Abstract
In B-cell chronic lymphocytic leukemia (B-CLL), malignant cells seem to be arrested in the G0/early G1phase of the cell cycle, and defective apoptosis might be involved in disease progression. However, increasing evidence exists that B-CLL is more than a disease consisting of slowly accumulating resting B cells: a proliferating pool of cells has been described in lymph nodes and bone marrow and might feed the accumulating pool in the blood. Rapamycin has been reported to inhibit cell cycle progression in a variety of cell types, including human B cells, and has shown activity against a broad range of human tumor cell lines. Therefore, we investigated the ability of rapamycin to block cell cycle progression in proliferating B-CLL cells. We have recently demonstrated that stimulation with CpG-oligonucleotides and interleukin-2 provides a valuable model for studying cell cycle regulation in malignant B cells. In our present study, we demonstrated that rapamycin induced cell cycle arrest in proliferating B-CLL cells and inhibited phosphorylation of p70s6 kinase (p70s6k). In contrast to previous reports on nonmalignant B cells, the expression of the cell cycle inhibitor p27 was not changed in rapamycin-treated leukemic cells. Treatment with rapamycin prevented retinoblastoma protein (RB) phosphorylation in B-CLL cells without affecting the expression of cyclin D2, but cyclin D3 was no longer detectable in rapamycin-treated B-CLL cells. In addition, rapamycin treatment inhibited cyclin-dependent kinase 2 activity by preventing up-regulation of cyclin E and cyclin A. Interestingly, survivin, which is expressed in the proliferation centers of B-CLL patients in vivo, is not up-regulated in rapamycin-treated cells. Therefore, rapamycin interferes with the expression of many critical molecules for cell cycle regulation in cycling B-CLL cells. We conclude from our study that rapamycin might be an attractive substance for therapy for B-CLL patients by inducing a G1 arrest in proliferating tumor cells.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is characterized by the progressive accumulation of small malignant B cells, which are arrested in the G0 phase of the cell cycle.1 In the peripheral blood, B-CLL cells express high levels of the cell cycle inhibitor p27,2 but B-CLL cells with low expression of p27 have been identified in so-called proliferation centers in lymph nodes and bone marrow.3Interestingly, clusters of survivin and Ki67-positive cells were observed in the bone marrow and lymph nodes of B-CLL patients, interspersed with CD3-positive T lymphocytes.4 Survivin is a member of the family of inhibitors of apoptosis proteins (IAP)5 and is expressed in the G2/M phase of the cell cycle.6 Proliferation of B-CLL cells might be dependent on the pseudofollicle microenvironment,7 where infiltrating autologous T cells can provide CD40 stimulation and cytokines.4,8,9,10 These cycling cells are thought to represent the proliferating compartment of B-CLL and might be important for disease progression.4 Therefore, effective drugs for treatment of this still-incurable disease should be targeted against the proliferating pool rather than the accumulating pool of cells. However, B-CLL cells separated from the peripheral blood display a marked hyporesponsiveness to a variety of polyclonal B-cell activators.11,12 Thus, the effect of chemotherapeutic agents or cell cycle inhibitors on cycling cells has not been investigated in B-CLL. However, normal and malignant B cells are highly susceptible to stimulation with immunostimulatory CpG-oligonucleotides (ODN).13-15
We have previously shown that a high percentage of B-CLL cells traverse the cell cycle upon stimulation with CpG-ODN and interleukin-216 (IL-2) and have investigated the expression of the cyclin-dependent kinase (cdk) inhibitor p27, cyclin D2, and cyclin D3 in this in vitro model of cycling B-CLL cells.17
Rapamycin is a streptomyces derivative that modulates signal transduction pathways that link mitogenic stimuli to the synthesis of proteins regulating cell cycle progression.18 Rapamycin is widely used as an immunosuppressant in organ transplant recipients and has shown limited toxicities even in combination schedules with other immunosuppressants like cyclosporine or corticosteroids.19,20 The intracellular rapamycin receptor is a small protein termed FKBP12 (FK506-binding protein).21 The FKBP-rapamycin complex inhibits the function of a serine/threonine kinase, mTOR (mammalian target of rapamycin), thereby blocking stimulation of the ribosomal s6 kinase p70s6 kinase (p70s6k). P70s6k in turn phosphorylates the 40s ribosomal protein S6, which favors translation of mRNAs that encode ribosomal proteins and elongation factors.18,22 Another target of mTOR is a low-molecular-weight repressor of translation initiation termed phosphorylated heat- and acid-stable protein regulated by insulin (PHAS-I). Phosphorylation of PHAS-I results in its dissociation from eukaryotic initiation factor (eIF)–4E and increases eIF-4E–dependent translation initiation.23 24
While the potential of rapamycin varies between different cell types,18 it has been reported to inhibit cell cycle progression in a variety of cell types, including human T cells and B cells.25,26 In addition, rapamycin has in vitro and in vivo activity against a broad range of human tumor cell lines27-29 and is considered to represent a promising new class of cytostatic anticancer agents.30
In the present study, we have analyzed the effect of rapamycin on cell cycle progression and expression of cell cycle regulatory molecules in a model of proliferating B-CLL cells to better define a potential role for this drug in therapy of B-CLL patients.
Patients, materials, and methods
Cell samples
After informed consent, peripheral blood was obtained from patients with the diagnosis of B-CLL according to clinical and immunophenotypic criteria. Patients were either untreated or had not received cytoreductive chemotherapy for a period of at least 3 months prior to investigation. At the time of analysis all patients were clinically stable, free from infectious complications, and undergoing routine clinical outpatient review.
Reagents and antibodies
The CpG ODN DSP30 (TCGTCGCTGTCTCCGCTTCTTCTTGCC)13was used single stranded, phosphorothioate stabilized, and synthesized by TibMolBiol (Berlin, Germany). Murine monoclonal antibodies (mAbs) specific for p27, cyclin D2, cyclin D3, and retinoblastoma protein were purchased from Pharmingen (San Diego, CA). Phospho-p 70S6k (Thr389) mAb, p70S6k mAb, and total cell extracts from 293 cells, prepared with and without serum, were purchased from New England Biolabs (Schwalbach, Germany). mAbs specific for cdk2, cdk4, cyclin A, cyclin E, and survivin were from Santa Cruz Biotechnology (Santa Cruz, CA); mAb specific for actin was obtained from Sigma (Deisenhofen, Germany). IL-2 was obtained from Pepro Tech (London, United Kingdom), and IL-10 was purchased from R&D Systems (Wiesbaden, Germany). Rapamycin was obtained from Sigma.
Separation procedures
Peripheral blood mononuclear cells (PBMNCs) were isolated from heparinized blood samples by centrifugation over a Ficoll-Hypaque layer (Biochrom, Berlin, Germany) of 1.077 g/mL density. For separation of CLL-B cells, PBMNCs were incubated with anti-CD2 and anti-CD14 magnetic beads (Dynabeads M450; Dynal, Oslo, Norway) according to the manufacturer's instructions. Such prepared B cells from CLL patients were more than 98% pure as assessed by direct immunofluorescence using a Coulter Epics XL (Coulter, Hamburg, Germany). Purification of tonsillar B-cell fraction was performed by negative selection as described for CLL-B cells.
Culture conditions
Purified normal and leukemic B cells were cultured in RPMI 1640 medium (Biochrom) supplemented with 10% fetal calf serum (Biochrom); penicillin/streptomycin, 50 IU/mL; Na-pyruvate, 1 mM;l-glutamine, 2 mM; L-asparagine, 20 μg/mL; 2-mercaptoethanol, 0.05 mM; HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid), 10 mM; and minimum essential medium (MEM) nonessential amino acids, 0.7 × (Biochrom) at 37°C and 5% CO2 in a fully humidified atmosphere in 12-well plates at 5 × 106 cells in a total volume of 5 mL. HeLa cells stably expressing the CD40 ligand (CD40LH) were kindly provided by C. Wendtner, Medical Clinic III, University Hospital Grosshadern, Ludwig-Maximilians-University, Munich.
[3H]-thymidine uptake
Purified B cells were cultured at 105 cells/200 μL in round-bottom wells with or without DSP30 at 1 μM and IL-2 (100 U/mL) for induction of B-cell proliferation. For CD40 ligand–induced activation, CD40LH cells were irradiated (10 000 rad), plated at 104 cells/well in flat-bottom wells, and incubated overnight. Then, 105 B-CLL cells were added, and coculture was performed in a total volume of 200 μL. At the indicated time points, cells were pulsed with [3H]-thymidine (Du Pont, Paris, France) and harvested in a PHD-Cell Harvester (Dunn Labortechnik, Asbach, Germany) after 8 hours. Thymidine incorporation was quantified in a B-counter (Beckmann, Munich, Germany).
Cell cycle analysis
Approximately 106 cells were collected and fixed overnight in 70% ethanol at 4°C. Cells were then washed and stained with propidium iodide (PI; Sigma) 5 μg/mL in the presence of DNAse-free RNAse (Sigma). After 30 minutes at room temperature, the cells were analyzed via flow cytometry using a Coulter Epics XL cytofluorometer (Hamburg, Germany), acquiring 10 000 events.
Immunoblotting
A total of 1 to 2 × 107 cells were lysed as previously described17 in lysis buffer (10 mM Tris [tris(hydroxymethyl)aminomethane]–HCL [ph 7.4], 5 mM EDTA [ethylenediaminetetraacetic acid], 130 mM NaCl, 1% Triton, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, and 10 mg/mL each phenantroline, aprotinin, leupeptin, and pepstatin) for 20 minutes at 4°C. Lysates were spun at 12 000 rpm for 20 minutes and supernatant collected. Protein concentration was assessed by the Bio-Rad assay method (Bio-Rad Laboratories, Munich, Germany). Total extracts (50 μg/lane) were subjected to 12% SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) (7.5% for detection of the RB protein, 10% for detection of p70s6 kinase), and blotting was performed on polyvinylidenefluoride membranes (Immobilon-P; Millipore GmbH, Bedford, MA). Blots were developed using SuperSignal chemoluminescent substrates from Pierce Chemical Company (KMF GmbH, Sankt Augustin, Germany). To control for equal protein loading, amido black staining was performed (data not shown).
In vitro kinase assay
For RB kinase assays, whole-cell extracts were prepared by resuspending the cells on ice in Tween 20 lysis buffer, 50 mM HEPES (ph 7.5 150 mM NaCl, 1 mM EDTA [ph 8.0], 2.5 mM EGTA [ethyleneglycoltetraacetic acid] [ph 8.0], 0.1% Tween 20, 10% glycerol, 1 mM DTT [dichlorodiphenyltrichloroethane], 1 mM NaF, 0.1 mM Na3VO4, and 10 mg/mL of each phenantroline, aprotinin, leupeptin, and pepstatin). Cell lysates were precleared by incubation with a polyclonal rabbit anti–mouse antibody and protein A–Sepharose. Immunoprecipitation of cyclin E and cdk2 complexes was performed as described previously.17 After washing 2 times in Tween 20 lysis buffer, once in Tween lysis buffer containing 500 mM NaCl, once in H20, and 3 times in kinase buffer, 50 mM HEPES (ph 7.5 10 mM MgCL2, 1 mM DTT, 2 mM EGTA, 1 mM NaF, 0.1 mM Na3VO4, 10 mM β-glycerophosphat), the reaction was started in 25 μL kinase buffer containing 20 μmol/L adenosine triphosphate (ATP), 0.5 μg histone H1 (Upstate Biotechnology, Lake Placid, NY), 10 μCi (0.37 MBq) γ32P ATP (5000 Ci [185 000 GBq]/mmol; Amersham, Freiburg, Germany). After 30 minutes at 30°C, the reaction was terminated by boiling the samples in 2× SDS sample buffer for 10 minutes. The phosphorylated substrate was then identified by resolution in 10% SDS-PAGE followed by drying and autoradiography.
Flow cytometric analysis of apoptosis
To determine apoptosis-associated parameters, purified B cells were cultured either in medium alone or together with DSP30 (1 μM) and IL-2 (100 U/mL) with or without rapamycin at 50 ng/mL. The samples were washed with phosphate buffered saline (PBS) and resuspended in 500 μL binding buffer (Annexin V–FITC Kit; Immunotech, Marseille, France), containing 1 μL of annexin V–fluorescein isothiocyanate (FITC) stock and 5 μL of 20 μg/mL PI for determination of phosphatidylserine (PS) exposure on the outer plasma membrane. After incubation for 10 minutes at room temperature in a light-protected area, the specimens were quantified by flow cytometry using a Coulter Epics XL cytofluorometer, acquiring 5000 events. Viable cells were annexin V– and PI-negative.
DNA fragmentation assay
To identify fragmented DNA, a terminal deoxynucleotidyl transferase (TdT)–mediated dUTP nick-end labeling (TUNEL) assay was performed and assessed by cytometry (Boehringer Mannheim, Indianapolis, IN). Then, 106 cells per sample were washed in PBS and fixed in 2% paraformaldehyde for 30 minutes at room temperature. After fixation, the cells were washed twice in PBS containing 0.01% bovine serum albumin and resuspended in TUNEL reaction mixture containing fluorescein dUTP. Fluorescein incorporated in DNA strand breaks was detected by flow cytometry.
Results
Rapamycin induces G1 arrest in cycling B-CLL cells
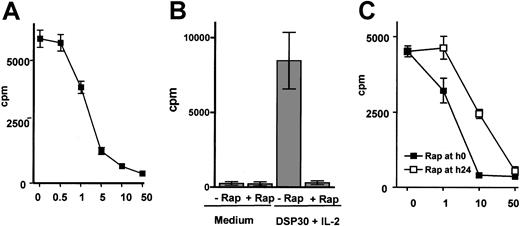
The effect of rapamycin on cycling B-CLL cells was investigated in B-CLL cells activated with the CpG-ODN DSP30 and IL-2. Rapamycin was added at concentrations ranging from 0.5 to 50 ng/mL to activated B-CLL cells. After 72 hours, thymidine incorporation was strongly reduced in B-CLL cells cocultured with rapamycin in concentrations above 1 ng/mL, and maximal suppression was observed at concentrations above 10 ng/mL. One representative experiment of 3 performed is demonstrated in Figure1A. In addition, we observed a near to complete inhibition of thymidine incorporation in 13 different patient samples using rapamycin at a concentration of 50 ng/mL (Figure 1B). The patient characteristics are provided in Table1.
Rapamycin inhibits proliferation of B-CLL cells stimulated with CpG-ODN DSP30 and IL-2.
B-CLL cells were cultured at 106 cells/mL in medium alone or in the presence of 1 μm DSP30 and 100U/mL IL-2 with or without the indicated concentrations of rapamycin. Thymidine incorporation was analyzed after 72 hours of culture, and results are shown as means ± SEMs of triplicates. Panel A shows 1 representative experiment (means ± SEM of triplicate cultures) of 3 performed. Inhibition of thymidine incorporation in stimulated B-CLL cells (DSP30 and IL-2) from 13 patients by rapamycin at 50 ng/mL is shown in panel B as means ± SEMs. In 3 additional experiments, rapamycin was either added at the indicated concentrations from the beginning of the culture (▪) or after 24 hours of stimulation (■). Thymidine incorporation was measured after 72 hours as described in “Patients, materials, and methods.” Panel C shows one representative experiment (means ± SEMs of triplicates).
Rapamycin inhibits proliferation of B-CLL cells stimulated with CpG-ODN DSP30 and IL-2.
B-CLL cells were cultured at 106 cells/mL in medium alone or in the presence of 1 μm DSP30 and 100U/mL IL-2 with or without the indicated concentrations of rapamycin. Thymidine incorporation was analyzed after 72 hours of culture, and results are shown as means ± SEMs of triplicates. Panel A shows 1 representative experiment (means ± SEM of triplicate cultures) of 3 performed. Inhibition of thymidine incorporation in stimulated B-CLL cells (DSP30 and IL-2) from 13 patients by rapamycin at 50 ng/mL is shown in panel B as means ± SEMs. In 3 additional experiments, rapamycin was either added at the indicated concentrations from the beginning of the culture (▪) or after 24 hours of stimulation (■). Thymidine incorporation was measured after 72 hours as described in “Patients, materials, and methods.” Panel C shows one representative experiment (means ± SEMs of triplicates).
Patient characteristics
| No. . | Sex . | Age, y . | White blood count, ×109/L . | Binet stage . | Previous therapies . |
|---|---|---|---|---|---|
| 1 | F | 69 | 31 | A | 0 |
| 2 | F | 77 | 144 | B | 2 |
| 3 | M | 61 | 125 | C | 3 |
| 4 | M | 81 | 83 | C | 2 |
| 5 | F | 70 | 38 | A | 0 |
| 6 | M | 66 | 112 | C | 3 |
| 7 | F | 72 | 39 | B | 0 |
| 8 | M | 67 | 92 | B | 0 |
| 9 | F | 73 | 42 | A | 0 |
| 10 | M | 67 | 112 | C | 5 |
| 11 | F | 69 | 49 | B | 1 |
| 12 | F | 77 | 74 | C | 2 |
| 13 | M | 63 | 41 | A | 0 |
| No. . | Sex . | Age, y . | White blood count, ×109/L . | Binet stage . | Previous therapies . |
|---|---|---|---|---|---|
| 1 | F | 69 | 31 | A | 0 |
| 2 | F | 77 | 144 | B | 2 |
| 3 | M | 61 | 125 | C | 3 |
| 4 | M | 81 | 83 | C | 2 |
| 5 | F | 70 | 38 | A | 0 |
| 6 | M | 66 | 112 | C | 3 |
| 7 | F | 72 | 39 | B | 0 |
| 8 | M | 67 | 92 | B | 0 |
| 9 | F | 73 | 42 | A | 0 |
| 10 | M | 67 | 112 | C | 5 |
| 11 | F | 69 | 49 | B | 1 |
| 12 | F | 77 | 74 | C | 2 |
| 13 | M | 63 | 41 | A | 0 |
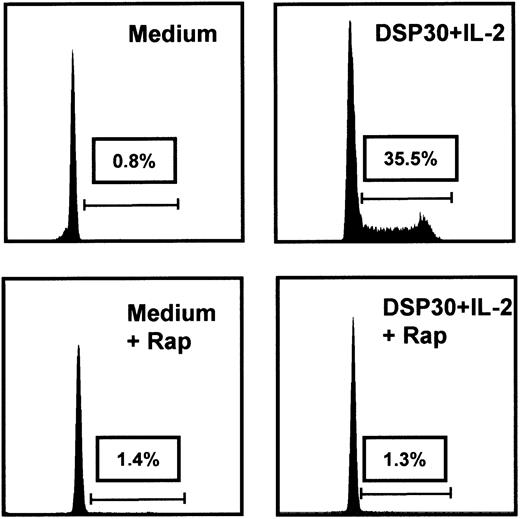
Inhibition of cell cycle progression also could be observed when rapamycin was added at a later time point, but maximal suppression of thymidine incorporation was noted at higher concentrations (Figure 1C). To further investigate the effect of rapamycin on cycling B-CLL cells, PI stains were performed in 3 independent samples. One representative example is depicted in Figure 2.
Rapamycin induces a G1 arrest in cycling B-CLL cells.
Cell cycle analyses were performed after 48 hours' culture in medium or DSP30 and IL-2 with or without rapamycin (Rap) at 50 ng/mL. One representative PI stain from a B-CLL sample is shown; 2 additional experiments gave similar results.
Rapamycin induces a G1 arrest in cycling B-CLL cells.
Cell cycle analyses were performed after 48 hours' culture in medium or DSP30 and IL-2 with or without rapamycin (Rap) at 50 ng/mL. One representative PI stain from a B-CLL sample is shown; 2 additional experiments gave similar results.
While nearly all cells were arrested in the G0/G1 phase after culture in medium alone, stimulation with DSP30 and IL-2 caused a significant entry of B-CLL cells into the cell cycle with a large fraction of cells in the S/G2/M phase. However, addition of rapamycin induced a complete G1arrest in these activated B-CLL cells (Figure 2). We confirmed our results in a coculture system with CD40 ligand expressing HeLa cells (CD40LH cells) in 3 independent samples. In line with previous results,12 CD40 ligation alone was not sufficient to induce strong proliferation in 2 of 3 B-CLL samples. Therefore, we also stimulated B-CLL cells with CD40LH, IL-2 (100 U/mL), and IL-10 (1 ng/mL), a combination known to provide maximal stimulation for B-CLL cells.31 Table 2 shows the results of a thymidine assay that was performed with 3 different samples. Importantly, rapamycin was able to inhibit the proliferation induced by CD40LH alone or by the combination of CD40LH, IL-2, and IL-10. Rapamycin also inhibited DSP30-IL-2–induced proliferation in cells from these patients (Table 2).
Rapamycin inhibits proliferative signals mediated by CD40 ligand
| . | Medium . | CD40LH . | CD40LH + rapamycin . | CD40LH + IL-2 + IL-10 . | CD40LH + IL-2 + IL-10 + rapamycin . |
|---|---|---|---|---|---|
| 1 | 540 ± 105 | 14 085 ± 1134 | 596 ± 30 | 13 336 ± 3064 | 480 ± 46 |
| 2 | 410 ± 80 | 558 ± 133 | 540 ± 80 | 10 305 ± 1007 | 2610 ± 341 |
| 3 | 353 ± 104 | 397 ± 38 | 410 ± 112 | 21 984 ± 6402 | 374 ± 67 |
| . | Medium . | CD40LH . | CD40LH + rapamycin . | CD40LH + IL-2 + IL-10 . | CD40LH + IL-2 + IL-10 + rapamycin . |
|---|---|---|---|---|---|
| 1 | 540 ± 105 | 14 085 ± 1134 | 596 ± 30 | 13 336 ± 3064 | 480 ± 46 |
| 2 | 410 ± 80 | 558 ± 133 | 540 ± 80 | 10 305 ± 1007 | 2610 ± 341 |
| 3 | 353 ± 104 | 397 ± 38 | 410 ± 112 | 21 984 ± 6402 | 374 ± 67 |
105 purified B-CLL cells were cultured in medium alone or in the presence of 104 irradiated HeLa cells stably expressing the CD40 ligand (CD40LH), IL-2 (100 U/mL), IL-10 (1 ng/mL), or rapamycin (50 ng/mL) as indicated. After 120 hours, thymidine was added for an additional 8 hours of culture, and thymidine incorporation was assessed as described in “Patients, materials, and methods.”
Purified B-CLL cells 105 were cultured in medium alone or in the presence of 104 irradiated HeLa cells stably expressing the cd40 ligand (CD40LH), IL-2 (100 U/mL), IL-10 (1 ng/mL), or rapamycin (Rap) (50 ng/mL), as indicated. After 120 hours, thymidine was added for an additional 8 hours of culture, and thymidine incorporation was assessed as described in “Patients, materials, and methods.” In an independent experiment, cells from the same patients were cultured with DSP30 and IL-2 with or without rapamycin for 72 hours, and thymidine incorporation was quantified as described in “Patients, materials, and methods.”
Rapamycin inhibits p70s6 kinase phosphorylation in B-CLL cells stimulated with DSP30 and IL-2
Treatment of lymphoid or nonlymphoid cells with rapamycin virtually abrogates the activation of p70s6 kinase by a diversity of mitogenic stimuli, but other mechanisms might be involved.18 To date, activation of the p70s6khas not been described in B-CLL. Phosphorylation of p70 threonin 389 is closely associated with kinase activity.32 Therefore, we analyzed phosphorylation of p70s6k in B-CLL cells stimulated with DSP30 and IL-2 with or without rapamycin using an mAb specific for p70s6k phosphorylated at threonin 389. As a positive and negative control, we included total cellular extracts from 293 cells cultured with serum (positive control) or without serum (negative control). While low levels of phosphorylation were observed at early time points, phosphorylation increased after 12 and 24 hours of stimulation and could be completely inhibited by rapamycin (Figure3).
p70S6k phosphorylation in B-CLL cells is inhibited by rapamycin.
(A) p70S6k expression and phosphorylation at threonin 389 (p-p70s6k) during 24 hours of activation with DSP30 and IL-2 were analyzed using specific antibodies. Cellular extracts of 293 cells cultured in the absence of or in the presence of serum were used as a positive (pc) and negative control (nc), respectively. (B) The same experiment was performed in the presence of 50 ng/mL rapamycin. Similar results were obtained in 2 additional experiments (data not shown).
p70S6k phosphorylation in B-CLL cells is inhibited by rapamycin.
(A) p70S6k expression and phosphorylation at threonin 389 (p-p70s6k) during 24 hours of activation with DSP30 and IL-2 were analyzed using specific antibodies. Cellular extracts of 293 cells cultured in the absence of or in the presence of serum were used as a positive (pc) and negative control (nc), respectively. (B) The same experiment was performed in the presence of 50 ng/mL rapamycin. Similar results were obtained in 2 additional experiments (data not shown).
Analysis of proteins regulating early G1 progression in cycling B-CLL cells in response to rapamycin
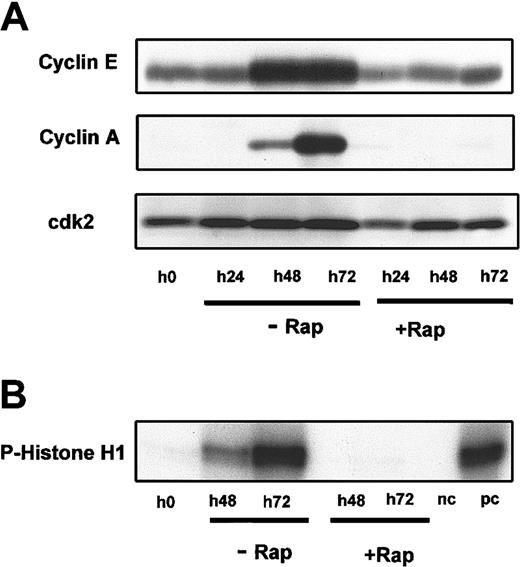
We have previously demonstrated that G1 progression in B-CLL cells is controlled by cyclin D2, cyclin D3, the cdk inhibitor p27, and cdk4.17 To determine whether rapamycin affected the expression of cyclin D2, cyclin D3, cdk4, or the cdk inhibitor p27, total cell lysates were subjected to Western blotting after 24 to 72 hours of culture in the presence of DSP30 and IL-2 with or without rapamycin. The time kinetics of thymidine incorporation over a period of 72 hours also are provided in Figure4A. In contrast to normal tonsillar B cells (Figure 4D), p27 levels were not restored to baseline levels but remained undetectable in rapamycin-treated B-CLL cells. Up-regulation of cyclin D2, which is strongly expressed in activated B-CLL cells, was not affected but seemed to be rather prolonged, with a stronger expression after 72 hours of culture. In contrast, cyclin D3 expression was not detectable when cells were cultured in the presence of rapamycin. The expression of cdk4, which is the catalytic partner of cyclin D2 and cyclin D3 in B-CLL cells, and cdk6 were not significantly changed in rapamycin-treated cells (Figure 4B). In line with the proliferation data, RB phosphorylation was diminished in these cells, although expression was slightly increased (Figure 4C).
Effect of rapamycin on the expression of proteins regulating early G1 progression in activated B-CLL cells.
Purified B-CLL cells were cultivated for up to 72 hours in the presence of DSP30 and IL-2. Rapamycin (Rap) was added where indicated at 50 ng/mL. (A) To quantify DNA synthesis, [3H]-thymidine was added for 8 hours at the indicated times (24, 48, or 72 hours), and 3H incorporation was measured. p27 was revealed by immunoblotting with mAb in total cell lysates. (B) The same membrane was sequentially stripped and probed with anti–cyclin D2, anti–cyclin D3, and anti-cdk4. (C) Hypophosphorylated (pRB) and hyperphosphorylated RBs (ppRB) were detected with the G3-245 mAb. (D) Tonsillar B cells were stimulated with DSP30 and IL-2 and cultured with or without rapamycin and analyzed for p27 expression. Protein concentrations were normalized by the Bio-Rad assay method. To control for equal protein loading, amido black staining was performed (data not shown).
Effect of rapamycin on the expression of proteins regulating early G1 progression in activated B-CLL cells.
Purified B-CLL cells were cultivated for up to 72 hours in the presence of DSP30 and IL-2. Rapamycin (Rap) was added where indicated at 50 ng/mL. (A) To quantify DNA synthesis, [3H]-thymidine was added for 8 hours at the indicated times (24, 48, or 72 hours), and 3H incorporation was measured. p27 was revealed by immunoblotting with mAb in total cell lysates. (B) The same membrane was sequentially stripped and probed with anti–cyclin D2, anti–cyclin D3, and anti-cdk4. (C) Hypophosphorylated (pRB) and hyperphosphorylated RBs (ppRB) were detected with the G3-245 mAb. (D) Tonsillar B cells were stimulated with DSP30 and IL-2 and cultured with or without rapamycin and analyzed for p27 expression. Protein concentrations were normalized by the Bio-Rad assay method. To control for equal protein loading, amido black staining was performed (data not shown).
Analysis of proteins regulating late G1 progression and G1/S transition in cycling B-CLL cells in response to rapamycin
Expression of cyclin E, cyclin A, and cdk2, which regulate G1 progression and the G1/S transition in normal and malignant cells,33 has not been described in proliferating B-CLL cells so far. Cdk 2 and cyclin E were both expressed in nonstimulated B-CLL cells. While cdk2 was regulated only slightly upon stimulation with DSP30 and IL-2, cyclin E expression was strongly increased in activated cells. However, up-regulation of cyclin E was not observed in rapamycin-treated B-CLL cells. In addition, cyclin A, which is not only involved in G1/S phase transition but also in S phase progression,34 was expressed at later time points in proliferating B-CLL cells, but cyclin A expression was not observed in rapamycin-treated cells (Figure5A). To further examine whether reduced levels of cyclin E and cyclin A were associated with reduced kinase activity, we performed in vitro histone H1 kinase assays with cdk2 immunoprecipitates. In vitro cdk2 kinase activity could be detected after 48 hours in activated B-CLL cells and peaked at 72 hours. We included cdk2 immunoprecipitates from proliferating and serum-starved raji-lymphoma cells as positive and negative controls, respectively. Corresponding to the reduced cyclin E and cyclin A expression, in vitro cdk2 kinase activity was reduced to baseline levels in B-CLL cells that were cultured in the presence of rapamycin (Figure 5B).
Effect of rapamycin treatment on the expression of proteins regulating late G1 progression and cdk2 kinase activity.
(A) Immunoblots were performed with mAb against cyclin E, cyclin A, and cdk2 in activated B-CLL cells with or without rapamycin (Rap) at 50 ng/mL. (B) The result of an in vitro kinase assay using histone H1 as a substrate for cdk2 immunoprecipitates of activated B-CLL cells cultured with or without rapamycin. Each experiment was repeated twice with similar results.
Effect of rapamycin treatment on the expression of proteins regulating late G1 progression and cdk2 kinase activity.
(A) Immunoblots were performed with mAb against cyclin E, cyclin A, and cdk2 in activated B-CLL cells with or without rapamycin (Rap) at 50 ng/mL. (B) The result of an in vitro kinase assay using histone H1 as a substrate for cdk2 immunoprecipitates of activated B-CLL cells cultured with or without rapamycin. Each experiment was repeated twice with similar results.
Rapamycin prevents up-regulation of survivin in activated B-CLL cells
Survivin is a member of the family of inhibitor of apoptosis proteins5 and interfaces cell cycle progression and apoptosis.6 A prominent role in the pathophysiology of B-CLL has been described recently when survivin was found to be expressed in proliferation centers in lymph nodes and bone marrow.4 Therefore, we analyzed the expression of survivin using Western blots in activated B-CLL cells and B-CLL cells treated with rapamycin. In 3 samples tested, we observed no baseline survivin expression, but strong expression was noted in B-CLL cells stimulated with CpG-ODN and IL-2 after 72 hours of activation. Survivin could not be detected when B-CLL cells were cultured in the presence of rapamycin at 50 ng/mL. One representative experiment is shown in Figure6.
Rapamycin inhibits up-regulation of survivin in B-CLL cells stimulated with DSP30 and IL-2.
Survivin expression was analyzed in B-CLL cells stimulated for up to 72 hours with DSP30 and IL-2 with or without rapamycin (Rap) at 50 ng/mL. The same membrane was sequentially stripped and probed with antiactin to control for equal protein loading.
Rapamycin inhibits up-regulation of survivin in B-CLL cells stimulated with DSP30 and IL-2.
Survivin expression was analyzed in B-CLL cells stimulated for up to 72 hours with DSP30 and IL-2 with or without rapamycin (Rap) at 50 ng/mL. The same membrane was sequentially stripped and probed with antiactin to control for equal protein loading.
Rapamycin does not affect apoptosis in cycling or resting B-CLL cells
Rapamycin is a potent inducer of G1 arrest in cycling B-CLL cells. Its potential to induce or prevent apoptosis remains controversial.18,26-29 Although rapamycin's mode of action is thought to be cytostatic and not cytotoxic,30 a G1 arrest in the proliferating compartment of B-CLL cells might theoretically result in decreased apoptosis. This would be a strong argument against rapamycin as a new drug for B-CLL patients. Therefore, we analyzed the effect of rapamycin on apoptosis of activated and resting B-CLL cells and performed annexin V assays after 48 and 72 hours in B-CLL cells cultured in medium alone or with DSP30 and IL-2 (stimulated/activated). The mean percentages of viable cells (annexin and PI negative) from 5 independent experiments are shown in Figure 7A. We confirmed our results with TUNEL staining in 2 different samples, which demonstrated a slight increase of DNA degradation. One experiment is shown in Figure7B. Therefore, rapamycin has only limited effects on apoptosis in cycling or resting B-CLL cells.
Rapamycin does not affect apoptosis in cycling or resting B-CLL cells.
(A) Annexin V and PI staining were performed in B-CLL cells after 48 or 72 hours of culture in the presence of medium alone or DSP30 and IL-2 (stimulated/activated[stim]) with or without rapamycin (Rap). Viable cells were annexin- and PI-negative. Results were obtained from 5 different samples and are presented as means ± SEMs. (B) For TUNEL staining, cells were cultured for 72 hours using the same conditions as described above. One additional independent experiment gave similar results.
Rapamycin does not affect apoptosis in cycling or resting B-CLL cells.
(A) Annexin V and PI staining were performed in B-CLL cells after 48 or 72 hours of culture in the presence of medium alone or DSP30 and IL-2 (stimulated/activated[stim]) with or without rapamycin (Rap). Viable cells were annexin- and PI-negative. Results were obtained from 5 different samples and are presented as means ± SEMs. (B) For TUNEL staining, cells were cultured for 72 hours using the same conditions as described above. One additional independent experiment gave similar results.
Discussion
B-CLL cells are arrested in G0 phase of the cell cycle1 and demonstrate a marked hyporesponsiveness to a variety of polyclonal B-cell activators.11,12 However, strong proliferative responses can be induced in T-cell B-CLL cocultures9,10 or in the presence of CD40 ligand–transfected cell lines together with IL-2 and IL-10.31 These proliferative signals can be derived from infiltrating T helper cells,8,9,10 which are part of the microenvironment of B-CLL proliferation centers in vivo.4,7 Cycling B-CLL cells from this proliferating compartment might feed the pool of small resting B-CLL cells in the accumulating compartment.4 These cells have been described to express survivin and Ki-674 and display decreased expression of the cdk inhibitor p27.3
We recently have described an in vitro model of proliferating B-CLL cells that allows investigation of cell cycle regulatory proteins in the absence of “contaminating” T cells or HeLa cells.16 A high percentage of B-CLL cells traverse the cell cycle upon stimulation with CpG-ODN and IL-2, and G1progression is controlled by cyclin D2, cyclin D3, p27, and cdk4.17
Rapamycin and its derivative, CCI-779, are considered to represent a new class of “cytostatic” anticancer agents.30 These substances have displayed in vitro and in vivo activity against a large range of solid tumors and lymphomas,28-30,35,36 and CCI-779 is currently being tested in phase 1 clinical trials.37 Phosphatase and tensin homolog deleted on chromosome 10 (PTEN)–deficient tumors and tumors with constitutively activated akt kinase or p70s6 kinase seemed to be especially susceptible to rapamycin treatment.38-40 P70s6 kinase function is essential for G1 progression by regulating the translation of a subset of mRNAs whose protein products are required for progression through the G1 phase of the cell cycle.41 42
As expected, rapamycin did not affect cell cycle distribution of resting B-CLL cells (Figure 2). However, addition of rapamycin caused a dose-dependent inhibition of thymidine incorporation in B-CLL cells activated with CpG-ODN DSP30 and IL-2 (Figure 1A). At a concentration of 50 ng/mL, a near to complete inhibition of proliferation was observed in 13 different B-CLL samples (Figure 1B,P < .001). PI stains confirmed that rapamycin caused a G1 arrest in activated B-CLL cells (Figure 2). Importantly, cell cycle arrest also occurred after delayed addition of rapamycin to the cultures.
We were able to confirm the results of our in vitro model in B-CLL cells activated with CD40LH with or without IL-2 and IL-10 (Table 2). CD40 ligand, IL-2, and IL-10 are considered to be in vivo proliferation signals provided by autologous T cells.7 However, stimulation with CpG-ODN and IL-2 was more reliable and allowed us to perform analysis of cell cycle regulatory proteins in the absence of “contaminating” HeLa cells.
Because rapamycin exerts much of its activities through inhibition of p70S6k phosphorylation18,22 and p70S6k recently has been reported to regulate cell cycle progression in human B cells,43 we analyzed phosphorylation of p70S6k in B-CLL cells activated with DSP30 and IL-2. We observed Thr 389 phosphorylation of p70S6k in B-CLL cells upon activation with DSP30 and IL-2 that could be inhibited by rapamycin (Figure 3). Phosphorylation peaked at late time points, which might be explained by the formation of IL-2 high-affinity receptors upon stimulation with DSP3022 and subsequent IL-2 signaling events.
Rapamycin can block G1 to S-phase transition in a number of cell types by blocking growth factor–stimulated elimination of the cdk inhibitor p27.26,33,44 In contrast to our findings in tonsillar B cells, we observed no up-regulation of p27 in rapamycin-treated B-CLL cells (Figure 4). Therefore, we went on to analyze the expression of cyclin D2, cyclin D3, cyclin E, and cyclin A, which are important for regulating progression through the G1 phase and transition from the G1 to S phase of the cell cycle.34 Treatment with rapamycin prevented RB phosphorylation in B-CLL cells without affecting the expression of cyclin D2, but cyclin D3 was no longer detectable in rapamycin-treated B-CLL cells (Figure 4). Although expression of early D-type cyclins was not influenced by rapamycin in activated T cells,45rapamycin caused cyclin D3 down-regulation in a breast cancer cell line and in primary cultures of spermatogonia,46 47 suggesting that cyclin D3 expression is differentially regulated in different cell types.
In mid to late G1, cdk2-cyclin E and cdk2-cyclin A are sequentially activated and are needed for complete phosphorylation of RB.34,48 In addition to the inhibition of cyclin D3 up-regulation, we observed a striking effect of rapamycin on the expression of cyclin E and cyclin A, which resulted in a reduction of cdk2 kinase activity to baseline values (Figure 5A-B). Depending on the cell type, cyclin E expression was reported to be inhibited by rapamycin or not in previous studies.49,50 Cyclin A also has been described to be down-regulated by rapamycin treatment,26,50 but the observed reduced expression might also be a consequence of RB inactivation.51 Therefore, rapamycin inhibits cell cycle progression by targeting cyclin D3, cyclin A, and cyclin E, which are all required for entry into S phase.34
Recently, survivin, which is a member of the IAP family of proteins,4 was demonstrated to be expressed in cycling B-CLL cells in proliferation centers in vivo, and survivin expression was induced in vitro upon CD40 stimulation.4 It was postulated that survivin expression is important in the pathophysiology of B-CLL by interfacing proliferation and apoptosis.6Therefore, targeting survivin expression is a promising approach in tumor therapy.52-54 Regulation of survivin expression by rapamycin has not been reported so far, but we observed a complete inhibition of survivin expression in rapamycin-treated cells (Figure6). Suppression of survivin expression in cycling B-CLL adds to the powerful effects of rapamycin treatment on cycling B-CLL cells.
Rapamycin failed to significantly enhance the percentage of cells undergoing apoptosis in cycling or resting B-CLL cells (Figure 7). However, imatinib mesylate (Gleevec), which is the most successful example for targeted therapy,55 also acts by inhibiting proliferation rather than by inducing apoptosis.55,56 We are currently investigating whether rapamycin sensitizes cycling B-CLL cells to apoptosis induced by chemotherapeutic drugs, as has been reported in lymphoblastoid cells.57
The inhibitory effect of rapamycin on cycling B-CLL cells might be exploited therapeutically because activated B-CLL cells can be identified in the proliferation centers of lymph nodes, bone marrow, and the spleen and contribute to disease progression.3,4In addition, the inhibitory effect of rapamycin on activated T cells, which might provide survival signals in proliferation centers,7-10 is well documented and might contribute to a therapeutic effect.26 Rapamycin might also reduce angiogenesis in the bone marrow by interfering with vascular endothelial growth factor (VEGF) signaling.58 Increased angiogenesis and VEGF production both have been implicated in B-CLL pathophysiology.59 60
Rapamycin is widely used as an immunosuppressant in solid-organ transplant recipients with limited side effects, even in combination schedules.19,20 In addition, it has demonstrated a great potential to inhibit stent restenosis in patients with coronary heart disease.61 Pharmacologic studies have revealed that plasma levels above 15 ng/mL are easily achievable and were well tolerated in patients treated with rapamycin.62 This is important in view of the strong inhibitory effect of rapamycin on proliferating B-CLL cells at concentrations above 5 ng/mL (Figure 1A).
Because relapse seems to be inevitable in B-CLL cells even after complete remissions have been achieved,63 a maintenance therapy with rapamycin might be of value in delaying time to tumor progression, especially in patients at high risk for disease progression. Such a treatment schedule might not only be able to improve survival but might also strongly improve patients' quality of life. The potential for long-term therapy with rapamycin and its analog CCI-779 has been recognized recently in cancer patients,64 and phase 1/2 studies of CCI-779 in cancer patients are currently being performed.37Therefore, phase 1/2 studies with rapamycin will soon be initiated in patients with B-CLL at our institution. Rapamycin also might be combined with other chemotherapeutic drugs because it was well tolerated in combination with other immunosuppressants and corticosteroids.19,20 62 Further in vitro studies will deal with the effect of combination schedules of chemotherapeutic agents and rapamycin on cycling and resting B-CLL cells.
Supported by a research grant from the Technical University of Munich (KKF H30-97) and a grant from the Deutsche Forschungsgemeinschaft DE 771.
Submitted January 23, 2002; accepted August 1, 2002. Prepublished online as Blood First Edition Paper, August 29, 2002; DOI 10.1182/blood-2002-01-0189.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thomas Decker, 3rd Department of Medicine, Technical University of Munich, Ismaninger Str 15, 81675 Munich, Germany; e-mail: t.decker@lrz.tu-muenchen.de.


![Fig. 4. Effect of rapamycin on the expression of proteins regulating early G1 progression in activated B-CLL cells. / Purified B-CLL cells were cultivated for up to 72 hours in the presence of DSP30 and IL-2. Rapamycin (Rap) was added where indicated at 50 ng/mL. (A) To quantify DNA synthesis, [3H]-thymidine was added for 8 hours at the indicated times (24, 48, or 72 hours), and 3H incorporation was measured. p27 was revealed by immunoblotting with mAb in total cell lysates. (B) The same membrane was sequentially stripped and probed with anti–cyclin D2, anti–cyclin D3, and anti-cdk4. (C) Hypophosphorylated (pRB) and hyperphosphorylated RBs (ppRB) were detected with the G3-245 mAb. (D) Tonsillar B cells were stimulated with DSP30 and IL-2 and cultured with or without rapamycin and analyzed for p27 expression. Protein concentrations were normalized by the Bio-Rad assay method. To control for equal protein loading, amido black staining was performed (data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/1/10.1182_blood-2002-01-0189/6/m_h80133615004.jpeg?Expires=1767708311&Signature=NimMK-BG8myCtNqIEfCdlNiUpt1aXUEBH9wS~NQOeC5YLNpaaP73HCbANPn48ohKbMHgw3-VQ0-V~rov6Pf19vviedqvVPjTylzYWPJpSsPl5L3num~RfAfybXuQmDUhOUnnvRrf0mHRORthIhmHfZJcp4mAx5hj0aJZ0ZuL6b9gINMrHOfq830I-ZtjY49Io9emEhvvvv1ZtZKip~Hfh~AmVzOW9fF83HOA5RQQWa1E-7ahF7XYBQVtwuJYNsxmuxu9WqU~gZEpEHeSF3oUyBvX5udL0~tZfj8YX1-~KwaAm2RvLfLanWhzYnBx1se05oPs1wH8eSePoaIVmvEnbA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 7. Rapamycin does not affect apoptosis in cycling or resting B-CLL cells. / (A) Annexin V and PI staining were performed in B-CLL cells after 48 or 72 hours of culture in the presence of medium alone or DSP30 and IL-2 (stimulated/activated[stim]) with or without rapamycin (Rap). Viable cells were annexin- and PI-negative. Results were obtained from 5 different samples and are presented as means ± SEMs. (B) For TUNEL staining, cells were cultured for 72 hours using the same conditions as described above. One additional independent experiment gave similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/1/10.1182_blood-2002-01-0189/6/m_h80133615007.jpeg?Expires=1767708311&Signature=Jp1-X6-Ufkov-GU41ljNYIjnP1CpfpFvPVU3ENKLyP9wnXrwKAn866alRn~GYvlc-JTYXPkv5OW3wZX1vdmZQ1pJQiF5wHAk3cngzW1xQwgL1gPryHahtZXUyKdlIdFEhdOilwXcjGgWVe04Tb6OAriBXh7ZMnUqnjQEItV8Hbf2fiNEZ9L6fWtuA1eITPvG0baeTKIMCQOMO5JBx6pps~u6w4K6a2Bt6nPulinwm5Opou0IO0Tqi6TVcraB9hYrCC2ClLCAhKP3~boi0kV8Eki0YqCxwK~2rpDSuYWbrJrKcl7esS1gL9cWgsDq6F31YIaKlujssZzXRywX8P77zA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal