Abstract
The role of ursodeoxycholic acid (UDCA) in the prevention of hepatic complications after allogeneic stem cell transplantation was studied in a prospective randomized open-label multicenter trial. A total of 242 patients were allocated to receive (n = 123) or not to receive (n = 119) UDCA in the dose of 12 mg/kg/d orally from the day preceding the conditioning until day 90 after transplantation. In the UDCA-treated group a significantly smaller proportion of patients developed a serum bilirubin level exceeding 50 μM (18 of 123 versus 31 of 119, P = .04), and similarly a smaller proportion of patients exceeded the alanine aminotransferase level of 100 U/L. There was no difference in the incidence of veno-occlusive disease of the liver. Compared to the control group, in the UDCA-treated group there was a nonsignificant trend toward a lower overall incidence of acute graft-versus-host disease (GVHD) and a significantly lower incidence of grade III to IV acute GVHD (5 of 123 versus 17 of 119,P = .01), stage II to IV liver and intestinal GVHD, and stage III to IV skin GVHD. There was no difference in the incidence of chronic GVHD or in the relapse rate. Among the patients given UDCA, the survival at 1 year was significantly better, 71% versus 55% (P = .02), and the nonrelapse mortality rate was lower, 19% versus 34% (P = .01), than in the control group. There were significantly more deaths in GVHD in the control group. In conclusion, UDCA administration reduced hepatic problems and severe acute GVHD and improved survival. These results suggest a role for UDCA in the prevention of transplant-related complications in allogeneic transplantation.
Introduction
Liver problems are very common after allogeneic stem cell transplantation, and only a minority of patients get through the treatment with normal liver findings. Often the abnormalities are mild, but severe problems, particularly veno-occlusive disease (VOD) of the liver and graft-versus-host disease (GVHD) affecting the liver, are relatively common. Liver GVHD is prevented and managed as GVHD in general. There has been no generally accepted and effective prophylaxis for VOD, and the treatment has been problematic, though defibrotide has recently proved promising as an effective agent.1 2
Ursodeoxycholic acid (UDCA) is a hydrophilic bile acid that constitutes less than 5% of bile acids in normal bile.3 This proportion can be increased to 40% to 50% by oral administration.3 The concentration of hydrophobic bile acids is thereby reduced.4-6 Hydrophobic bile acids are toxic to liver parenchymal cells in direct contact, which takes place in disorders damaging bile ducts, whereas hydrophilic bile acids are nontoxic. UDCA has also been shown to have other effects that may be useful in the prophylaxis and management of liver problems. It stabilizes hepatocyte cell membrane by altering the lipid composition,7 and it has been shown to reduce the release and expression of inflammatory cytokines such as tumor necrosis factor α (TNF-α) and interleukin 1α (IL-1α) in a hepatoblastoma cell line8 and the production of IL-2, IL-4, and γ-interferon by peripheral blood mononuclear cells.9 It has also been shown to have other immunomodulatory effects.3 9
UDCA has been studied as treatment for several liver problems. In primary biliary cirrhosis it has a positive effect on biochemical parameters, but the effect on the long-term outcome has remained less clear.3,10-12 It may have some effect in the prevention of rejection episodes after liver transplantation.13 In 2 randomized studies UDCA prophylaxis was reported to reduce the incidence of VOD after allogeneic stem cell transplantation.14,15 UDCA has also been reported to be effective in the treatment of manifest GVHD.16 17 The present large randomized multicenter trial of the effects of prophylactic UDCA administration in allogeneic transplantation shows that this treatment has beneficial effects, not only on biochemical liver parameters, but also on GVHD and survival.
Patients and methods
Study design
This is a prospective randomized, open-label multicenter study. All patients undergoing allogeneic hematopoietic stem cell transplantation during the period from January 1996 to November 1998 at the 3 participating centers, a total of 244 patients, were randomized to receive or not to receive UDCA. The randomization was stratified according to the disease category (low or high risk), type of donor (HLA-identical sibling, other related, or unrelated), conditioning (total body irradiation [TBI], yes or no), and center. Low-risk patients were those with acute leukemia in first remission, chronic myeloid leukemia in first chronic phase, aplastic anemia, or hereditary disorder. All others were high-risk patients.
HLA typing
Approximately one half of the patients and donors were typed by standard serologic cytotoxicity tests and the other half by polymerase chain reaction (PCR)–based methods for class I alleles both in transplantations from a sibling and in those from an unrelated donor. When serology was used, uncertain findings were confirmed with PCR-based techniques. Class II alleles were typed by PCR methods, in approximately one half of the cases with low-resolution techniques and in the other half with highresolution techniques.
Study drug and other treatment
According to randomization, the patients received or did not receive UDCA (Adursal, Leiras, Helsinki, Finland). The drug was given as tablets of 150 mg, which could be halved. The dose was 12 mg/kg/d divided into 2 doses, and the daily dose was rounded upward to the closest dose. The administration was initiated on the day preceding the first dose of conditioning and continued until day 90 after transplantation.
All other treatment was given according to local routine and identically in both study groups.
Study parameters
The recipient's and the donor's cytomegalovirus (CMV) and hepatitis C virus antibodies and hepatitis B virus surface antigen as well as the patient's antibodies against hepatitis B virus surface antigen before transplantation were recorded. The serum total bilirubin and alanine aminotransferase (ALAT) concentrations were measured before the transplantation, twice weekly until day 28, and thereafter weekly until day 91 after transplantation. The actual doses of UDCA taken were recorded daily during the hospitalization. The cyclosporine concentrations were monitored according to local routine. The doses of cyclosporine per recipient weight were registered. VOD of the liver was diagnosed according to the criteria of McDonald et al18and Jones et al.19 Acute20,21 and chronic22 23 GVHD were graded according to previously published criteria. All cases of gastrointestinal GVHD were verified by biopsy. Liver GVHD was documented either by liver biopsy or by biopsy-proven gastrointestinal GVHD and simultaneous clinical and laboratory findings compatible with liver GVHD. Skin GVHD was in most cases diagnosed on clinical grounds. Other parameters recorded were relapse, survival, cause of death, and adverse effects of UDCA.
Statistics
The primary parameter was the proportion of patients with a total serum bilirubin concentration exceeding the level of 50 μM (2.5 times the upper limit of normal) during the first 90 days after transplantation. The sample size of 120 patients per group provided 87% power at the significance level of .05 to detect a minimum reduction from 25% to 10% in the proportion of patients with the maximum total bilirubin concentration exceeding this level. The cumulative risk of elevated serum total bilirubin and ALAT concentrations, acute GVHD and nonrelapse mortality, and survival were analyzed by Kaplan-Meier curves and compared by the log-rank test. The proportions between the groups were compared by the χ2test or Fisher exact test. The differences between the groups in the daily doses of cyclosporine were tested using the Mann-Whitney test. All statistics were performed with SPSS software for Windows 95.
Results
A total of 244 patients were randomized. One patient in each arm did not undergo the transplantation, one due to death before transplantation (before the conditioning and the initiation of the study drug) and the other due to cancellation of the transplantation. Thus 242 patients remained for evaluation.
Patient characteristics
The characteristics of the patients are shown in Table1. There were no statistically significant differences between the study groups in sex, age, diseases, proportion of low- and high-risk patients, type of donor, type of conditioning, type of graft, or GVHD prophylaxis. Most patients received cyclosporine A and a short course of methotrexate for GVHD prophylaxis. In 2 of the participating centers low-dose corticosteroid was added.24 All transplantations for malignant disorders were conventional full-intensity transplantations.
Patient characteristics
| . | Treatment group . | |
|---|---|---|
| With UDCA . | Without UDCA . | |
| Patients | ||
| Total number | 123 | 119 |
| Female | 62 | 58 |
| Male | 61 | 61 |
| Age, median y (range) | 38 (5-59) | 40 (1-58) |
| Disease | ||
| AML | 38 | 34 |
| CML | 31 | 37 |
| ALL | 26 | 24 |
| MDS | 11 | 12 |
| MM | 2 | 3 |
| NHL | 2 | 2 |
| CLL | 2 | 2 |
| MF | 2 | 1 |
| ET | 0 | 1 |
| NK cell leukemia | 0 | 1 |
| HES | 1 | 0 |
| SAA | 3 | 0 |
| Fanconi | 1 | 0 |
| Amegakaryocytic thrombocytopenia | 1 | 0 |
| CGD | 1 | 1 |
| FHL | 1 | 1 |
| AGU | 1 | 0 |
| Low risk | 75 | 65 |
| High risk | 48 | 54 |
| Donor | ||
| HLA-identical sibling | 64 | 68 |
| Unrelated | 57 | 51 |
| Other family member | 2 | 0 |
| Conditioning | ||
| TBI containing | 112 | 107 |
| Cytostatic drugs only | 11 | 12 |
| Busulphan | 6 | 11 |
| ATG | 56 | 51 |
| Type of graft | ||
| Bone marrow | 97 | 93 |
| Blood stem cells | 26 | 26 |
| GVHD prophylaxis | ||
| CSA + MTX | 63 | 60 |
| CSA + MTX + MP | 57 | 55 |
| CSA + MTX + TCD | 3 | 0 |
| CSA + MP | 0 | 3 |
| MTX + MP | 0 | 1 |
| . | Treatment group . | |
|---|---|---|
| With UDCA . | Without UDCA . | |
| Patients | ||
| Total number | 123 | 119 |
| Female | 62 | 58 |
| Male | 61 | 61 |
| Age, median y (range) | 38 (5-59) | 40 (1-58) |
| Disease | ||
| AML | 38 | 34 |
| CML | 31 | 37 |
| ALL | 26 | 24 |
| MDS | 11 | 12 |
| MM | 2 | 3 |
| NHL | 2 | 2 |
| CLL | 2 | 2 |
| MF | 2 | 1 |
| ET | 0 | 1 |
| NK cell leukemia | 0 | 1 |
| HES | 1 | 0 |
| SAA | 3 | 0 |
| Fanconi | 1 | 0 |
| Amegakaryocytic thrombocytopenia | 1 | 0 |
| CGD | 1 | 1 |
| FHL | 1 | 1 |
| AGU | 1 | 0 |
| Low risk | 75 | 65 |
| High risk | 48 | 54 |
| Donor | ||
| HLA-identical sibling | 64 | 68 |
| Unrelated | 57 | 51 |
| Other family member | 2 | 0 |
| Conditioning | ||
| TBI containing | 112 | 107 |
| Cytostatic drugs only | 11 | 12 |
| Busulphan | 6 | 11 |
| ATG | 56 | 51 |
| Type of graft | ||
| Bone marrow | 97 | 93 |
| Blood stem cells | 26 | 26 |
| GVHD prophylaxis | ||
| CSA + MTX | 63 | 60 |
| CSA + MTX + MP | 57 | 55 |
| CSA + MTX + TCD | 3 | 0 |
| CSA + MP | 0 | 3 |
| MTX + MP | 0 | 1 |
AML indicates acute myeloid leukemia; CML, chronic myeloid leukemia; ALL, acute lymphatic leukemia; MDS, myelodysplastic syndrome; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; CLL, chronic lymphatic leukemia; MF, myelofibrosis; ET, essential thrombocythemia; NK, natural killer; HES, hypereosinophilic syndrome; SAA, severe aplastic anemia; CGD, chronic granulomatous disease; FHL, familial hemophagocytic lymphohistiocytosis; AGU, aspartylglucosaminuria; ATG, antithymocyte globulin; CSA, cyclosporine A; MTX, methotrexate; MP, methylprednisolone; and TCD, T-cell depletion.
The patients and donors were HLA A, B, DR-matched except in 5 transplantations from an unrelated donor in each randomization group. In the group of patients given prophylactic UDCA there was one HLA A antigen mismatch in 3 cases, one HLA B antigen mismatch in 1 case, and one DR subtype mismatch in 1 case. In the control group there was one HLA B antigen mismatch in 1 case, one HLA B subtype mismatch in 3 cases, and one DR subtype mismatch in 1 case. Both related donors other than sibling were HLA A, B, DR matched with the recipient.
There was no statistically significant difference in the virologic status of the recipients or donors between the study groups. Among the patients randomized to receive UDCA 70% were anti-CMV+, and the corresponding figure among those not given UDCA was 73%. Sixty-two percent and 64% of the donors were anti-CMV+, respectively.
Compliance
Because the study drug was given orally and many patients have difficulties in taking drugs by mouth during the first few weeks after the transplantation, the actual doses of the study drug taken were registered daily during the hospitalization. During each week more than half of the patients were able to take all tablets. The proportion of all prescribed UDCA tablets actually taken was 94% during the week preceding the transplantation, 79% during the first week, 71% during the second week, 78% during the third week, and 83% during the fourth week after the transplantation. During the same weeks the proportion of patients who took more than 80% of the tablets was 91%, 67%, 66%, 74%, and 81%, respectively.
Bilirubin and ALAT
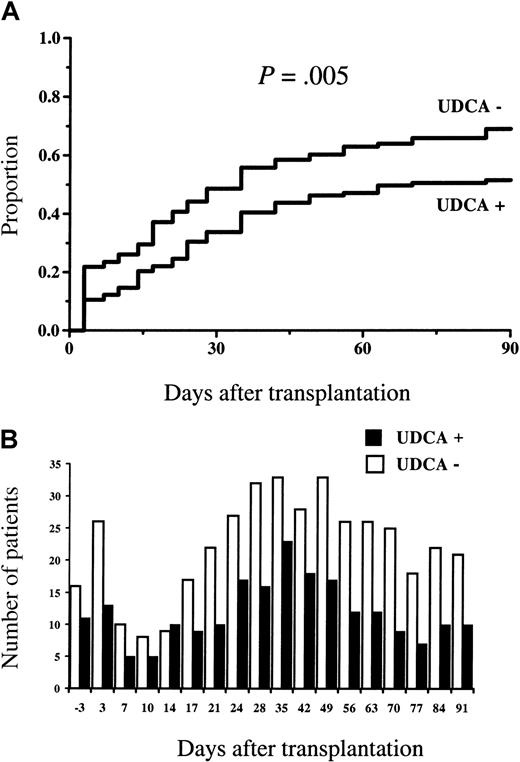
Statistically significantly fewer patients in the group given UDCA developed a serum bilirubin concentration of higher than 50 μM (2.5 times the upper limit of normal) (18 of 123 versus 31 of 119,P = .04). Figure 1A shows the cumulative proportion of patients exceeding this blood level. Figure 1B shows the numbers of patients with serum bilirubin higher than 50 μM at each time of measurement. With one exception, at 7 days after the transplantation, the number of patients exceeding this level was always higher in the study group not given UDCA.
Serum bilirubin concentrations.
(A) The cumulative proportion of patients developing a serum bilirubin concentration higher than 50 μM, and (B) the number of patients with serum bilirubin higher than 50 μM at each time of measurement. Statistically significantly fewer patients in the group given UDCA exceeded this blood level (2.5 times the upper limit of normal), and, with one exception, the number of patients exceeding this level was always higher in the group not given UDCA.
Serum bilirubin concentrations.
(A) The cumulative proportion of patients developing a serum bilirubin concentration higher than 50 μM, and (B) the number of patients with serum bilirubin higher than 50 μM at each time of measurement. Statistically significantly fewer patients in the group given UDCA exceeded this blood level (2.5 times the upper limit of normal), and, with one exception, the number of patients exceeding this level was always higher in the group not given UDCA.
The number of patients who developed a serum ALAT concentration higher than 100 U/L (2.5 times the upper limit of normal) was statistically significantly less in the study group given UDCA (64 of 123 versus 80 of 119, P = .02). Figure 2A shows the cumulative proportion of patients exceeding this blood level. Figure 2B shows the number of patients with ALAT higher than 100 U/L at each time of measurement. With one exception, at 14 days after the transplantation, the number of patients exceeding this ALAT blood level was higher among those not given the study drug.
Serum ALAT concentrations.
(A) The cumulative proportion of patients developing a serum ALAT concentration higher than 100 U/L, and (B) the number of patients with serum ALAT higher than 100 U/L at each time of measurement. Statistically significantly fewer patients in the group given UDCA exceeded this blood level (2.5 times the upper limit of normal), and, with one exception, the number of patients exceeding this level was always higher in the group not given UDCA.
Serum ALAT concentrations.
(A) The cumulative proportion of patients developing a serum ALAT concentration higher than 100 U/L, and (B) the number of patients with serum ALAT higher than 100 U/L at each time of measurement. Statistically significantly fewer patients in the group given UDCA exceeded this blood level (2.5 times the upper limit of normal), and, with one exception, the number of patients exceeding this level was always higher in the group not given UDCA.
VOD
There was no statistically significant difference between the study groups in the incidence of VOD. According to the criteria of Jones et al,19 3 of 123 (2%) of the patients randomized to receive UDCA and 5 of 119 (4%) of those not given this drug developed VOD, and the corresponding figures with the criteria of McDonald et al18 were 14 of 123 (11%) and 14 of 119 (12%).
GVHD
There was a trend toward a lower incidence of acute GVHD among the patients given UDCA, but in the overall incidence there was no statistically significant difference (Figure3). However, the incidence of grade III to IV acute GVHD was statistically significantly lower among the patients who received UDCA (5 of 123 versus 17 of 119 patients,P = .01, cumulative incidence shown in Figure 3). Table2 shows the incidence of acute GVHD by organ and stage. In the group given UDCA the incidence of stage II to IV and III to IV liver GVHD, stage III to IV skin GVHD, and stage II to IV intestinal tract GVHD was statistically significantly lower than in the control group. There was no statistically significant difference in the incidence or severity of chronic GVHD until 1 year after transplantation; 50 of 107 and 40 of 89 patients at risk for at least 100 days had developed chronic GVHD by 1 year in the groups given and not given UDCA, respectively.
Incidence of acute GVHD.
Cumulative incidence of acute GVHD grade I to IV (▨) and III to IV (▨▨▨▨) among patients given and not given UDCA. There was a nonsignificant trend toward lower overall incidence of acute GVHD in the group given UDCA, and a statistically significantly lower incidence of grade III to IV acute GVHD in the UDCA-treated group.
Incidence of acute GVHD.
Cumulative incidence of acute GVHD grade I to IV (▨) and III to IV (▨▨▨▨) among patients given and not given UDCA. There was a nonsignificant trend toward lower overall incidence of acute GVHD in the group given UDCA, and a statistically significantly lower incidence of grade III to IV acute GVHD in the UDCA-treated group.
Acute GVHD, stage by organ
| . | With UDCA, n = 123 . | Without UDCA, n = 119 . | P . |
|---|---|---|---|
| No. of patients . | No. of patients . | ||
| Liver | |||
| II to IV | 6 | 15 | .04 |
| III to IV | 3 | 12 | .02 |
| Skin | |||
| II to IV | 38 | 45 | .28 |
| III to IV | 7 | 17 | .03 |
| Intestinal tract | |||
| II to IV | 7 | 17 | .03 |
| III to IV | 5 | 12 | .08 |
| . | With UDCA, n = 123 . | Without UDCA, n = 119 . | P . |
|---|---|---|---|
| No. of patients . | No. of patients . | ||
| Liver | |||
| II to IV | 6 | 15 | .04 |
| III to IV | 3 | 12 | .02 |
| Skin | |||
| II to IV | 38 | 45 | .28 |
| III to IV | 7 | 17 | .03 |
| Intestinal tract | |||
| II to IV | 7 | 17 | .03 |
| III to IV | 5 | 12 | .08 |
There was no statistically significant difference in the cyclosporine blood concentrations between the study groups at any time. In the groups given and not given UDCA, the mean cyclosporine blood concentrations at 28 days were 206 and 208 μg/L, at 56 days 184 and 175 μg/L, and at 84 days 153 and 164 μg/L, respectively. Neither were there statistical differences between the cyclosporine doses, except on day 42 when the doses were slightly higher in the group given UDCA (mean ± SE 5.7 ± 1.6 versus 5.1 ± 1.5 mg/kg/d,P = .04).
Severe liver problems
Because the principal target of this study was to evaluate the possible role of UDCA in preventing liver problems, we compared the number of patients with stage III to IV liver GVHD, VOD (the criteria of Jones et al19), or fatal non-GVHD liver failure not fulfilling Jones' criteria for VOD in the 2 study groups. There were 6 such patients in the group given UDCA and 19 in the group not given this drug. The difference is statistically highly significant (P = .009).
Eight patients in the group randomized not to receive prophylactic UDCA received this drug for the treatment of liver complications. At the initiation of UDCA treatment the serum bilirubin concentration was 67 to 408 μM. This treatment was given for 7 to 48 days (median, 28 days). All these patients died.
Relapses, survival, and causes of death
There was no statistically significant difference in the relapse rate during the first year; 17 of the 115 patients with a malignant disease had relapse in the group given UDCA and 21 of the 117 patients in the control group. Among the low-risk patients with malignant disease the corresponding figures were 3 of 67 and 5 of 63.
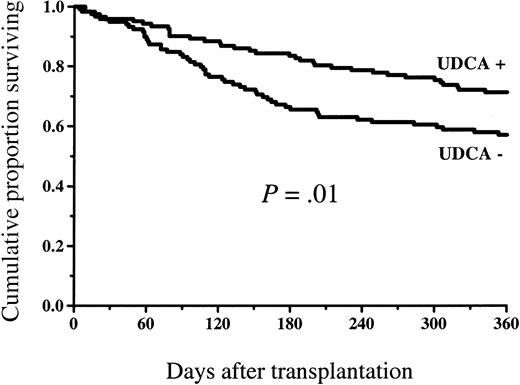
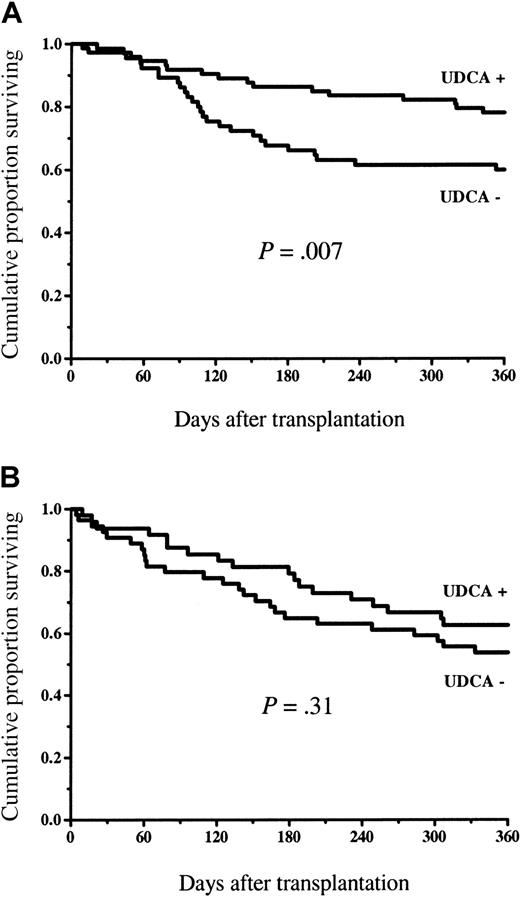
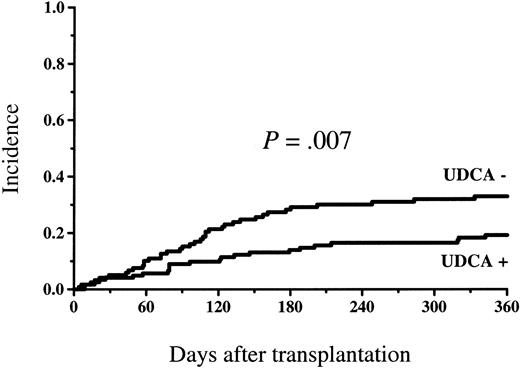
Figure 4 shows the survival in the study groups. At 1 year, 87 of 123 patients (71%) survived in the UDCA-treated group and 66 of 119 patients (55%) survived in the control group (P = .02). The survival difference was statistically significant among the low-risk patients (58 of 75 = 77% versus 38 of 65 = 58%, P = .03), but there was no statistically significant difference in the high-risk group (29 of 48 = 60% versus 28 of 54 = 52%, P = .50; Figure5). The incidence of nonrelapse mortality was lower in the group randomized to receive UDCA (23 of 123 = 19% versus 40 of 119 = 34%, P = .01) at 1 year (cumulative incidence Figure 6). The principal causes of death are shown in Table 3. The proportion of patients who died of GVHD was statistically significantly greater in the group not given UDCA (17 of 119 versus 7 of 123,P = .03). There were 2 deaths in VOD and 2 in non-GVHD liver failure not fulfilling VOD criteria in the control group, whereas among the UDCA-treated patients there was no death with non-GVHD liver failure as the principal cause.
Overall survival among patients given and not given UDCA.
The survival was statistically significantly better in the group of patients given UDCA.
Overall survival among patients given and not given UDCA.
The survival was statistically significantly better in the group of patients given UDCA.
Overall survival in low- and high-risk patients.
Overall survival among low-risk (A) and high-risk (B) patients given and not given UDCA. There was a statistically significant difference among the low-risk patients in favor of those who received UDCA, but no significant difference among the high-risk patients.
Overall survival in low- and high-risk patients.
Overall survival among low-risk (A) and high-risk (B) patients given and not given UDCA. There was a statistically significant difference among the low-risk patients in favor of those who received UDCA, but no significant difference among the high-risk patients.
Cumulative incidence of nonrelapse mortality among patients given and not given UDCA.
The incidence was statistically significantly lower in the group given UDCA.
Cumulative incidence of nonrelapse mortality among patients given and not given UDCA.
The incidence was statistically significantly lower in the group given UDCA.
Causes of death
| . | With UDCA . | Without UDCA . |
|---|---|---|
| Relapse, progressive disease | 11 | 14 |
| GVHD | 7 | 173-150 |
| Infection | 14 | 16 |
| VOD | 0 | 2 |
| Liver failure, non-VOD | 0 | 2 |
| TTP | 1 | 1 |
| ARDS | 1 | 1 |
| Not known | 1 | 0 |
| Total | 35 | 53 |
| . | With UDCA . | Without UDCA . |
|---|---|---|
| Relapse, progressive disease | 11 | 14 |
| GVHD | 7 | 173-150 |
| Infection | 14 | 16 |
| VOD | 0 | 2 |
| Liver failure, non-VOD | 0 | 2 |
| TTP | 1 | 1 |
| ARDS | 1 | 1 |
| Not known | 1 | 0 |
| Total | 35 | 53 |
TTP indicates thrombotic thrombocytopenic purpura; ARDS, acute respiratory distress syndrome.
Risk of dying of GVHD significantly higher among patients not given UDCA, P = .03.
Adverse events
No adverse event regarded as being caused by the study drug was identified in any patient.
Discussion
In the present study the prophylactic use of UDCA resulted in statistically significant and clinically beneficial effects in patients undergoing allogeneic transplantation. The proportion of patients who developed severe liver problems indicated by a markedly increased bilirubin level (> 50 μM) was smaller in the group given UDCA, and the same was seen in the serum ALAT concentrations (> 100 U/L). There was also a reduction in the incidence of severe acute GVHD and nonrelapse mortality, and the survival was improved. In contrast to previous studies, no effect on the incidence of VOD could be observed.
In the 2 previous randomized prophylaxis studies, Essel et al observed that UDCA reduced bilirubin levels,14 whereas no effect on bilirubin was seen in the study of Ohashi et al.15 No difference in ALAT levels was seen15 or reported.14 These studies focused mainly on VOD.14,15 In both studies the incidence of VOD in the control group was clearly higher than in the present study, in the study of Essell et al14 40% and in the study of Ohashi et al15 18.5%, whereas in the present study the incidence in the control group was 4% with the criteria of Jones et al19 and 12% with those of McDonald et al.18 The range of the reported incidences of VOD is wide, from 1% to 70%.14,15,25-27 This probably reflects differences in the patient materials and, particularly, in the conditioning given. All patients in the study of Essell et al14 received conditioning with busulphan and cyclophosphamide, and busulphan conditioning has been shown to be a risk factor for the development of VOD.15,26,28 In the present material only a small minority of patients received busulphan; their findings did not differ from those of the rest of the patients. The low overall incidence of VOD in the present material probably at least partly explains the difference from the earlier studies in the efficacy of UDCA to prevent VOD. The VOD incidence of the patients in the present study is similar to the long-term experience in the participating centers; an earlier large study from the same study group showed, using the criteria of Jones et al,19 an incidence of VOD of 1% among the patients given TBI conditioning and 12% among those given conditioning with busulphan.28
Of considerable interest is the effect of UDCA prophylaxis on acute GVHD. Although there was a slight trend toward a lower overall incidence of GVHD among the patients given UDCA, this difference did not reach statistical significance. However, there were statistically significantly fewer patients with severe GVHD among those given the study drug, and this reduction in the incidence was seen in liver, skin, and intestinal tract GVHD of stage II to IV or III to IV. UDCA has been shown to induce immunomodulation by reducing the expression of class I antigens on hepatocytes in cholestatic liver diseases.29 Therefore, it seems plausible that UDCA could reduce the antigenic stimulus for GVHD-inducing immunologic cells. The effect could also be more nonspecific. UDCA may reduce the damage caused by GVHD reaction by stabilizing cell membranes and thereby reducing cell destruction,7 by reducing the production of cytokines,9 and by making the bile less toxic to liver parenchymal cells. A trend toward a lower overall incidence of acute GVHD in the group given UDCA was seen in the study of Essell et al,14 but there was no difference in the incidence of liver GVHD. In the study of Ohashi et al15 UDCA had no effect on the incidence of GVHD. The larger number of patients in the present study may at least partly explain the differences between the findings in the 3 studies.
One mechanism by which UDCA might have modified acute GVHD could be a change in the pharmacokinetics of the agents used for GVHD prophylaxis. Cyclosporine blood levels were monitored, and there were no statistically significant differences between the study groups at any time. Cyclosporine doses were also similar in the 2 groups and they differed statistically significantly at only one single time point. Thus altered cyclosporine pharmacokinetics is an unlikely mechanism of the effects of UDCA observed in the present study. An effect of UDCA on the pharmacokinetics of methotrexate and low-dose corticosteroid used in 2 centers, or cyclophosphamide used in the conditioning, cannot be excluded.
In the present study the patients given UDCA had a statistically significantly better survival up to 1 year than the control patients. In the 2 previous randomized studies,14,15 no significant effect on survival was observed, though in the study of Essell et al14 a trend toward the same direction as in the present study was observed. The larger patient material in the present study may partly explain this difference. The higher mortality in the control group was mainly due to more deaths in GVHD. There were also 4 deaths with non-GVHD liver failure as the principal cause in the control group but none in the group given UDCA.
What the optimal dose of UDCA in prophylactic use for allogeneic stem cell transplantation patients is, is not clear. In the present study we gave 12 mg/kg/d, whereas the dose was 600 mg/d in the studies of Essell et al14 and Ohashi et al,15 except 900 mg to patients weighing more than 90 kg in the former study. Thus, the doses were somewhat higher in the present study. Whether this might explain some of the differences seen remains unclear. Recently, Angulo et al published a study of a comparison of 3 doses of UDCA in the treatment of primary biliary cirrhosis.30 They observed that 5 to 7 mg/kg/d was a suboptimal dose, whereas there was no difference between 13 to 15 mg and 23 to 25 mg/kg/d. The dose of 13 to 15 mg/kg/d was recommended. The dose used in the present study is close to this recommendation.
UDCA is an easy drug to use. It is given orally, it has practically no side effects, and it is inexpensive. The only side effect reported is diarrhea, which is seen in less than 5% of patients.3There was no indication of an increased relapse risk in the present or previous studies. Neither in the present study nor in the 2 previous randomized prophylactic studies among allogeneic transplantation patients were any adverse effects attributable to the study drug seen. There may be temporary problems with oral administration of drugs during the early period after the transplantation due to mucositis and nausea. However, in the present study more than half of the patients could take the drug according to the protocol at all times, and about two thirds of the patients were able to take more than 80% of the planned dose even during the early period after transplantation.
This study shows that the prophylactic administration of UDCA results in clinically relevant beneficial effects, particularly in the reduction of the incidence of severe GVHD and in improved survival. The drug is easy to use and inexpensive, and it causes practically no side effects. The present findings indicate a role for UDCA in the prevention of transplant-related complications after allogeneic stem cell transplantation.
Prepublished online as Blood First Edition Paper, May 24, 2002; DOI 10.1182/blood-2001-12-0159.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Tapani Ruutu, Helsinki University Central Hospital, Department of Medicine, Division of Hematology, POB 340, FIN-00029 HUS, Helsinki, Finland; e-mail: tapani.ruutu@hus.fi.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal