Abstract
Immunologic reactions against gene therapy products may prove to be a frequent problem in clinical gene therapy protocols. Enhanced green fluorescence protein (EGFP) is commonly used as a marker in gene transfer protocols, and immune responses against EGFP-expressing cells have been documented. The present study was designed to investigate the effect of a pharmacologic, nonmyeloablative, conditioning regimen on the development of EGFP+ donor/recipient mixed bone marrow chimerism and ensuing tolerance to EGFP-expressing transplants. To this end, C57BL/6J (B6) mice were treated with soluble formulations of either busulfan (Busulfex) or the closely related compound treosulfan, followed by transplantation of bone marrow cells from EGFP-transgenic (B6-EGFP.Tg) donor mice. Such conditioning regimens resulted in long-term persistence of donor EGFP+ cells among various hematopoietic lineages from blood, bone marrow, and thymus. Stable hematopoietic chimeras transplanted at 10 to 17 weeks after bone marrow transplantation (BMT) with B6-EGFP.Tg skin grafts all accepted their transplants, whereas non-EGFP chimeric B6 control animals were able to mount rejection of the EGFP+ B6 skin grafts. Control third-party grafts from major histocompatibility complex (MHC)–mismatched mice were rejected within 20 days, indicating that acceptance of EGFP-expressing skin grafts was the result of specific immune tolerance induction by the transplantation of EGFP-transgenic bone marrow. Long-term tolerance to EGFP in chimeric recipients was confirmed by the absence of anti-EGFP–reactive T cells and antibodies. These results broaden the therapeutic potential for using hematopoietic molecular chimerism in nonmyeloablated recipients as a means of preventing rejection of genetically modified cells.
Introduction
Gene therapy continues to hold great promise in the treatment of various diseases.1 Major advances in this field have come from the improvement of gene delivery systems, the identification of critical target cell populations, and the establishment of optimal transduction protocols. However, clinical and animal studies have revealed potential limitations posed by the recognition and consequent rejection by the host immune system of a number of newly introduced therapeutic and marker transgene products, including neomycin phosphotransferase (neo),2,3 hygromycin phosphotransferase (Hy) and/or herpes simplex virus thymidine kinase (HSV-TK),3, 4, 5 erythropoietin,6 α-L-iduronidase (α-ID)7 and α1-antitrypsin.8 To improve the efficacy of gene therapy protocols, it is essential to eliminate host responses to these and hitherto undefined immunogenic gene products. Although treatment with conventional immunosuppressive agents may prolong the clinical benefit of gene therapy, the adverse effects associated with long-term immune suppression make this approach undesirable.
To overcome these limitations, we developed a combination protocol of gene therapy with tolerance induction to eliminate the immunogenicity of the transgene-product enhanced green fluorescence protein (EGFP) in a murine model. This follows from recent studies in BALB/c mice, which are capable of rejecting syngeneic sarcoma and leukemia cells through the production of cytotoxic T-lymphocytes (CTLs) that recognize the foreign EGFP peptides presented in the context of major histocompatibility complex (MHC) class 1 molecules.9,10 Such murine studies are entirely consistent with recent primate studies in which EGFP-specific CTL responses were observed in rhesus monkeys receiving a nonmyeloablative dose of radiation before autologous bone marrow transplantation (BMT) of EGFP-transduced hematopoietic cells. The lack of hematopoietic cell engraftment in these animals has been attributed to the clearance of EGFP-expressing hematopoietic cells as a result of their immunogenicity.11
It has been demonstrated that specific immune tolerance for allogeneic tissues can be induced by establishing stable mixed hematopoietic chimerism after donor hematopoietic stem cell (HSC) transplantation (reviewed in Wekerle and Sykes12 ). This concept has been extended to tolerance induction through the establishment of molecular chimerism by the transfer of class 1 or class 2 histocompatibility genes into the hematopoietic compartment.13, 14, 15, 16, 17 In the present study, we have explored whether a similar approach can be applied for a model transgene but with a less toxic nonmyeloablative conditioning regimen in which bone marrow (BM) cells modified to express EGFP were transplanted in murine recipients pretreated with low doses of busulfan or treosulfan. In addition, the monitoring of EGFP expression in transplanted cells provides a valuable marker system for identifying those cell types that confer immunologic tolerance. Our results point to the therapeutic potential of establishing immunologic hyporesponsiveness through gene-transduced hematopoietic cells using nonmyeloablative conditioning and thereby preventing the rejection of cells and tissues bearing the same transgene.
Materials and methods
Animals
All mice were obtained from the Jackson Laboratory (Bar Harbor, ME). At 3 months of age, male or female C57BL/6 (B6: H-2b/CD45.2) mice were used as bone marrow transplant recipients. Congenic B6-Ly5.1 mice (PtprcaPep3b/BoyJ [CD45.1]) or hemizygous transgenic B6-EGFP.Tg mice (C57BL/6-TgN [ACTbEGFP]10sb)18,19 were used as a source of donor bone marrow. B6-EGFP.Tg and B10.A (B10.A: H2a, Kk, I-Ak, I-Ek, Dd) or B6.C-H2d mice were used as donors for skin grafting.
Recipient mice treatment and bone marrow transplantation
Busulfan (Busulfex; Orphan Medical, Minnetonka, MN) was obtained as a 6 mg/mL busulfan solution dissolved in N,N-dimethylacetamide (33% wt/wt) and polyethylene glycol 400 (67% wt/wt) and diluted in sterile saline to 1.0 mg/mL and injected intraperitoneally in volumes of 0.1 mL/10 g body weight (10 mg/kg) at –3 and –2 days relative to BMT. This dose of busulfan was determined to be only approximately one fifth the maximal tolerated dose (MTD ≈100 mg/kg in 4 daily fractions) in these mice. This MTD is equivalent to 350 mg/m2,20 which, on a dose per surface area basis, is approximately 70% of the high-dose busulfan commonly used in clinical myeloablative transplantation regimens.21 Treosulfan (Ovastat; Medac, Wedel, Germany) was dissolved in 37°C sterile water at 50 mg/mL and injected intraperitoneally in volumes of 0.1 mL/10 g body weight (500 mg/kg) at –4, –3, and –2 days relative to BMT. The MTD for treosulfan has been determined to be approximately 6000 mg/kg delivered in 3 daily doses22 ; thus, the dose used in the present study (approximately one quarter of the MTD) should also be considered nonmyeloablative.
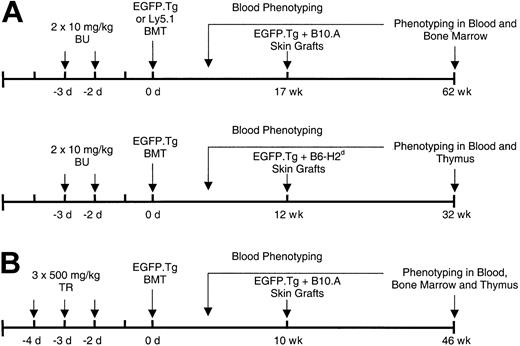
Bone marrow cell suspensions in Hanks buffered salt solution (HBSS) were prepared from the long bones of sex-matched B6-EGFP.Tg or B6-Ly5.1 mice, and the busulfan- or treosulfan-pretreated B6 recipients were intravenously infused through the tail vein with 15 × 106 nucleated cells. Figure 1 describes the different treatment and transplantation protocols.
Treatment and transplantation protocols. Experiments were conducted with (A) busulfan and (B) treosulfan BMT conditioning followed by donor-type and third-party skin grafting.
Treatment and transplantation protocols. Experiments were conducted with (A) busulfan and (B) treosulfan BMT conditioning followed by donor-type and third-party skin grafting.
Hematologic monitoring
Leukocyte, platelet, erythrocyte, hemoglobin, and hematocrit levels were assessed in tail blood samples using a Coulter Ac.T diff Analyzer with veterinary applications software (Coulter, Miami, FL). Granulocyte counts were determined from forward-versus side-scatter profiles, and T- and B-lymphocytes were distinguished by anti-CD3 and anti-B220 staining, respectively, in flow cytometry (FCM).
Posttransplantation FCM monitoring
Blood samples were treated twice with ACK lysis buffer (Bio-Whittaker, Walkersville, MD) at room temperature for 5 minutes to remove erythrocytes. For washing and dilutions, fluorescence-activated cell sorter (FACS) buffer consisted of phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (BSA) and 0.01% sodium azide. Fc receptors were blocked using anti-CD16/32 (clone 2.4G2) prepared in-house at 5 μg/sample for 5 to 10 minutes at room temperature. Other antibodies were obtained from BD PharMingen (San Diego, CA) and were added at 10 μg/mL in 100 μL FACS buffer. Samples were incubated for 30 minutes at 4°C and then washed twice with 3 mL FACS buffer. The different peripheral blood leukocyte (PBL) populations were also distinguished by their forward scatter (FSC) and side scatter (SSC) properties: FSC low and SSC low (lymphocytes), SSC high (granulocytes), and FSC high and SSC low (monocytes). Exclusion of dead cells was performed using propidium iodide (PI) staining and live gating on PI-negative cells.
For donor analysis, cells were stained with phycoerythrin (PE) antimouse CD45.1 (clone A20) and PE mouse immunoglobulin G2a (IgG2a) (isotype control). For lineage analysis, cells were stained with PE-antimouse CD3e (clone 145-2C11), PE hamster IgG (isotype control), or PE antimouse CD45R/B220 (clone RA3-6B2); isotype control was PE rat IgG2a (isotype control).
Femoral BMCs were harvested from animals on termination of the experiments. BMCs (5 × 105) were blocked with 5 μg anti-CD16/32 (clone 2.4G2) for 5 to 10 minutes at room temperature. Samples were incubated 30 minutes at 4°C with 10 μg/mL antibody in FACS buffer and then washed twice with 3 mL FACS buffer.
For FCM analysis of bone marrow, cells were stained with PE antimouse CD117 (clone ACK45), PE rat IgG2b (isotype control), PE antimouse CD45.1 (clone A20), and PE mouse IgG2a (isotype control). Acquisition and analysis were performed as described above.
BMCs were also plated in methylcellulose medium (MethoCult GF434; Stem Cell Technologies, Vancouver, BC, Canada), and the resultant hematopoietic colonies forming at 7 days were assessed by FCM for the expression of EGFP or PE antimouse CD45.1. After the methyl cellulose media were scraped into at least 4 volumes of PBS, the cells were centrifuged and resuspended in FACS buffer. PI (0.5-1.0 μg/tube) was added to exclude nonviable cells during analysis.
Analysis of thymic CD11c and CD8α+ cells
Thymuses were finely minced in 2000 U collagenase type IV (Sigma) in 1 mL Dulbecco PBS containing CaCl2 and MgCl2 (Gibco BRL, Grand Island, NY) and 50 U DNase (Sigma) and were incubated for 45 minutes. Samples were then passed through an 18-gauge hypodermic needle until a single-cell suspension was obtained.
Pooled samples were stained with biotinylated antimouse CD11c (clone HL3) using streptavidin–allophycocyanin and PE-conjugated antimouse CD8α (clone 53-6.7) (BD PharMingen). Nonviable cells were discarded based on DAPI (4′6-diamino-2-phenyl-indole dihydrochloride; BD PharMingen) exclusion and 4-color analysis performed for EGFP expression versus CD11c and CD8α using a Becton Dickinson FACSVantage SE with CellQuest software.
Skin grafts from EGFP transgenic and third-party donor mice
Sex-matched B6-EGFP.Tg mice, at 8 to 12 weeks of age, served as a source of donor tail skin grafts along with third-party skin grafts from MHC-incompatible B10.A (H-2a, Kk, I-Ak, I-Ek, Dd) or B6.C-H2d mice. Full-thickness tail skin grafts were implanted according to Sharabi and Sachs.23 Grafts were defined as accepted if they were in uniform viable condition, with tail hairs and scales, and were considered rejected at the time of complete sloughing or when they formed a dry scab.
Measurement of anti-EGFP T-cell response by ELISPOT
T-cell responses were determined in splenocyte preparations from animals at 32 weeks after EGFP-BMT preconditioned with or without busulfan. All mice were challenged 7 days previously with an intraperitoneal dose (7.5 × 107 nucleated cells) of irradiated (20 Gy) B6-EGFP.Tg splenocytes. Spleens of the recipient mice were then removed, minced, and filtered, and the cell suspensions were separated over a Lympholyte-M density gradient (Cedarlane Laboratories, Hornby, ON, Canada).
Ninety-six–well enzyme-linked immunosorbent assay (ELISA) spot plates (Polyfiltronics, Rockland, MA) were coated with a capture monoclonal antibody (mAb) in sterile PBS overnight. Anti–interleukin-2 (anti–IL-2) (JES6-1A12), anti–interferon-γ (anti–IFN-γ) (R4-6A2), and anti–IL-4 (11B11) capture mAbs were used at 3, 4, 2, and 5 μg/mL (BD PharMingen). On the day of the experiment, the plates were washed twice with sterile PBS, blocked for 1.5 hours with PBS containing 1% bovine serum albumin (BSA), then washed 3 times with sterile PBS. To measure the anti-EGFP response, recipient spleen mononuclear cells were added to wells previously filled with intact irradiated (20 Gy) spleen donor cells (EGFP.Tg cells) or with control syngeneic B6 or allogeneic BALB/c-irradiated spleen cells as previously described.24,25 Cells were incubated for different periods of time, depending on the cytokine measurement: 24 hours for IL-2 and IFN-γ and 36 hours for IL-4. Plates were washed 3 times with PBS, then 4 times with PBS containing 0.025% Tween (PBST). Biotinylated antilymphokine detection mAbs (IL-2, JES6-5H4; IFN-γ, XMG1.2; IL-4, BVD4-1D11) were added at 2 μg/mL (BD PharMingen) for 5 hours at room temperature or overnight at 4°C. After washing 3 times with PBST, avidin-horseradish peroxidase (1/2000) was added to each well for 1.5 hours. Finally, 4 washes with PBS were performed before the spots were revealed by the addition of the developing solution composed of 800 μL 3-amino 9-ethylcarbazole (AEC) (Sigma; 10 mg dissolved in 1 mL dimethyl formamide) in 24 mL 0.1 M sodium acetate, pH 5.0, catalyzed by 12 μL H2O2. Resultant spots were counted and analyzed on a computer-assisted ELISA spot image analyzer (C.T.L., Cleveland, OH).
Measurement of anti-EGFP antibodies
B6-EGFP.Tg splenocytes were harvested, irradiated with 30 Gy, and prepared in an emulsion with complete Freund adjuvant (Sigma). Groups of 5 animals at 61 weeks after B6-EGFP.Tg or B6-Ly5.1 BMT were immunized with an intraperitoneal dose of 5 × 107 splenocytes per animal. Blood plasma was taken before immunization and 7 days after immunization. Antibody levels were determined using ELISA. Ninety-six–well plates were coated with EGFP protein (Clontech, Palo Alto, CA). Serial dilutions of the preimmunization and postimmunization samples were added to the plates, and antibody binding was detected with goat antimouse IgG–horseradish peroxidase (Southern Biotech, Birmingham, AL). Enzyme activity was measured using TMB substrate (Sigma), and the reaction was stopped with 0.5 M H2SO4. Color development was determined at 450-nm absorbance. A control mouse anti-EGFP monoclonal antibody (Clontech) was used as a positive control.
Results
Minimal myelosuppression after low-dose busulfan treatment
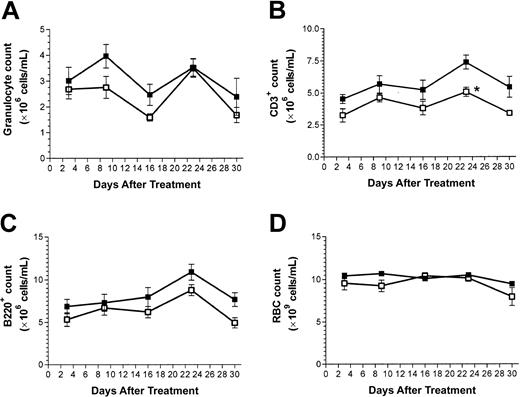
A minimal nonmyeloablative busulfan-based preparative regimen was evaluated for efficacy and hematologic toxicity. As shown in Figure 2, this treatment protocol had a minor and mostly insignificant effect on relevant blood cell parameters, even in the absence of BMT. The minimal myelosuppressive properties of this dose of busulfan are comparable to the recent finding of Adams et al,26 who also reported that this dose of busulfan was milder than leukocyte depression elicited by a nonmyeloablative dose (3 Gy) of radiation. Minimal effects on leukocyte and red blood cell parameters were similarly seen in the recipients of treosulfan (3 × 500 mg/kg) and BMT (data not shown).
Peripheral blood cell counts. (A) Granulocytes. (B) T lymphocytes. (C) B lymphocytes. (D) Erythrocytes. Counts were taken in C57BL/6 mice following treatment with busulfan (2 × 10 mg/kg, days –1 and 0). Shown are mean values (± 1 SEM) for groups of 4 (untreated, ▪) and 5 (treated, □) mice. No significant difference of treated versus untreated mice (P > .05 using the Mann-Whitney U test) was observed for any of the cell counts except for CD3+ cells at the 16-day time point (*P = .03). Similarly, no statistically significant differences were observed for total leukocyte, hematocrit, hemoglobin, or platelet levels (data not shown).
Peripheral blood cell counts. (A) Granulocytes. (B) T lymphocytes. (C) B lymphocytes. (D) Erythrocytes. Counts were taken in C57BL/6 mice following treatment with busulfan (2 × 10 mg/kg, days –1 and 0). Shown are mean values (± 1 SEM) for groups of 4 (untreated, ▪) and 5 (treated, □) mice. No significant difference of treated versus untreated mice (P > .05 using the Mann-Whitney U test) was observed for any of the cell counts except for CD3+ cells at the 16-day time point (*P = .03). Similarly, no statistically significant differences were observed for total leukocyte, hematocrit, hemoglobin, or platelet levels (data not shown).
Recipients pretreated with nonmyeloablative conditioning regimens consisting of busulfan or treosulfan all survived in good health throughout the experiments (up to 62 weeks) without any significant loss in body weight or other apparent health complications.
Chimerism following low-dose busulfan conditioning and transplantation of EGFP+ or Ly5.1+ BM cells
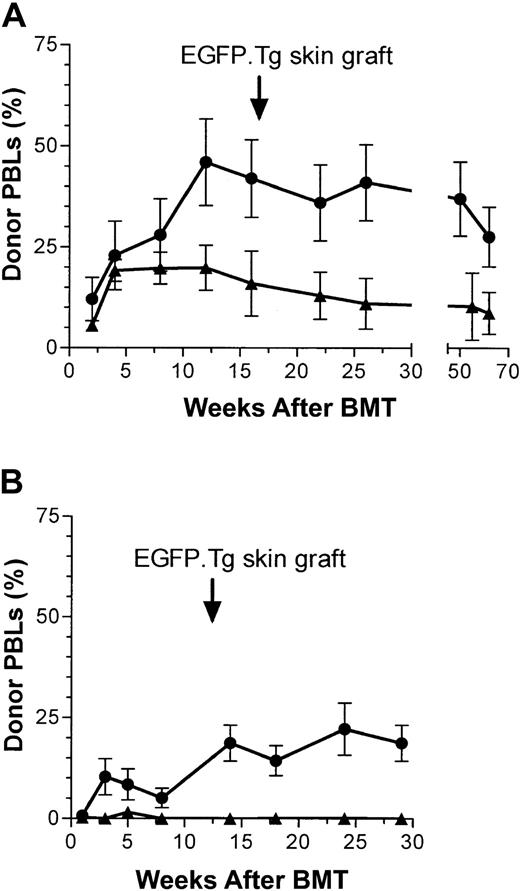
Treatment with a low dose of 20 mg/kg busulfan, representing one fifth of the maximal tolerated dose, followed by the injection of 1.5 × 107 EGFP+ BM cells in 2 experiments, allowed high levels of donor chimerism (20%-40%) in the PBLs to 32 or 62 weeks (Figure 3). Similar expression was observed among blood granulocytes, T cells, and B cells and in bone marrow progenitors (Table 1). Chimerism was also obtained after transplantation of Ly5.1 congenic BMCs, albeit at lower average levels (Figure 3A; Table 1). In the second experiment, injection of the same dose of EGFP+ BM into untreated recipients failed to produce detectable EGFP chimerism among peripheral blood cells (Figure 3B).
Chimerism after busulfan. (A) PBL EGFP chimerism (mean ± SEM) in 5 female B6 mice after recipient pretreatment with busulfan (BU, 2 × 10 mg/kg) followed by transplantation (15 × 106 BMCs) from B6-EGFP.Tg (•) or B6-Ly5.1 (▴) donor mice. (B) EGFP chimerism (mean ± SEM) in male B6 recipients pretreated with saline (4 mice, ▴) or busulfan (2 × 10 mg/kg) (5 mice, •) followed by transplantation (15 × 106 BMCs) from B6-EGFP.Tg donor mice.
Chimerism after busulfan. (A) PBL EGFP chimerism (mean ± SEM) in 5 female B6 mice after recipient pretreatment with busulfan (BU, 2 × 10 mg/kg) followed by transplantation (15 × 106 BMCs) from B6-EGFP.Tg (•) or B6-Ly5.1 (▴) donor mice. (B) EGFP chimerism (mean ± SEM) in male B6 recipients pretreated with saline (4 mice, ▴) or busulfan (2 × 10 mg/kg) (5 mice, •) followed by transplantation (15 × 106 BMCs) from B6-EGFP.Tg donor mice.
Percent donor EGFP or Ly5.1 expression in different cells and tissues at 62 weeks after 2 × 10 mg/kg busulfan and BMT (± 1 SEM)
Group . | Donor PBL Granulocytes . | Donor PBL B220 . | Donor PBL CD3 . | Donor BM c-kit . | Donor BM CFU-Cs . |
|---|---|---|---|---|---|
| B6-EGFP.Tg BMT, n = 5 | 19.5 ± 8.8 | 32.2 ± 7.7 | 36.4 ± 11.1 | 28.7 ± 7.1 | 38.8 ± 4.8 |
| B6-Ly5.1 BMT, n = 5 | 6.2 ± 5.9 | 12.4 ± 8.8 | 19.3 ± 12.2 | 18.2 ± 14.4 | 10.3 ± 8.1 |
Group . | Donor PBL Granulocytes . | Donor PBL B220 . | Donor PBL CD3 . | Donor BM c-kit . | Donor BM CFU-Cs . |
|---|---|---|---|---|---|
| B6-EGFP.Tg BMT, n = 5 | 19.5 ± 8.8 | 32.2 ± 7.7 | 36.4 ± 11.1 | 28.7 ± 7.1 | 38.8 ± 4.8 |
| B6-Ly5.1 BMT, n = 5 | 6.2 ± 5.9 | 12.4 ± 8.8 | 19.3 ± 12.2 | 18.2 ± 14.4 | 10.3 ± 8.1 |
Corrected for EGFP and Ly5.1 expression in respective B6-EGFP.Tg and B6-Ly5.1 control mice.
Analysis of the thymic CD11c+, CD8α+ cell subpopulation 32 weeks after BMT revealed the presence of EGFP in 23% of this cell subset (Figure 4), a level comparable to that observed in peripheral leukocytes of the same animals (average, 18.7%).
FCM analysis of collagenase-digested thymocytes. Analysis showed EGFP expression in cells coexpressing CD11c and CD8α. Cells were pooled from 5 recipients of busulfan and EGFP.Tg bone marrow at 32 weeks after BMT.
FCM analysis of collagenase-digested thymocytes. Analysis showed EGFP expression in cells coexpressing CD11c and CD8α. Cells were pooled from 5 recipients of busulfan and EGFP.Tg bone marrow at 32 weeks after BMT.
Survival of EGFP.Tg skin grafts in recipients of low-dose busulfan and EGFP.Tg BMT
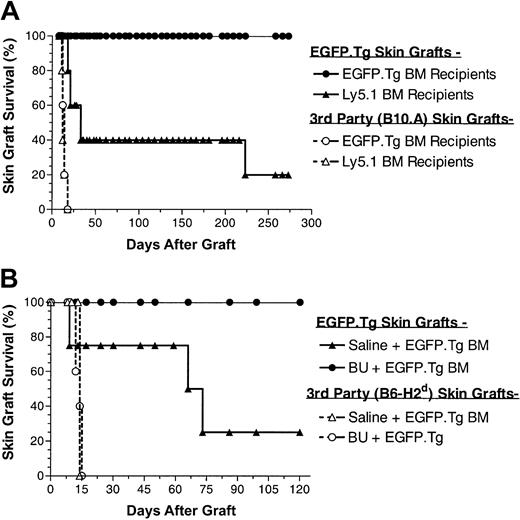
To assess whether stable EGFP+ chimeric B6 mice developed immunologic unresponsiveness toward EGFP-expressing tissues, such chimeric hosts were challenged with EGFP.Tg and third-party skin grafts, at 17 and 12 weeks after BMT in the first and second experiments, respectively. Skin grafting results from these experiments (Figure 5) show that all 10 recipients of EGFP.Tg bone marrow grafts subsequently and permanently accepted their EGFP.Tg skin grafts, whereas 7 of 9 control recipients (receiving either the same treatment and EGFP nonexpressing BM or no treatment and EGFP.Tg BM) rejected EGFP-expressing skin grafts. Figure 6 shows the appearance of an accepted skin graft with uniform green fluorescence under ultraviolet illumination in a recipient that exhibited EGFP expression in blood cells. Allogeneic skin transplantation was performed to establish that mice used in this study were immunocompetent after the conditioning regimens applied. In both experiments, third-party grafts from MHC-mismatched B10.A or B6-H2d mice were promptly rejected among all BMT groups.
Skin graft survival after busulfan. (A) Skin graft survival (EGFP.Tg and B10.A) performed at 17 weeks after BMT in 5 female B6 recipients of busulfan (BU, 2 × 10 mg/kg) and BMCs from B6-EGFP.Tg or B6-Ly5.1 donor mice. (B) Skin graft survival (EGFP.Tg and B6-H2b) performed at 12 weeks after BMT in male B6 recipients of saline (4 mice) or BX (2 × 10 mg/kg) (5 mice) and BMCs from B6-EGFP.Tg donor mice.
Skin graft survival after busulfan. (A) Skin graft survival (EGFP.Tg and B10.A) performed at 17 weeks after BMT in 5 female B6 recipients of busulfan (BU, 2 × 10 mg/kg) and BMCs from B6-EGFP.Tg or B6-Ly5.1 donor mice. (B) Skin graft survival (EGFP.Tg and B6-H2b) performed at 12 weeks after BMT in male B6 recipients of saline (4 mice) or BX (2 × 10 mg/kg) (5 mice) and BMCs from B6-EGFP.Tg donor mice.
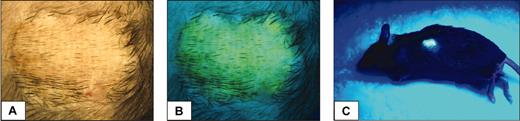
Appearance of an accepted skin graft. Under light (A) or ultraviolet illumination (B,C) in an EGFP chimeric animal (19% EGFP-positive leukocytes) at 32 weeks after busulfan and BMT.
Appearance of an accepted skin graft. Under light (A) or ultraviolet illumination (B,C) in an EGFP chimeric animal (19% EGFP-positive leukocytes) at 32 weeks after busulfan and BMT.
EGFP.Tg skin graft acceptance is associated with an abrogation of anti-EGFP T-cell responses
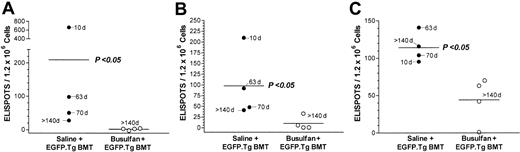
We next investigated the antidonor immune response in chimeric mice that had permanently accepted their EGFP-expressing skin grafts. To test this, mice that had been treated with busulfan and EGFP.Tg BMT and that had accepted EGFP skin grafts for more than 140 days were challenged intraperitoneally with 7.5 × 106 irradiated (20 Gy) EGFP splenocytes. Seven days after challenge, spleen T cells were collected, and the frequencies of reactive T cells producing type 1 (IL-2 and IFN-γ) and type 2 (IL-4) cytokines were measured by ELISPOT as previously described.24 Nonchimeric mice that had received saline and EGFP.Tg BMT and had rejected their EGFP grafts were used as positive controls. As observed in Figure 7, no EGFP-specific T cells secreting either type 1 or type 2 cytokines were found in chimeric mice (P > 0.05 compared with syngeneic [B6] stimulator controls). In contrast, significant numbers of type 1 and type 2 cytokine-producing T cells were detected in nonchimeric control mice that had rejected their grafts (P < .5 compared with chimeric mice or with syngeneic [B6] stimulator controls). Interestingly, the frequency of type 1 ELISPOTS was inversely related to the time to EGFP.Tg skin rejection with the one nonchimeric mouse that had accepted its graft for more than 140 days and that had the least number of spots. These data indicate that tolerance to the EGFP.Tg skin graft in chimeric mice is strictly associated with the abrogation of anti-EGFP T-cell responses. Both groups consistently showed strong responses for all cytokines (above 217, 64, and 142 ELISPOTS per 1.2 × 106 cells for IFN-γ, IL-2, and IL-4, respectively) to third-party allogenic (BALB/c) stimulators, confirming that the tolerance was indeed donor specific.
Splenic ELISPOTS. Frequency of splenic ELISPOTS secreting (A) IFN-γ, (B) IL-2, and (C) IL-4 in response to EGFP.Tg stimulators in individual mice at 32 weeks after treatment with saline (nonchimeric mice) or busulfan (chimeric mice) before B6-EGFP.Tg BMT. Significant differences of nonchimeric versus chimeric mice are shown for all responses (P < .05 using the Mann-Whitney U test).
Splenic ELISPOTS. Frequency of splenic ELISPOTS secreting (A) IFN-γ, (B) IL-2, and (C) IL-4 in response to EGFP.Tg stimulators in individual mice at 32 weeks after treatment with saline (nonchimeric mice) or busulfan (chimeric mice) before B6-EGFP.Tg BMT. Significant differences of nonchimeric versus chimeric mice are shown for all responses (P < .05 using the Mann-Whitney U test).
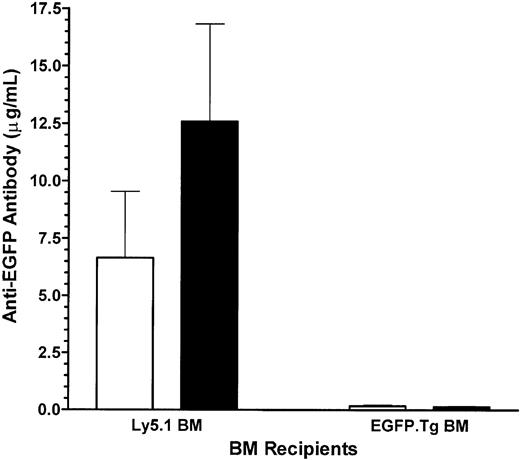
A lack of host response against EGFP in EGFP-chimeric recipients was also seen with respect to the generation of anti-EGFP antibodies. Control recipients of Ly5.1 bone marrow developed high levels of anti-EGFP antibodies, most likely induced during the cell-mediated destruction of the EGFP.Tg skin graft. Challenge with EGFP.Tg splenocytes induced approximately a 2-fold increase in antibody levels in these mice. Experimental recipients of EGFP-transgenic bone marrow had much lower levels of anti-EGFP antibodies, and immunization with the EGFP.Tg splenocytes had no effect on these antibody levels (Figure 8).
Antibody levels. Blood anti-EGFP antibody levels in Ly5.1 (control) and EGFP-chimeric (experimental) mice grafted with EGFP-expressing skin before (□) and after (▪) challenge with EGFP.Tg splenocytes (SPL). Values represent mean ± 1 SEM for groups of 5 mice at 62 weeks after BMT.
Antibody levels. Blood anti-EGFP antibody levels in Ly5.1 (control) and EGFP-chimeric (experimental) mice grafted with EGFP-expressing skin before (□) and after (▪) challenge with EGFP.Tg splenocytes (SPL). Values represent mean ± 1 SEM for groups of 5 mice at 62 weeks after BMT.
EGFP expression and EGFP.Tg skin graft survival after low-dose treosulfan conditioning and EGFP.Tg BMT
Analysis of EGFP expression in leukocytes showed that treosulfan allowed the induction of constant levels of mixed chimerism of approximately 10% over a period of 46 weeks (Figure 9A). As seen with busulfan, EGFP expression was seen at similar levels among all hematopoietic cell lineages tested on termination of the experiment (Table 2). In particular, EGFP expression of approximately 14% was observed in the CD11c and CD8α population residing in the thymuses of these animals (data not shown), with the implication that these cells might be responsible for the induction of immunologic tolerance as demonstrated by the long-term acceptance of EGFP-expressing skin grafts in all 5 EGFP-chimeric animals (Figure 9B) compared with rejection in all 5 control mice. Rapid rejection of third-party B10.A skin grafts again indicated the specificity of this tolerance induction by transplantation of EGFP-expressing BMCs. The ELISPOT assay was also performed on the splenocytes of these mice, and all the chimeric recipients showed abrogation of anti-EGFP T-cell responses (data not shown), similar to that described above for busulfan-conditioned animals.
Chimerism and skin graft survival after treosulfan. (A) PBL donor-type chimerism (mean ± SEM) after recipient pretreatment with treosulfan (3 × 500 mg/kg) followed by transplantation (15 × 106 BMCs) from B6-EGFP.Tg donor mice (•). ▴ indicates saline control mice. (B) Skin graft survival (EGFP.Tg and B10.A) performed 10 weeks after BMT. Each group contained 5 mice.
Chimerism and skin graft survival after treosulfan. (A) PBL donor-type chimerism (mean ± SEM) after recipient pretreatment with treosulfan (3 × 500 mg/kg) followed by transplantation (15 × 106 BMCs) from B6-EGFP.Tg donor mice (•). ▴ indicates saline control mice. (B) Skin graft survival (EGFP.Tg and B10.A) performed 10 weeks after BMT. Each group contained 5 mice.
Percent donor EGFP expression in different cells and tissues at 46 weeks after 3 × 500 mg/kg treosulfan and BMT (± 1 SEM)
Group . | Donor PBL granulocytes . | Donor PBL B220 . | Donor PBL CD3 . | Donor BM c-kit . |
|---|---|---|---|---|
| Untreated | ||||
| controls, n = 5 | 0 | 0 | 0 | 0 |
| B6-EGFP.Tg | ||||
| BMT, n = 5 | 8.7 ± 2.0 | 11.0 ± 1.3 | 7.1 ± 0.5 | 8.2 ± 0.9 |
Group . | Donor PBL granulocytes . | Donor PBL B220 . | Donor PBL CD3 . | Donor BM c-kit . |
|---|---|---|---|---|
| Untreated | ||||
| controls, n = 5 | 0 | 0 | 0 | 0 |
| B6-EGFP.Tg | ||||
| BMT, n = 5 | 8.7 ± 2.0 | 11.0 ± 1.3 | 7.1 ± 0.5 | 8.2 ± 0.9 |
Corrected for EGFP expression in B6-EGFP.Tg control mice.
Discussion
The immunogenicity of transferred gene products continues to be a major hurdle in the development of efficient clinical gene therapy protocols. A possible strategy to overcome this problem would be to “tolerize” the host to the foreign products before applying the gene therapy protocol. Immune tolerance to foreign (donor) products can be reproducibly achieved through the establishment of donor/recipient mixed hematopoietic chimerism.12,27 The general use of such an approach is, however, limited clinically because it requires stringent myeloablative conditioning regimens with undesirable toxicity, and nonmyeloablative protocols currently being evaluated are associated with a high rate of graft-versus-host disease.28,29 Because it is well established experimentally and clinically that, with decreasing antigenic disparity, engraftment is easier to achieve and graft-versus-host disease is less frequent and severe, we postulated that a single gene product intended for use in a gene therapy protocol could be used to establish hematopoietic molecular chimerism using a mild conditioning regimen. The gene encoding EGFP was selected for this purpose because this marker protein has been shown to be immunogenic in mice and primates.9,11 The present study was also an extension of earlier experiments in which transplanted donor BM cells were transduced with MoMLV-based retroviral vectors expressing the EGFP gene, but in recipients that were preconditioned using a standard myeloablative (10 Gy total body irradiation [TBI]) protocol.30 In this case, skin grafts from EGFP-transgenic mice were accepted long-term only in those animals receiving the transduced bone marrow. It was especially striking that EGFP-transduced BMT appeared to prevent the rejection of EGFP.Tg skin, even under conditions in which EGFP expression in PBLs declined to undetectable levels. Thus, our earlier data suggest that specific immune tolerance can be induced to a single immunogenic protein expressed on autologous cells and that conditioning requirements for transplantation of molecularly modified autologous cells are less than those required for allogeneic transplants.
Previous murine experiments using syngeneic BMT have shown that, with the exception of high cell doses BMT,31,32 a certain degree of hematopoietic stem cell depletion in the host BM by agents such as busulfan, or the closely related compound treosulfan, is necessary to establish long-term engraftment and hematopoietic chimerism of transplanted donor stem cells.33,34 When allogeneic BM cells are transplanted, additional suppression of host immunity with agents such as cyclophosphamide, fludarabine, or T-cell–depleting antibodies is also required to establish stable chimerism.34, 35, 36, 37 In the present study, EGFP-expressing syngeneic BM readily engrafted in recipients conditioned only with low-dose busulfan or treosulfan in the absence of any immune suppression (Figures 3 and 9). Multilineage chimerism was detected in BM and in peripheral blood. Indeed, the levels of blood and BM chimerism were slightly higher in the experimental mice than in the control group, which received Ly5.1 (CD45.1)–marked BM. This observation lends support to the recent finding that the antigenicity of CD45.1 can confer resistance to BM engraftment at low radiation doses.38
Our results are consistent with those of Kang et al39 in that the pretreatment of C57BL/6 recipients with radiation doses as low as 1 Gy allowed long-term peripheral EGFP expression after the transplantation of EGFP-transduced BM, though the cell dose used was approximately 10-fold higher than in our current studies (120 vs 15 million cells). These data are in contrast with those of Rosenzweig et al,11 who reported that EGFP-transduced autologous BM cells transplanted into rhesus monkeys under nonmyeloablative conditioning (240 cGy TBI) underwent immune rejection of the EGFP-expressing cells. The authors cite 2 additional primate studies in which increasing the dose of TBI to 320 to 500 cGy (still at nonmyeloablative levels) resulted in persistent transgene expression 40,41 to indicate that the efficacy of TBI is sensitive to relatively small changes in radiation dose. Because TBI is known to have immunosuppressive properties and stem cell-depleting capacity, it remains unclear whether the lowest dose of TBI used in the Rosenzweig experiments provided insufficient stem cell depletion or whether stem cell depletion alone was insufficient to achieve engraftment. If EGFP elicits a relatively strong immune response in primates, then some degree of immune suppression may also be required to achieve engraftment.
Animals exhibiting stable hematopoietic chimerism involving EGFP showed long-term acceptance of EGFP.Tg. skin grafts (Figures 5 and 9). In contrast, most (12 of 14) control mice that were EGFP-negative in the hematopoietic compartment rejected the EGFP+ skin grafts within 250 days. Immunologic competence in EGFP-tolerant mice was demonstrated by prompt rejection of MHC-mismatched skin grafts within 20 days. The more rapid rejection of the MHC-mismatched grafts over that of EGFP-only disparate grafts may be attributed to stronger immune responses against greater MHC antigenic disparity. The operational tolerance to EGFP skin grafts also correlated with the absence of T-cell and antibody reactivity to EGFP (Figures 7 and 8, respectively). These data confirmed that the host immune responses toward the introduced EGFP protein were specifically down-modulated, a clear indication of an effective, antigen-specific, immune tolerance. It is likely that the mechanism of tolerance induction in our model relies on the same pathways as that of control tolerance in BM chimeras made from fully disparate MHC donors12 —clonal deletion and peripheral regulatory mechanisms.
In agreement with the possible induction of central T-cell tolerance by our procedure, we have observed that the thymuses of EGFP-expressing, but not of EGFP-negative, mice contained a small minority of cells expressing CD11c, CD8α, and EGFP (Figure 4). Lymphoid CD11c+, CD8α+, CD45– dendritic cells (DCs) are the principal thymic antigen-presenting cells42 and have been shown to be involved in the induction of operational tolerance to allogeneic heart transplants43 and in the control of peripheral tolerance to self-antigens.44 It is, therefore, conceivable that this cell type, expressing EGFP intrathymically, participates in the negative selection process of anti-EGFP T-cell receptor (TCR) clones in our model. Although donor APCs appear to be directly involved in the selection process that leads to donor antigen-specific tolerance, their finite lifespan requires a continuing replenishment from marrow-derived precursors. Thus, the engraftment of self-renewing, pluripotent donor stem cells may be essential for long-term stable tolerance induction.
Our mouse model also provides a system in which the importance of varying degrees of immunogenicity of a single antigen can be addressed. Using EGFP-expressing tumor models, investigators have indicated that the immune response to EGFP is much stronger in BALB/c mice (H-2d)9,10 than in C57BL/6 mice (H-2b).45,46 Preliminary studies in our laboratory suggest a more rapid onset of rejection of EGFP-transgenic skin among C57BL/6 congenic mice bearing the H-2d haplotype compared with standard C57BL/6 (H-2b) mice. Because busulfan is one of the few agents with stem cell-depleting capacity though it lacks immune suppressive properties, it can be used with or without immunosuppressive agents to dissect conditioning requirements for a given antigen or set of antigens. Studies have been initiated using our rodent model to address these issues.
In conclusion, the current studies demonstrate that operational tolerance can be induced to nonhematopoietic antigens by their ectopic expression in the hematopoietic compartment. They also serve to emphasize that with minimal antigenic differences, mild nonmyeloablative protocols are sufficient for establishing molecular chimerism and inducing tolerance. Busulfan, or its soluble form Busulfex, is one of the few agents that is effective in depleting hematopoietic stem cells, a requirement for engraftment. Although TBI is also effective in this capacity, it has immunosuppressive properties as well, which complicates attempts to define minimal requirements for engraftment. Although similar engraftment and skin tolerance results have been achieved with the dihydroxy-busulfan derivative treosulfan, this drug may also have broader effects and may confer some immunosuppression in addition to stem cell depletion, particularly at higher doses.47 Given that busulfan treatment only has a minimal effect on T lymphocytes (Figure 2), it can be titrated with or without immunosuppressive agents to determine an optimal balance of these 2 components for engraftment. It is notable that these murine studies support the recent clinical success of using low-dose busulfan before gene-corrected autologous stem cell transplantation of patients with adenosine deaminase (ADA)–deficient severe combined immunodeficiency (SCID).48 With the advent of improved gene-delivery vectors and transduction protocols, it is anticipated that the use of such nonmyeloablative preparative regimens will similarly enable the approach of establishing immunologic tolerance toward gene therapy products through molecular chimerism to become a clinical reality.
Prepublished online as Blood First Edition Paper, February 6, 2003; DOI 10.1182/blood-2002-06-1649.
Supported by National Institutes of Health grant 1R43CA090097-1A1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Meaghan Boston, Merrill Shaffer, Kenol Etienne, Lana Popova, Peter Morgan, Angela Sullivan, and Susan Dearborn for their excellent technical expertise, David Dombkowski (Massachusetts General Hospital) for the cytometric analysis of thymic cells, Dr Joachim Baumgart (Medac, Wedel, Germany) for the supply of treosulfan, and Drs Clive Patience and Barbara Wallner for their constructive critique of the manuscript.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal