymphocytes, have been shown to produce TNF-α upon stimulation.5,6 In chronic lymphocytic leukemia (CLL), the neoplastic lymphocytes release TNF-α spontaneously in vitro,7 and exposure to TNF-α in vitro increases the proliferation and viability of leukemic lymphocytes.8 It was postulated that TNF-α acts as an autocrine growth factor in CLL; the work published by Cordingley et al9 supports this hypothesis. The in vitro observations described above and evidence of an increased TNF-α serum level in patients having CLL7 prompted the evaluation of TNF-α levels in a large cohort of such patients to determine if the TNF-α level correlated with unfavorable features and might reflect clinical behavior.
Patients and methods
Subjects
Peripheral blood samples were obtained from 150 consecutive patients having CLL during routine diagnostic evaluation in the Department of Leukemia at The University of Texas M. D. Anderson Cancer Center. Of these patients, 92 were previously untreated and 58 were previously treated outside our institution. All our previously treated patients had been off therapy for several months at the time samples were obtained. The diagnosis of CLL was established based on morphology, flow cytometric analysis (CD5+, CD19+, and CD23+), and molecular analysis (JH and κ- or λ-chain rearrangement). All samples were obtained in accordance with protocols approved by the M. D. Anderson Cancer Center Human Subjects Committee (Houston, TX), and all patients gave informed consent. Peripheral blood samples obtained from 20 normal healthy donors were used as controls. Patient characteristics are illustrated in Table1.
Patient characteristics
| Characteristic . | . |
|---|---|
| Median age, y (range) | 62 (32-81) |
| Sex, no. (%) | |
| Female | 55 (37) |
| Male | 95 (63) |
| Rai stage (n = 142), no. (%) | |
| 0-II | 99 (70) |
| III-IV | 43 (30) |
| Binet stage (n = 141), no. (%) | |
| A | 63 (45) |
| B | 41 (29) |
| C | 37 (26) |
| Median β2M serum level, nM (range) (mg/L [range]) | 254 (118-1303); (3 [1.4-15.4]) |
| Previously treated, no. (%) | 58 (39) |
| Characteristic . | . |
|---|---|
| Median age, y (range) | 62 (32-81) |
| Sex, no. (%) | |
| Female | 55 (37) |
| Male | 95 (63) |
| Rai stage (n = 142), no. (%) | |
| 0-II | 99 (70) |
| III-IV | 43 (30) |
| Binet stage (n = 141), no. (%) | |
| A | 63 (45) |
| B | 41 (29) |
| C | 37 (26) |
| Median β2M serum level, nM (range) (mg/L [range]) | 254 (118-1303); (3 [1.4-15.4]) |
| Previously treated, no. (%) | 58 (39) |
Samples
Peripheral venous blood samples were collected in sterile test tubes. On the same day, plasma was separated from each specimen and stored at −70°C until the time of analysis.
Plasma TNF-α immunoassay
TNF-α levels were assayed in duplicate, with all of the levels expressed as the mean of the 2 determinations. A standard curve was generated with the use of known concentrations of human TNF-α. The concentration of TNF-α in both patient and control plasma samples was obtained from the standard curve. The assessment of TNF-α immunoreactivity used a validated commercial enzyme-linked immunosorbent assay (ELISA) (Quantikine Human TNF-α Immunoassay; R&D Systems, Minneapolis, MN). This assay uses a solid-phase monoclonal anti–TNF-α antibody bound to microtiter plates. Unbound protein is removed by washing; a polyclonal anti–TNF-α antibody conjugated to horseradish peroxidase is then added, with excess conjugated antibody removed by further washing. Next, a substrate solution containing stabilized hydrogen peroxide and chromogen is added; color develops in proportion to the amount of TNF-α bound in the initial step. The reaction is stopped after 20 minutes of incubation at room temperature, and the color intensity is quantified by means of a microtiter plate reader at a wavelength of 450 nm. The ELISA has been reported by the manufacturer to measure the total amount of TNF-α present, free TNF-α, and TNF-α bound to the soluble receptors. No significant cross-reactivity was observed with recombinant human TNF-β, (rhTNF-β), rhTNF-R1, or rhTNF-R2.
Statistical analysis
All data were collected while reviewing the patients' records and compiled in the Department of Leukemia database. Survival was plotted by means of Kaplan-Meier plots and compared by means of the log-rank test. The Cox proportional hazards model was used to predict survival for the continuous variables in the univariate and multivariate analyses. Categorical comparison was performed by means of the Kruskal-Wallis test. Finally, the Spearman rank correlation coefficient was used for correlation testing.
Results
Increased TNF-α plasma concentrations in patients having CLL
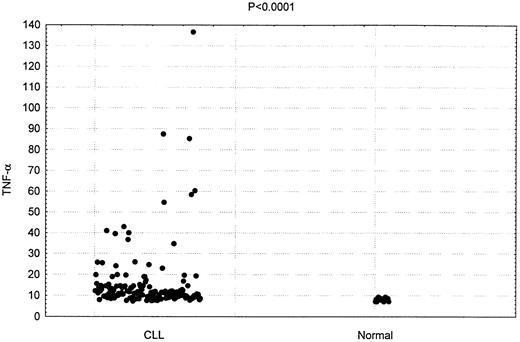
TNF-α plasma concentration was measured in 150 patients with CLL. As shown in Figure 1, the mean plasma concentration in these patients was 16.4 pg/mL. This was significantly higher than the mean plasma concentration detected in the 20 hematologically normal subjects (8.7 pg/mL;P < .0001).
Increased TNF-α plasma concentrations in patients having CLL.
Comparison of TNF-α levels in 150 CLL patients and 20 healthy donors (P < .0001)
Increased TNF-α plasma concentrations in patients having CLL.
Comparison of TNF-α levels in 150 CLL patients and 20 healthy donors (P < .0001)
Correlation of TNF-α levels with Rai and Binet staging
TNF-α plasma levels correlated significantly with stage of disease. Patients with Rai stage III or IV disease and Binet stage C disease had higher TNF-α levels. The mean TNF-α level was 22.3 pg/mL (P < .004) in patients having Rai stage III or IV disease and 23.8 pg/mL (P < .0003) in those having Binet stage C disease. The mean values and SEs are depicted in Figure2.
Correlation of TNF-α levels with Rai and Binet staging.
TNF-α plasma levels in CLL patients according to Rai staging in all patients (panel A), of previously untreated patients (panel B), and according to Binet staging in all patients (panel C). Similar results were obtained when only previously untreated patients were considered.
Correlation of TNF-α levels with Rai and Binet staging.
TNF-α plasma levels in CLL patients according to Rai staging in all patients (panel A), of previously untreated patients (panel B), and according to Binet staging in all patients (panel C). Similar results were obtained when only previously untreated patients were considered.
Correlation of TNF-α plasma level with patient characteristics and prognostic markers
The TNF-α plasma concentrations in the patients with CLL correlated strongly and directly with the serum β2-microglobulin (β2M) levels (SpearmanR = 0.6, P < .0001, andR = 0.51, P < .0001, respectively); mildly with CD38 expression (R = 0.43, P < .0001) (Figure 3); and slightly with total white blood cell count (R = 0.27, P < .0006). There was an moderate inverse correlation between the TNF-α level and the hemoglobin (R = −0.37, P < .0001) and platelet values (R = −0.35, P < .0001). Similar correlation was seen when only previously untreated patients were considered (Table 2).
Correlation between CD38 expression and TNF-α.
Scatter plot showing the correlation between CD38 expression in the leukemic cells and TNF-α levels.
Correlation between CD38 expression and TNF-α.
Scatter plot showing the correlation between CD38 expression in the leukemic cells and TNF-α levels.
Correlation between TNF-α level and clinical variables
| Clinical variable . | All patients . | Previously untreated patients . | ||
|---|---|---|---|---|
| R . | P . | R . | P . | |
| WBC | 0.27 | .0006 | 0.39 | .0001 |
| Hemoglobin | − 0.37 | .0001 | − 0.28 | .0057 |
| Platelet | − 0.35 | .0001 | − 0.24 | .0202 |
| CD38 expression | 0.43 | .0001 | 0.39 | .0030 |
| β2M level (serum) | 0.60 | .0001 | 0.49 | .0000 |
| Clinical variable . | All patients . | Previously untreated patients . | ||
|---|---|---|---|---|
| R . | P . | R . | P . | |
| WBC | 0.27 | .0006 | 0.39 | .0001 |
| Hemoglobin | − 0.37 | .0001 | − 0.28 | .0057 |
| Platelet | − 0.35 | .0001 | − 0.24 | .0202 |
| CD38 expression | 0.43 | .0001 | 0.39 | .0030 |
| β2M level (serum) | 0.60 | .0001 | 0.49 | .0000 |
WBC indicates white blood count.
Presence of high TNF-α levels in patients carrying unfavorable cytogenetic abnormalities
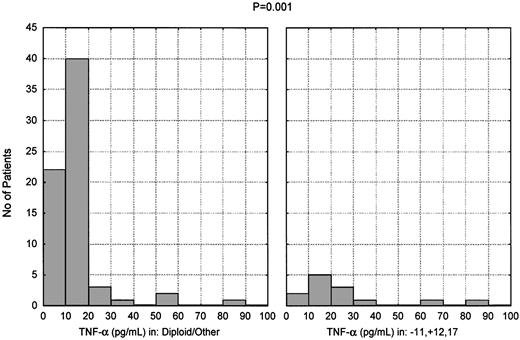
Karyotypic analysis at the time of testing was available for 82 of the 150 patients studied. The patients were assigned to 2 groups according to this analysis. The first group consisted of 13 patients (16%) who had one or more of the following: 11q deletion, trisomy 12, any chromosome 17 aberration. The second group consisted of the remaining 69 patients (84%), including 48 patients (59%) having a diploid karyotype. There was a significant difference in TNF-α level between the 2 groups: as shown in Figure4, the mean TNF-α level was 27.5 pg/mL in the first group and 14.2 pg/mL in the second group (P < .001).
Presence of high TNF-α levels in patients carrying unfavorable cytogenetic abnormalities.
TNF-α plasma levels in CLL patients subdivided according to cytogenetic analysis. Thirteen patients had 11q deletion, trisomy 12, or chromosome 17 abnormalities (alone or in combination); 48 patients had a diploid karyotype; and 21 had miscellaneous abnormalities (P < .001).
Presence of high TNF-α levels in patients carrying unfavorable cytogenetic abnormalities.
TNF-α plasma levels in CLL patients subdivided according to cytogenetic analysis. Thirteen patients had 11q deletion, trisomy 12, or chromosome 17 abnormalities (alone or in combination); 48 patients had a diploid karyotype; and 21 had miscellaneous abnormalities (P < .001).
Correlation of a high TNF-α plasma level with shorter survival
To evaluate the correlation between the TNF-α level and survival duration, the TNF-α level was entered as a continuous variable in the Cox proportional hazards model. In this model, the TNF-α level was a strong predictor of survival, and higher levels of TNF-α correlated with shorter survival duration (P = .0001). A multivariate Cox proportional hazards model incorporating the TNF-α, β2M levels, hemoglobin levels, and Rai stage also showed that the TNF-α was an independent prognostic factor (Table3).
Multivariate Cox proportional hazards analysis of overall survival
| Variable . | Coefficient . | SE . | Multivariate P . |
|---|---|---|---|
| β2M level | .10 | 0.17 | .43 |
| TNF-α level | .09 | 0.03 | .005 |
| Hemoglobin level | −.95 | 0.31 | .002 |
| Platelet level | −.01 | 0.006 | .02 |
| Rai stage | −.70 | 0.38 | .06 |
| WBC | .002 | 0.002 | .30 |
| Prior therapy | 2.85 | 1.30 | .02 |
| Variable . | Coefficient . | SE . | Multivariate P . |
|---|---|---|---|
| β2M level | .10 | 0.17 | .43 |
| TNF-α level | .09 | 0.03 | .005 |
| Hemoglobin level | −.95 | 0.31 | .002 |
| Platelet level | −.01 | 0.006 | .02 |
| Rai stage | −.70 | 0.38 | .06 |
| WBC | .002 | 0.002 | .30 |
| Prior therapy | 2.85 | 1.30 | .02 |
WBC indicates white blood count.
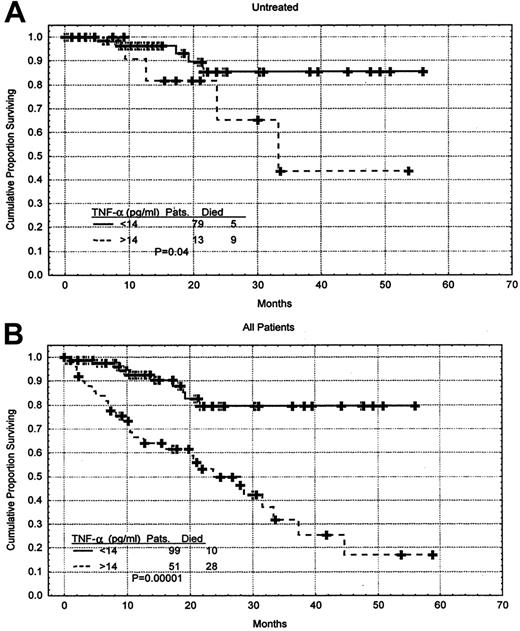
Furthermore, the TNF-α level was a predictor of survival when only the 92 previously untreated patients were considered (P = .006) as well as when only patients having early-stage disease (Rai 0-II, 103 patients) were evaluated (P = .02). Survival rates were also analyzed with the use of the mean value of the untreated patients, which was 14 pg/mL. Previously untreated patients having a TNF-α level above the mean value had a shorter survival duration when compared with those having a TNF-α level below the mean (P = .04) (Figure5A). This was also true when all patients were considered (P = .000 01) (Figure 5B). The cutoff point of 14 was reached with the use of the Martingale residual plot. The Martingale residual plot correlates the residual risk of death with the level of TNF-α. The plot showed change in the risk of death to be around 14 pg/mL, and for simplicity, we elected to use 14 pg/mL, which corresponds to the mean of the untreated patients. The characteristics of the previously untreated patients who had high levels of TNF-α were not significantly different from the rest of the patients. They had median hemoglobin level of 125 g/L (12.5 g/dL), median platelet count of 163 × 109/L (163 × 103/μL), median white blood cell count of .099 × 109/L (99 × 103/μL), median age of 64, and median β2M level of 294 nM (3.47 mg/L).
Correlation between TNF-α level and survival duration.
(A) Survival of the previously untreated CLL patients stratified by TNF-α level. The mean TNF-α level in this group of patient was 14 pg/mL. Distributions were estimated by means of the Kaplan-Meier method. The tick marks indicate the point of last follow-up for one or more of the patients who did not die. (B) When all 150 patients were considered, higher levels of TNF-α correlated with shorter survival.
Correlation between TNF-α level and survival duration.
(A) Survival of the previously untreated CLL patients stratified by TNF-α level. The mean TNF-α level in this group of patient was 14 pg/mL. Distributions were estimated by means of the Kaplan-Meier method. The tick marks indicate the point of last follow-up for one or more of the patients who did not die. (B) When all 150 patients were considered, higher levels of TNF-α correlated with shorter survival.
Discussion
These results indicated that the TNF-α plasma level is increased in patients having CLL and that there is a correlation between the TNF-α plasma level and known prognostic factors in CLL. Expression of CD38 is a recently described prognostic factor in CLL10,11; specifically, a CD38 expression level greater than 20% in the bone marrow cells of patients having CLL is considered an adverse feature. In our study, CD38 expression was higher in patients having an elevated TNF-α level. Patients having an elevated TNF-α level also had an increased concentration of β2M, a poor prognostic factor in lymphoid malignancies.12
Genomic aberrations are another important predictor of survival in patients with CLL.13 In this study, patients having poor prognostic cytogenetic abnormalities that included 11q deletion, trisomy 12, and aberrations of chromosome 17 had a significantly higher TNF-α level when compared with patients having a diploid karyotype or other miscellaneous abnormalities.
As described above, the level of TNF-α correlated with survival. Specifically, patients having a higher level had a shorter survival time. This effect was independent of other prognostic factors. The correlation between TNF-α levels and survival was noted in patients having both early- and late-stage disease and in patients who both did and did not undergo treatment.
These observations suggest that TNF-α is involved in the progression of CLL. Furthermore, several studies have described the existence of autocrine as well as paracrine loops involving TNF-α and other cytokines, such as interleukin-1 (IL-1), IL-4, interferon-γ, IL-6, and IL-10 in CLL and other B-cell neoplasms. There is significant variability in the literature regarding the effect of TNF-α on CLL cells.7-9,14 We believe that this is a consequence of the complexity of the TNF-α system in vivo. TNF-α can be active in soluble and cell-bound form,15 and the 2 are in continuous equilibrium. Two transmembrane receptors for TNF-α have been identified: TNF-R1 (55 kd) and TNF-R2 (75 kd).16,17 These receptors are shaded by proteolytic cleavage and exist as soluble receptors. Neoplastic lymphocytes of patients having CLL express both receptors; TNF-R2 seems to be the dominant receptor in CLL cells, but it is not known which signaling pathways are activated upon binding of TNF-α to its corresponding receptor.17 TNF-α was shown to stimulate, inhibit, or have no effect on CLL cell proliferation.7-9,18 This is not surprising since TNF-α activates cell survival and cell death mechanisms simultaneously and can influence cell growth by apoptotic, nonapoptotic, and antiapoptotic mechanisms.19 As an example, TNF-α activates transcription factor NFκΒ (NFkB) and mitogen-activated protein kinases that are involved in cell proliferation, differentiation, and protection from apoptosis, and at the same time triggers cleavage of caspase 8 via the TRADD-FADD-FLICE pathway that will result in induction of apoptosis.20 Jabbar et al21 showed that the NFkB pathway was inducible in the malignant lymphocytes of 15 patients with CLL.
In our study population, we measured total TNF-α using a methodologically simple ELISA that can be applied to large clinical studies. The elevation of TNF-α observed in our study was associated with aggressive clinical behavior and shorter survival, suggesting that the TNF-α we measured has relevant biological activity.
Patients having an elevated TNF-α level also had significant anemia and thrombocytopenia. The anemia and thrombocytopenia in these patients may have been due to extensive bone marrow replacement by CLL but may also be attributed to the direct suppressive effect of TNF-α on the erythroid and thrombopoietic lineages. This is in agreement with the potent inhibitory effect of TNF-α on hematopoiesis in vitro described by our group and others22,23 and with the development of progressive anemia reported in patients who received TNF-α for prolonged time.24-26 Furthermore, Michalevevicz et al27 were able to restore in vitro normal hematopoiesis in 11 out of 15 patients having CLL using anti–TNF-α antibodies.
On the basis of these observations, it is possible that patients having an elevated TNF-α level may benefit from targeted interventions using specific TNF-α inhibitors, such as the soluble TNF-α receptor p75 and the anti–TNF-α monoclonal antibody inflixiMAB, that are available for clinical use. The p75 has been used to treat nonmalignant diseases such as rheumatoid arthritis and inflammatory bowel syndrome and appears to be effective and well tolerated.4 InflixiMAB was recently approved for the management of Crohn disease and rheumatoid arthritis on the basis of its clinical activity and safe toxicity profile.28 Specific inhibition of TNF-α could potentially result in the control of proliferation of the leukemic clone as well as elimination of the suppressive effects on other hematopoietic lineages.
We conclude that the TNF-α plasma level correlates with the extent of disease in patients with CLL and is a novel prognostic factor for survival. The TNF-α level should be monitored in conjunction with other disease indicators, and patients having an elevated TNF-α level should be considered for specific therapeutic intervention using TNF-α inhibitory molecules.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maher Albitar, Department of Hematopathology, Box 72, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: malbitar@mdanderson.org.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal