Ganciclovir effectively prevents cytomegalovirus (CMV) disease in the first 100 days after allogeneic hematopoietic stem cell transplantation (HSCT), but late-onset CMV disease is increasingly observed. We designed a prospective cohort study to define the incidence and risk factors for late CMV infection in patients who undergo HSCT. CMV-seropositive patients were studied prospectively for CMV infection (quantitative pp65 antigenemia, quantitative CMV-DNA, blood culture), T-cell immunity (CMV-specific CD4+ T-helper and CD8+ cytotoxic T-lymphocyte responses, CD4 and CD8 T-cell count, absolute lymphocyte count), and other transplantation-related factors. Univariate and multivariable analyses were used to assess the risk for late CMV infection and disease and to assess overall survival. Late CMV disease developed in 26 of 146 (17.8%) patients a median of 169 days after transplantation (range, 96-784 days); the mortality rate was 46%. Thirty-eight percent of patients surviving late disease had a second episode a median of 79 days after the first episode. At 3 months after transplantation, preceding detection of CMV pp65 antigenemia, CD4 T-cell counts lower than 50 cells/mm3, postengraftment absolute lymphopenia levels lower than 100 lymphocytes/mm3, undetectable CMV-specific T-cell responses, and graft-versus-host disease (GVHD) were associated with late CMV disease or death. After 3 months, continued detection of pp65 antigenemia or CMV DNA in plasma or peripheral blood leukocytes and lymphopenia (fewer than 300 lymphocytes/mm3) were strong predictors of late CMV disease and death. In conclusion, CMV viral load, lymphopenia, and CMV-specific T-cell immunodeficiency are predictors of late CMV disease and death after allogeneic stem cell transplantation. Prevention strategies should be targeted at patients in whom CMV reactivated during the first 3 months and those with poor CMV-specific immunity or low CD4 counts.
Introduction
Ganciclovir effectively prevents cytomegalovirus (CMV) disease during the first 3 months after allogeneic hematopoietic stem cell transplantation (HSCT) when it is administered to the patient during engraftment,1,2 for pp65 antigenemia,3or for the detection of CMV DNA by polymerase chain reaction (PCR),4 and its use improves survival in selected patients at high risk.4-7 However, recent clinical studies have shown that most cases of CMV disease now develop after 3 months,3,4 8-12 when many patients are treated by their referring physicians rather than at specialized cancer centers.
CMV-specific T-cell immunity and CMV viral load are important in predicting early CMV disease after HSCT.13-16 Ganciclovir therapy, as well as graft-versus-host disease (GVHD) and its treatment, can delay the recovery of CMV-specific T-cell immunity after marrow transplantation.17-19 Thus, CMV-specific immunodeficiency may persist after the discontinuation of ganciclovir therapy, leading to excess late CMV disease and CMV-related death. In the first 3 months after transplantation, CMV viremia, quantitative pp65 antigenemia, and DNA load are risk factors for the development of CMV disease, independent of GVHD.15,16,20,21 High CMV DNA load is also associated with poor survival in HIV-infected persons independent of CD4 lymphocytopenia and HIV load.22 Here we examine, in a prospective study, the interrelationship between CMV load, CMV-specific T-cell immunity, and death in patients who have undergone HSCT and are at risk for late CMV disease.
Patients, materials, and methods
Patients
CMV-seropositive subjects of all ages who were undergoing first allogeneic marrow transplantation in Seattle and who were alive without leukemic relapse at day 80 after transplantation were eligible for this prospective, longitudinal, observational study. Baseline testing was performed between days 80 and 100 after transplantation. At the time of study entry, patients were receiving either ganciclovir prophylaxis, started at engraftment, or pp65 antigenemia-guided early ganciclovir treatment, each of which was stopped at 3 months, when most patients were discharged to their referring physicians. After the first analysis 3 months after transplantation, there was no routine use of ganciclovir, foscarnet, high-dose acyclovir, or valacyclovir. White blood cell counts and differentials were monitored locally every 1 to 2 weeks. All study participants or their physicians were contacted by a research nurse on a regular basis to record clinical events. Patients were monitored for CMV disease and death for at least 2 years after transplantation. The protocol received Institutional Review Board approval, and signed, informed consent was obtained from each patient before study entry.
Virologic monitoring
Before study entry, only antigenemia testing was performed and viral blood cultures were grown. Between days 80 and 200 after transplantation or until the detection of disease, whichever occurred earlier, patients were monitored prospectively every 2 weeks for CMV pp65 antigenemia, CMV excretion from blood by conventional culture, and CMV DNA by PCR in peripheral blood leukocytes (PBLs) and plasma. Antigenemia and PCR testing were performed in batches from frozen slides or PBLs and plasma, respectively. CMV antigenemia testing was performed in duplicate, as described,23 and pp65 antigenemia was quantified as the average number of positive cells per 150 000 PBLs. For qualitative detection of CMV DNA by PCR, 105 PBLs were alkaline extracted, and DNA was amplified from the CMV UL 123 region, as described.24 Quantitative real-time PCR using the TaqMan (Perkin Elmer, Foster City, CA) system was performed on sequentially stored PBL samples from patients with positive findings by qualitative PCR or the antigenemia assay. Sequential quantitative PCR was performed on plasma in a subgroup of patients. Details of the assay have been described elsewhere.25 Quantitative PCR levels are shown as DNA copies per 106 copies of β-globin and as copies per milliliter plasma.
Immunologic monitoring
CMV-specific CD4+ T-helper (TH) and CD8+ cytotoxic T lymphocyte (CTL) responses, as well as CD4 and CD8 cell counts, were tested between days 80 and 100 and between days 140 and 150 after transplantation. In vitro expansion of CMV-specific CTLs was performed from peripheral blood mononuclear cells (PBMCs) as previously described using skin fibroblasts as stimulators and targets.19 To establish fibroblast lines, autologous skin or skin from HLA-matched donors was used. HLA-restricted CTL activity was determined by a 5-hour chromium-release assay. The panel of target cells used for each cytotoxicity assay included autologous and HLA-mismatched CMV-infected and mock-infected fibroblasts. Specific lysis was calculated by the standard formula, with maximum release reflecting counts per minute (cpm) from incubation of targets with 1% Nonidet P40-solution (Sigma, St Louis, MO), and spontaneous release, which should not exceed 30% of maximum release, reflecting cpm from targets incubated with medium alone. A positive result was defined as specific lysis of CMV-infected target cells greater than 10% above that of mock-infected targets.19 CMV-specific CD4+ Th responses and responses to phytohemagglutinin (PHA) were determined by a lymphoproliferative assay using 2 × 105 PBMCs per well in triplicate.19 A stimulation index of 3 or greater was considered positive.19 CD4 and CD8 T-lymphocyte counts were determined concurrently with CMV-specific T-cell responses (ie, days 80-100 and 140-150) by labeling PBMCs with specific monoclonal antibodies and subsequent analysis by 3-color flow cytometry.26
Definitions
CMV disease was defined as the demonstration of CMV in tissue by culture or histology or in bronchoalveolar lavage (BAL) by culture, direct fluorescence antibody stain, or cytology in the presence of new or changing pulmonary infiltrates.3 CMV retinitis was defined by ophthalmologic criteria. CMV-associated graft failure was defined as an absolute neutrophil count lower than 500 neutrophils/mm3 and the presence of CMV disease in marrow, detected by PCR and immunohistology, in the absence of other causes. CMV sinus disease was defined as the detection of CMV in sinus biopsy samples by culture or immunohistology and typical inflammatory changes in the absence of other pathogens. CMV-associated syndromes without tissue documentation were not considered. A second episode was defined as any manifestation that occurred after the completion of treatment and the disappearance of the initial symptoms. CMV-associated mortality was defined as death within 6 weeks of diagnosis of CMV disease or CMV identified in autopsy specimens.3 Acute and chronic GVHD were defined as described.27 28
Statistical analysis
CMV infection and disease incidence were estimated using cumulative incidence estimates, treating death before the event of interest as a competing risk event.29 Survival curves were estimated using the method of Kaplan and Meier.30 Hazard ratios and 95% confidence intervals were obtained for selected risk factors using univariate and multivariable Cox proportional hazards regression models for late CMV disease and death.31 In all time-to-event analyses, time was censored at second transplantation or at the end of follow-up. CMV virologic monitoring, chronic GVHD, CD4 counts, absolute lymphocyte counts, aspergillosis disease, and CMV disease (for death analysis, see Table 5) were included as time-dependent covariates in the regression models unless otherwise noted. Additional candidate risk factors were evaluated in the multivariable models as noted (Tables 1-7). Forward and backward elimination stepwise regression models were used to determine which variables should be included in the multivariable models. All reportedP values are 2-sided.
Patient data
| Characteristic . | Total (N = 146) . |
|---|---|
| Median age, y (range) | 37.9 (2.3-67.0) |
| Male, no. (%) | 80 (55) |
| CMV serostatus before transplantation, no. (%) | |
| Recipient CMV-positive | 146 (100) |
| Donor CMV-positive | 97 (66) |
| Disease stage at transplantation, no. (%) | |
| CML chronic phase | 44 (30) |
| Hematologic malignancy first remission | 17 (12) |
| Hematologic malignancy other than first remission | 70 (48) |
| Other | 15 (10) |
| HLA donor matching, no. (%) | |
| Matched related | 75 (51) |
| Mismatched related | 20 (14) |
| Unrelated | 51 (35) |
| Conditioning therapy, no. (%) | |
| Cyclophosphamide plus TBI | 63 (43) |
| Busulfan + cyclophosphamide | 48 (33) |
| Other | 35 (24) |
| GVHD prophylaxis, no. (%) | |
| CSA plus methotrexate | 92 (63) |
| Other | 54 (37) |
| GVHD incidence, no. (%)* | |
| Acute, grades 2-4 | 115 (80) |
| Acute, grades 3-4 | 102 (71) |
| Chronic, clinical-extensive | 86 (60) |
| Ganciclovir use before d 80, no. (%) | |
| At engraftment | 60 (41) |
| At pp65 antigenemia, as strategy | 86 (59) |
| pp65 antigenemia, actually present | 55 (38) |
| None | 31 (21) |
| Lymphopenia between d 40 and 95 after HSCT | |
| Fewer than 100 lymphocytes/mm3 | 80 (55) |
| Fewer than 300 lymphocytes/mm3 | 137 (94) |
| CD4 counts at baseline less than 50 cells/mm3 | 57 (43)† |
| CD8 counts at baseline less than 50 cells/mm3 | 44 (33)† |
| CMV disease before study entry, no. (%) | 3 (2) |
| Characteristic . | Total (N = 146) . |
|---|---|
| Median age, y (range) | 37.9 (2.3-67.0) |
| Male, no. (%) | 80 (55) |
| CMV serostatus before transplantation, no. (%) | |
| Recipient CMV-positive | 146 (100) |
| Donor CMV-positive | 97 (66) |
| Disease stage at transplantation, no. (%) | |
| CML chronic phase | 44 (30) |
| Hematologic malignancy first remission | 17 (12) |
| Hematologic malignancy other than first remission | 70 (48) |
| Other | 15 (10) |
| HLA donor matching, no. (%) | |
| Matched related | 75 (51) |
| Mismatched related | 20 (14) |
| Unrelated | 51 (35) |
| Conditioning therapy, no. (%) | |
| Cyclophosphamide plus TBI | 63 (43) |
| Busulfan + cyclophosphamide | 48 (33) |
| Other | 35 (24) |
| GVHD prophylaxis, no. (%) | |
| CSA plus methotrexate | 92 (63) |
| Other | 54 (37) |
| GVHD incidence, no. (%)* | |
| Acute, grades 2-4 | 115 (80) |
| Acute, grades 3-4 | 102 (71) |
| Chronic, clinical-extensive | 86 (60) |
| Ganciclovir use before d 80, no. (%) | |
| At engraftment | 60 (41) |
| At pp65 antigenemia, as strategy | 86 (59) |
| pp65 antigenemia, actually present | 55 (38) |
| None | 31 (21) |
| Lymphopenia between d 40 and 95 after HSCT | |
| Fewer than 100 lymphocytes/mm3 | 80 (55) |
| Fewer than 300 lymphocytes/mm3 | 137 (94) |
| CD4 counts at baseline less than 50 cells/mm3 | 57 (43)† |
| CD8 counts at baseline less than 50 cells/mm3 | 44 (33)† |
| CMV disease before study entry, no. (%) | 3 (2) |
TBI indicates total body irradiation; CSA, cyclosporine A.
Data available on 144 subjects.
Data available on 132 subjects.
Incidence of CMV infection after day 95
| CMV test method . | Positive CMV test result . | |||
|---|---|---|---|---|
| Number of patients tested . | All patients % . | Patients with positive CMV-specific CD4+ TH responses at 3 months . | Patients with negative CMV-specific CD4+TH responses at 3 months . | |
| Viremia (culture) pp 65 antigenemia* | 144 | 14.6 | 7.7 | 17.9 |
| Any | 145 | 47.6 | 32.7 | 54.7 |
| More than 5 | — | 26.2 | 17.3 | 29.8 |
| More than 10 | — | 22.7 | 13.5 | 27.4 |
| More than 50 | — | 12.4 | 5.8 | 16.7 |
| More than 100 | — | 9.0 | 3.9 | 11.9 |
| CMV DNA in PBL* | 139 | |||
| Any | — | 53.2 | 51.0 | 51.9 |
| More than 1 000 | — | 37.4 | 28.6 | 39.5 |
| More than 10 000 | — | 24.5 | 18.4 | 25.9 |
| More than 100 000 | — | 13.7 | 6.1 | 16.1 |
| More than 0.5 log10increase | — | 48.2 | 50.0 | 44.9 |
| CMV DNA in plasma* | 86 | |||
| Any | — | 53.5 | 34.5 | 61.2 |
| More than 1 000 | — | 43.0 | 27.6 | 46.9 |
| More than 10 000 | — | 29.1 | 13.8 | 34.7 |
| More than 100 000 | — | 14.0 | 3.5 | 20.4 |
| More than 0.5 log10increase | — | 41.9 | 31.0 | 42.9 |
| CMV test method . | Positive CMV test result . | |||
|---|---|---|---|---|
| Number of patients tested . | All patients % . | Patients with positive CMV-specific CD4+ TH responses at 3 months . | Patients with negative CMV-specific CD4+TH responses at 3 months . | |
| Viremia (culture) pp 65 antigenemia* | 144 | 14.6 | 7.7 | 17.9 |
| Any | 145 | 47.6 | 32.7 | 54.7 |
| More than 5 | — | 26.2 | 17.3 | 29.8 |
| More than 10 | — | 22.7 | 13.5 | 27.4 |
| More than 50 | — | 12.4 | 5.8 | 16.7 |
| More than 100 | — | 9.0 | 3.9 | 11.9 |
| CMV DNA in PBL* | 139 | |||
| Any | — | 53.2 | 51.0 | 51.9 |
| More than 1 000 | — | 37.4 | 28.6 | 39.5 |
| More than 10 000 | — | 24.5 | 18.4 | 25.9 |
| More than 100 000 | — | 13.7 | 6.1 | 16.1 |
| More than 0.5 log10increase | — | 48.2 | 50.0 | 44.9 |
| CMV DNA in plasma* | 86 | |||
| Any | — | 53.5 | 34.5 | 61.2 |
| More than 1 000 | — | 43.0 | 27.6 | 46.9 |
| More than 10 000 | — | 29.1 | 13.8 | 34.7 |
| More than 100 000 | — | 14.0 | 3.5 | 20.4 |
| More than 0.5 log10increase | — | 41.9 | 31.0 | 42.9 |
Quantitation defined in the text.
Risk factors for late CMV disease and death 3 months after transplantation
| Covariate . | Late CMV disease . | Death . | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate, RR . | 95% CI . | Multivariable, RR§ . | 95% CI . | Univariate, RR . | 95% CI . | Multivariable, RR∥ . | 95% CI . | |
| Absolute lymphocyte count after d 40, 100/mm3 or less | 2.5 | 1.1, 5.73-150 | — | — | 1.8 | 1.1, 3.03-150 | — | — |
| CD4 count at 3 mo, 50/mm3or less | 2.8 | 1.2, 6.33-150 | — | — | 1.9 | 1.1, 3.13-150 | — | — |
| CD8 count at 3 mo, 50/mm3 or less | — | — | — | — | 2.5 | 1.5, 4.23-151 | — | — |
| Undetectable CMV-specific CD4+ TH responses at 3 mo | — | — | — | — | 2.5 | 1.4, 4.53-150 | 2.1 | 1.1, 4.03-150 |
| CMV antigenemia before d 95 | ||||||||
| Positive, any level | 2.4 | 1.0, 5.73-150 | 3.4 | 1.2, 9.93-150 | — | — | — | — |
| More than 5 | 3.4 | 1.5, 7.53-151 | 3.5 | 1.5, 8.33-151 | 1.8 | 1.0, 3.13-150 | — | — |
| More than 10 | 2.6 | 1.1, 6.13-150 | 2.5 | 1.0, 6.153-150 | — | — | — | — |
| More than 100 | 3.8 | 1.1, 12.63-150 | — | — | — | — | — | — |
| Covariate . | Late CMV disease . | Death . | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate, RR . | 95% CI . | Multivariable, RR§ . | 95% CI . | Univariate, RR . | 95% CI . | Multivariable, RR∥ . | 95% CI . | |
| Absolute lymphocyte count after d 40, 100/mm3 or less | 2.5 | 1.1, 5.73-150 | — | — | 1.8 | 1.1, 3.03-150 | — | — |
| CD4 count at 3 mo, 50/mm3or less | 2.8 | 1.2, 6.33-150 | — | — | 1.9 | 1.1, 3.13-150 | — | — |
| CD8 count at 3 mo, 50/mm3 or less | — | — | — | — | 2.5 | 1.5, 4.23-151 | — | — |
| Undetectable CMV-specific CD4+ TH responses at 3 mo | — | — | — | — | 2.5 | 1.4, 4.53-150 | 2.1 | 1.1, 4.03-150 |
| CMV antigenemia before d 95 | ||||||||
| Positive, any level | 2.4 | 1.0, 5.73-150 | 3.4 | 1.2, 9.93-150 | — | — | — | — |
| More than 5 | 3.4 | 1.5, 7.53-151 | 3.5 | 1.5, 8.33-151 | 1.8 | 1.0, 3.13-150 | — | — |
| More than 10 | 2.6 | 1.1, 6.13-150 | 2.5 | 1.0, 6.153-150 | — | — | — | — |
| More than 100 | 3.8 | 1.1, 12.63-150 | — | — | — | — | — | — |
Additional covariate factors considered in the univariate baseline model included patient age and sex, CMV donor serostatus before transplantation, HSV recipient serostatus before transplantation, underlying disease risk status, HLA donor matching (matched-related vs unrelated or mismatched donor), conditioning regimen, GVHD prophylaxis regimen, presence of neutropenia between engraftment and study entry, GVHD (acute grades 2-4 and 3-4; chronic clinical-extensive), use of ganciclovir (preemptive therapy vs ganciclovir prophylaxis), invasive aspergillosis, year of transplantation, and CMV viremia by culture.
Covariates for late CMV disease considered for inclusion in the final multivariable model were aspergillosis, GVHD prophylaxis, CD4 cell count lower than 50/mm3, GVHD (grades 2-4 or chronic clinical-extensive), lack of CMV-specific T-cell responses, and postengraftment lymphocytopenia (<100/mm3). For pp65 antigenemia before day 95, each cut-off variable was entered singly in a model adjusted for aspergillosis, GVHD prophylaxis, and CD4 cell count lower than 50/mm3.
Covariates for death considered for inclusion in the final multivariable model were CMV donor serostatus, aspergillosis, GVHD prophylaxis, underlying disease, donor type, CD4 cell count lower than 50/mm3, GVHD (grades 2-4 or chronic clinical-extensive), lack of CMV-specific T-cell responses, and postengraftment lymphocytopenia (<100/mm3). For pp65 antigenemia, each cut-off variable was entered singly in a model adjusted for donor CMV serostatus, GVHD prophylaxis, CMV-specific T-cell negativity, and CD4 cell count lower than 50/mm3.
— indicates results not significant.
P ≤ .05 (because of the number of statistical tests being carried out, values between .05 and .01 should be considered suggestive and those less than .01 considered significant).
P ≤ .01.
Time-varying risk factors for lymphocytopenia after day 95
| Covariate . | Univariate, RR . | 95% CI . | Multivariable, RR . | 95% CI . |
|---|---|---|---|---|
| Lymphocytopenia, time dependent | ||||
| Fewer than 100 lymphocytes/mm3 | 7.0 | 1.6, 30.56-150 | — | — |
| Fewer than 300 lymphocytes/mm3 | 7.2 | 3.1, 17.06-150 | 9.46-151 | 3.8, 23.56-150 |
| Covariate . | Univariate, RR . | 95% CI . | Multivariable, RR . | 95% CI . |
|---|---|---|---|---|
| Lymphocytopenia, time dependent | ||||
| Fewer than 100 lymphocytes/mm3 | 7.0 | 1.6, 30.56-150 | — | — |
| Fewer than 300 lymphocytes/mm3 | 7.2 | 3.1, 17.06-150 | 9.46-151 | 3.8, 23.56-150 |
— indicates results not significant.
P ≤ .01.
Candidates for the multivariable model included chronic clinical-extensive GVHD, donor type, GVHD prophylaxis, antigenemia level greater than 100 per slide before day 95, CMV-specific T-cell negativity, and CD4 lymphocyte count less than 50/mm3 (all as time-varying covariates).
Time-varying risk factors for late CMV disease after day 95
| Covariate . | Univariate, RR . | 95% CI . | Multivariable, RR . | 95% CI . |
|---|---|---|---|---|
| CMV antigenemia, time dependent4-150 | ||||
| Positive, any level | 4.0 | 1.4, 11.7‡ | 5.34-155 | 1.5, 19.1‡ |
| More than 5 cells/slide | 4.0 | 1.3, 12.1‡ | 6.14-155 | 1.7, 21.5‡ |
| More than 10 cells/slide | 4.1 | 1.3, 13.5‡ | 6.64-155 | 1.8, 24.6‡ |
| More than 50 cells/slide | 5.5 | 1.5, 20.6‡ | 8.74-155 | 2.1, 36.5‡ |
| CMV DNA in PBL, time dependent4-150,4-151 | ||||
| Positive, any level | 3.2 | 1.0, 9.94-153 | — | — |
| 0.25 log10increase | 2.3 | 1.0, 5.24-153 | — | — |
| 0.5 log10 increase | 2.4 | 1.0, 5.34-153 | — | — |
| CMV DNA plasma, time dependent4-150,4-151 | ||||
| More than 103 copies/mL | 2.1 | 0.5, 8.3 | 6.24-155 | 1.0, 39.24-153 |
| More than 104 copies/mL | 3.0 | 0.8, 12.3 | 12.34-155 | 1.8, 85.1‡ |
| Covariate . | Univariate, RR . | 95% CI . | Multivariable, RR . | 95% CI . |
|---|---|---|---|---|
| CMV antigenemia, time dependent4-150 | ||||
| Positive, any level | 4.0 | 1.4, 11.7‡ | 5.34-155 | 1.5, 19.1‡ |
| More than 5 cells/slide | 4.0 | 1.3, 12.1‡ | 6.14-155 | 1.7, 21.5‡ |
| More than 10 cells/slide | 4.1 | 1.3, 13.5‡ | 6.64-155 | 1.8, 24.6‡ |
| More than 50 cells/slide | 5.5 | 1.5, 20.6‡ | 8.74-155 | 2.1, 36.5‡ |
| CMV DNA in PBL, time dependent4-150,4-151 | ||||
| Positive, any level | 3.2 | 1.0, 9.94-153 | — | — |
| 0.25 log10increase | 2.3 | 1.0, 5.24-153 | — | — |
| 0.5 log10 increase | 2.4 | 1.0, 5.34-153 | — | — |
| CMV DNA plasma, time dependent4-150,4-151 | ||||
| More than 103 copies/mL | 2.1 | 0.5, 8.3 | 6.24-155 | 1.0, 39.24-153 |
| More than 104 copies/mL | 3.0 | 0.8, 12.3 | 12.34-155 | 1.8, 85.1‡ |
— indicates results not significant.
Only late-disease events up to 14 days after the last tests were included. When events until 35 days after the last test value were considered, results were qualitatively similar (data not shown).
PCR results were tested at any positive level: greater than 103, greater than 104, greater than 105, 0.25 log10 and 0.5 log10increases.
P ≤ .01.
P ≤ .05. See also Table 2 footnote.
For virologic parameters each cut-off variable was entered singly into separate models, adjusting for lymphocytopenia (<300/mm3) and CD4 count less than 50/mm3, both as time-varying covariates. Adjusting for CMV-specific T-cell immunity did not qualitatively change results (data not shown).
Time-varying risk factors for death after day 95: lymphocytopenia
| Covariate . | Univariate, RR . | 95% CI . | Multivariable, RR . | 95% CI . |
|---|---|---|---|---|
| CMV disease, time dependent | 3.1 | 1.7, 5.67-150 | 2.37-151 | 1.2, 4.27-150 |
| Lymphocytopenia, time dependent | ||||
| Fewer than 100 lymphocytes/mm3 | 7.1 | 2.5, 19.97-150 | — | — |
| Fewer than 300 lymphocytes/mm3 | 5.9 | 3.3, 10.57-150 | 3.67-151 | 1.9, 6.77-150 |
| Covariate . | Univariate, RR . | 95% CI . | Multivariable, RR . | 95% CI . |
|---|---|---|---|---|
| CMV disease, time dependent | 3.1 | 1.7, 5.67-150 | 2.37-151 | 1.2, 4.27-150 |
| Lymphocytopenia, time dependent | ||||
| Fewer than 100 lymphocytes/mm3 | 7.1 | 2.5, 19.97-150 | — | — |
| Fewer than 300 lymphocytes/mm3 | 5.9 | 3.3, 10.57-150 | 3.67-151 | 1.9, 6.77-150 |
— indicates results not significant
P ≤ .01.
Candidates for the final multivariable model included CMV disease (viral monitoring parameters not included), aspergillosis, chronic clinical-extensive GVHD, CMV-specific T-cell negativity, CD4 cells, age, donor CMV serostatus, underlying disease, donor type, and lymphopenia.
Time-varying risk factors for death after day 95
| Covariate . | Univariate, RR . | 95% CI . | Multivariable, RR . | 95% CI . |
|---|---|---|---|---|
| CMV antigenemia, time dependent5-152 | ||||
| Positive, any level | 5.8 | 1.7, 19.65-150 | 5.05-155 | 1.5, 17.15-150 |
| More than 5 positive cells/slide | 7.1 | 2.2, 22.25-150 | 5.95-155 | 1.7, 20.45-150 |
| More than 50 positive cells/slide | 5.9 | 1.5, 22.85-151 | — | — |
| More than 100 positive cells/slide | 7.6 | 2.0, 29.35-150 | 7.25-155 | 1.5, 34.75-150 |
| CMV DNA in PBL, time dependent5-152,5-153 | ||||
| 0.25 log10 increase | 7.1 | 1.2, 44.25-150 | 6.45-155 | 1.0, 41.65-151 |
| 0.5 log10increase | 7.7 | 1.3, 46.95-150 | 6.85-155 | 1.1, 43.45-151 |
| CMV DNA plasma, time dependent5-152,5-153 | ||||
| Positive, any level | 11.6 | 1.4, 99.95-151 | 13.15-155 | 1.3, 132.75-151 |
| More than 103 copies/mL | 19.7 | 2.3, 169.65-150 | 22.65-155 | 2.2, 227.85-151 |
| CMV viremia by culture, time dependent | 6.0 | 1.6, 22.75-150 | — | — |
| Covariate . | Univariate, RR . | 95% CI . | Multivariable, RR . | 95% CI . |
|---|---|---|---|---|
| CMV antigenemia, time dependent5-152 | ||||
| Positive, any level | 5.8 | 1.7, 19.65-150 | 5.05-155 | 1.5, 17.15-150 |
| More than 5 positive cells/slide | 7.1 | 2.2, 22.25-150 | 5.95-155 | 1.7, 20.45-150 |
| More than 50 positive cells/slide | 5.9 | 1.5, 22.85-151 | — | — |
| More than 100 positive cells/slide | 7.6 | 2.0, 29.35-150 | 7.25-155 | 1.5, 34.75-150 |
| CMV DNA in PBL, time dependent5-152,5-153 | ||||
| 0.25 log10 increase | 7.1 | 1.2, 44.25-150 | 6.45-155 | 1.0, 41.65-151 |
| 0.5 log10increase | 7.7 | 1.3, 46.95-150 | 6.85-155 | 1.1, 43.45-151 |
| CMV DNA plasma, time dependent5-152,5-153 | ||||
| Positive, any level | 11.6 | 1.4, 99.95-151 | 13.15-155 | 1.3, 132.75-151 |
| More than 103 copies/mL | 19.7 | 2.3, 169.65-150 | 22.65-155 | 2.2, 227.85-151 |
| CMV viremia by culture, time dependent | 6.0 | 1.6, 22.75-150 | — | — |
— indicates results not significant
P ≤ .01.
P ≤ .05. See also Table 2 footnote.
Only late-disease events up to 14 days after the last tests were included. When events until 35 days after the last test value were considered, results were qualitatively similar (data not shown).
PCR results were tested at any positive level: greater than 103, greater than 104, greater than 105, 0.25 log10 and 0.5 log10increases.
For virologic parameters, each cut-off variable was entered singly in a separate model adjusting for lymphocytopenia, aspergillosis, chronic clinical-extensive GVHD, and donor CMV serostatus (CMV disease was not entered into these models).
Results
Incidence and outcome of late CMV infection and disease
One hundred forty-six consecutive CMV-seropositive recipients of transplanted allogeneic marrow underwent baseline immunologic evaluation and were followed up prospectively (Table1). Median follow-up among surviving patients was 4.9 years (range, 2.9-7.8 years). The incidence of CMV infection in the first 200 days is depicted in Table2. In approximately 50% of patients, CMV reactivated during follow-up. The median number of tests per patient after day 80 was similar for the 3 methods: pp65 antigenemia, 7 tests (range, days 1-32); plasma PCR, 6 tests (range, days 0-10); PBL PCR, 6 tests (range, days 0-11). The last tests were performed on days 169 (range, days 83-268), 169 (range, days 56-236), and 163 (range, days 77-245), respectively (Table 2).
CMV disease occurred in 26 (17.8%) patients (pneumonia [n = 10], pneumonia with gastrointestinal disease [n = 2], gastrointestinal disease [n = 10], sinus disease [n = 3], graft failure [n = 1]) at a median of 169 days after transplantation (range, 96 to 784 days). All first cases were diagnosed after death. Seventy-three percent of first cases of late disease occurred within the first year of transplantation, 92% within 18 months, and 96% within 2 years. The first case of CMV disease was fatal in 8 (31%) of 26 patients. Seven [39%] of 18 of those who survived the initial episode had a second episode of late CMV disease a median of 79 days (range, 54-703 days) after the first episode (pneumonia [n = 3], gastrointestinal disease [n = 3], sinus disease [n = 1]). In 5 (33%) of 15 episodes of CMV pneumonia, significant pulmonary copathogens were present (Aspergillus fumigatus [n = 2], respiratory syncytial virus [n = 2], Nocardia asteroides [n = 1]); 2 additional patients had concomitant disseminated candidiasis or gram-negative sepsis, respectively, without isolation of these pathogens from lung tissue or BAL.
Forty-six percent of patients with CMV disease died within 6 weeks of diagnosis, or CMV was detected in them at autopsy. More patients died of pneumonia than of other manifestations of late CMV disease (9 [60%] of 15 pneumonia episodes vs 4 [22%] of 18 other episodes, respectively). CMV pneumonia with pulmonary copathogens or concomitant severe disseminated infections was fatal in 6 (86%) of 7 patients compared with 3 (38%) of 8 in patients without copathogens. Of the 146 patients followed up in this cohort, 13 (8.9%) died with CMV disease.
Immune reconstitution
The proportion of patients with postengraftment lymphopenia and with CD4 and CD8 T-cell counts lower than 50 cells/mm3between days 80 and 100 are shown in Table 1. By day 150, increases to more than 50 cells/mm3 occurred in 39% (CD4) and 36% (CD8) of patients who had low counts at baseline, resulting in 26% and 19% of patients, respectively, with counts lower than 50 cells/mm3. During the surveillance period (starting at 95 days after transplantation), absolute lymphocyte counts lower than 100 lymphocytes/mm3 and lower than 300 lymphocytes/mm3 were detected in 16% and 39% of patients, respectively.
CMV-specific CD4+ TH responses were absent in 62% of 137 patients between days 80 and 100. Fifty-four percent of patients with detectable CD4+ T-helper responses at day 100 lost these responses by day 150 in association with corticosteroid use, whereas 12.5% of patients with negative responses at day 100 had positive responses by day 150. Overall, by day 150, 26 (35%) of 75 patients with follow-up test results had detectable CD4+ T-helper responses. CMV-specific CD8+ CTL responses were measured in a subset of patients. Twenty (67%) of 33 patients did not have CD8+ CTL responses at day 100. Four (40%) of 10 patients with detectable CD8+ CTL responses at day 100 lost these responses by day 150 in association with increased immunosuppression.
Failure of a CMV-specific CD4+ TH response to develop was highly associated with a nondetectable CD8+ CTL response (23 of 24 tests). There was only one weak-positive CD8+ CTL response (17% specific lysis) at day 150 in the absence of a positive CD4+ TH response. This occurred in a patient who had positive CD4+ THand CD8+ CTL responses at day 100 and was subsequently treated with steroids. Positive CD4+ THresponses were associated with positive CD8+ CTL responses in 10 (37%) of 27 patients. Because of the close association between negative CD4+ TH and CD8+ CTL responses in this and earlier studies,13 19 for subsequent analyses, the absence of CMV-specific CD4+ THresponses was used to assess the impact of CMV-specific T-cell responses.
Risk factors for late CMV disease
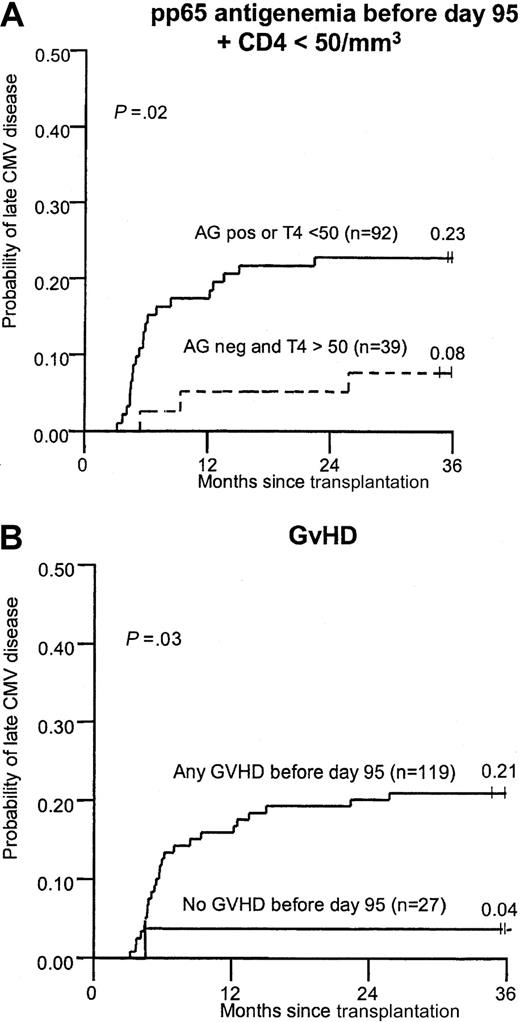
Baseline patient characteristics, quantitative CMV antigenemia before day 95, and CMV-specific immune reconstitution parameters between days 80 and 100 were analyzed first by Cox regression models and by cumulative incidence curves. Factors significant for the development of late CMV disease in univariate analysis were presence of antigenemia during the first 3 months, absolute lymphopenia after engraftment, low CD4 and CD8 counts, and acute grades 2-4 or chronic clinical-extensive GVHD. GVHD was significant in the time-to-event analysis but only suggestive in the regression models (Table 3, Figure1). In a multivariable model for late CMV disease using parameters that were known between days 80 and 100, antigenemia remained significant, yet the absence of CMV-specific T-cell responses, low CD4 counts, and GVHD were no longer statistically significant (Table 3). When lymphopenia was removed from the multivariable model (to account for the interrelationship of lymphocyte counts and CD4 subsets), low CD4 counts, GVHD, and antigenemia before day 80 remained significant for late CMV disease (data not shown). A model that included all patients with CMV disease, except 2 with isolated CMV sinusitis, confirmed the significance of CMV antigenemia before day 95 in univariate and multivariable models. In addition, CD4 counts remained significant in the multivariable model (relative risk [RR], 2.4; 95% confidence interval [CI], 1.0-5.9).
Cumulative incidence of late CMV disease in patients with or without risk factors at 3 months.
(A) Any pp65 antigenemia before day 95 or CD4 count lower than 50 cells/mm3. (B) GVHD (grades 2-4 or chronic clinical-extensive).
Cumulative incidence of late CMV disease in patients with or without risk factors at 3 months.
(A) Any pp65 antigenemia before day 95 or CD4 count lower than 50 cells/mm3. (B) GVHD (grades 2-4 or chronic clinical-extensive).
The significance of continued surveillance after the first analysis 3 months after transplantation was then evaluated in multivariable models, including virologic parameters and lymphopenia, as a time-varying parameter while controlling for additional factors. The first model evaluated absolute lymphopenia and showed a strong association of lymphopenia with late CMV disease (Table4). Then virologic surveillance parameters were analyzed in separate univariate and multivariable models (Table 5). This analysis showed a strong association between detection of CMV antigenemia and CMV DNA with CMV disease. This association persisted for antigenemia and plasma DNA detection after controlling for lymphopenia and CD4 T-cell counts (Table 5). Only late disease events up to 1 month after the last test were considered in these analyses. An analysis that excluded the 2 patients with isolated CMV sinusitis yielded results similar to those shown in Table 5 (data not shown).
In an additional analysis, the last test value was kept until the end of follow-up. In this analysis, viral monitoring parameters (antigenemia and CMV DNA in PBLs) were significantly associated with disease in univariate analysis but not in multivariable models (data not shown). Thus, antigenemia and CMV DNA load were significant predictors for late CMV disease only until approximately 1 month after the monitoring; the last test value was not predictive for long-term prediction of CMV disease. The cumulative incidence of late CMV disease in patients with and without CMV detection is shown in Figure2.
Cumulative incidence after day 95.
Cumulative incidence of CMV disease with different levels of pp65 antigenemia during surveillance after day 95 (A), with different levels of PCR positivity (B), and without positive test findings by either method (C). Quantitation is as outlined in “Virologic monitoring.”
Cumulative incidence after day 95.
Cumulative incidence of CMV disease with different levels of pp65 antigenemia during surveillance after day 95 (A), with different levels of PCR positivity (B), and without positive test findings by either method (C). Quantitation is as outlined in “Virologic monitoring.”
Risk factors for death
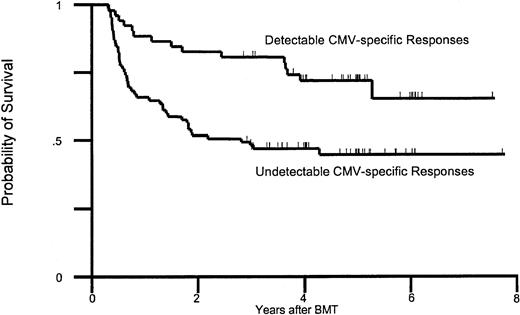
The first analysis included factors known at day 95 after transplantation. This analysis identified postengraftment lymphopenia, pp65 antigenemia before day 95, low CD4 and CD8 counts, and lack of CMV-specific T-cell responses in univariate analysis (Table 5). In a multivariable model that included these factors, GVHD, and other transplantation-related factors, only lack of CMV-specific T-helper responses remained significant. The impact on mortality of CMV-specific T-cell immunodeficiency at 3 months is shown in Figure 3.
Survival between days 80 and 100.
Probability of survival in patients relative to the presence of CMV-specific T-cell responses between days 80 and 100 after transplantation. BMT indicates bone marrow transplantation.
Survival between days 80 and 100.
Probability of survival in patients relative to the presence of CMV-specific T-cell responses between days 80 and 100 after transplantation. BMT indicates bone marrow transplantation.
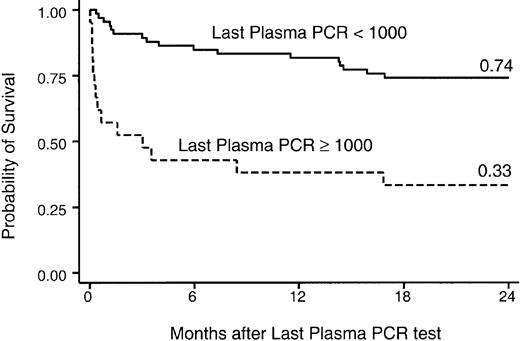
The significance of surveillance parameters and late transplantation events—CMV disease, GVHD, late aspergillosis—on survival were then analyzed in a time-dependent fashion. The first model evaluated late CMV disease and lymphopenia as time-varying covariates (without inclusion of virologic surveillance parameters) and showed a strong association with mortality in univariate and multivariable models (Table 6). The association of CMV detection by different methods and at different levels was then analyzed in separate models for each cut-off level (Table7). A strong association with overall mortality was found in univariate and multivariable models, which controlled for lymphopenia, chronic GVHD, invasive aspergillosis, and CMV donor status (Table 5). When the last test value of viral monitoring was evaluated for impact on subsequent death, CMV DNA in PBLs (more than 103 copies/106 copies β-globin; RR, 2.4; P = .03) and CMV DNA in plasma (more than 104 copies/mL; RR, 4.1; P < .01) were associated with death in multivariable models that included lymphopenia and GVHD. The association of the last plasma PCR test with death is shown in Figure 4. Among 17 patients who died within 1 year of last positive findings on plasma PCR test, 6 (35%) deaths were related to CMV disease, 3 (18%) to bacterial or fungal infection, 3 (18%) to pulmonary or multiorgan failure without pathogen identification, 1 (6%) to Epstein-Barr virus (EBV), and 4 (23%) to relapse of the underlying disease.
Survival after monitoring period.
Probability of survival after the active monitoring period. Shown are outcomes relative to the last PCR result, expressed per milliliter plasma.
Survival after monitoring period.
Probability of survival after the active monitoring period. Shown are outcomes relative to the last PCR result, expressed per milliliter plasma.
Discussion
This study demonstrates that late CMV disease is frequent in seropositive allograft recipients who receive ganciclovir before day 100, has a high relapse rate, is associated with poor outcome, and can be predicted by immunologic and viral factors. Reactivation of CMV during the first 3 months and lack of CMV-specific immunity appear to be the underlying pathophysiologic processes in this late reactivation. The incidence rate of 17.8% may be an underestimation of the true incidence because we only considered biopsy- or BAL-proven cases. CMV pneumonia is the leading manifestation of late disease, followed by gastrointestinal disease. The fatality rate for late CMV disease is similar to that for the first 100 days after transplantation. Relapses occurred in 39% of patients who survived the first episode of late CMV disease after a median of 79 days. Thus, patients with late disease should receive extended maintenance treatment of at least 3 months and perhaps longer with continued immunosuppression. Alternatively, close virologic monitoring should be continued after standard courses of antiviral treatment.
An important question was whether late CMV disease and overall outcomes can be assessed 3 months after transplantation, when most published anti-CMV strategies end and patients are often sent back to their referring physicians.32 CMV antigenemia during the first 3 months was the strongest risk factor in multivariable models; however, delayed lymphocyte engraftment, low CD4 counts, and GVHD also seemed to be important. Whether GVHD itself or its treatment is responsible for the observed risk cannot be determined from these data. The association of CMV-specific immunodeficiency at 3 months with late CMV infection and disease was modest after controlling for other factors, and the reason may be twofold. First, only 37% of patients with detectable CD4+ Th responses had positive CD8+ CTL responses—the type of response that most likely confers protection from disease.13 This dissociation was higher than that previously observed,13,19 possibly because of the use of ganciclovir. Second, a loss of CMV-specific CD4+ Th and CD8+ CTL responses occurred in 52% and 40%, respectively, of patients who had detectable responses at 3 months, usually in association with increased immunosuppression to treat chronic GVHD. T-cell responses seemed to be weaker and of shorter durability in patients who received ganciclovir, possibly because of lower CMV-specific CD8+ CTL and CD4+ Th precursor frequencies. Thus, CMV-specific immune reconstitution remains a dynamic process after day 100. Even at day 150, approximately two thirds of patients in this cohort had undetectable responses. Continued immunologic monitoring would be required to accurately predict all cases of late CMV disease.33 Recent advances in tetramer technology and intracellular cytokine detection by flow cytometry increase the feasibility of CMV-specific immune surveillance.34 35Although undetectable CMV-specific T-cell responses at 3 months were only moderately associated with late CMV disease, they were strongly associated with overall mortality.
Perhaps the most unexpected result of this study is the strong association of late death with virologic factors, independent of GVHD, CMV-specific T-cell function, lymphopenia, and CMV disease. Although an association of high CMV viral load and death has been reported in patients who undergo HSCT or who have HIV infection,21,22,36 these studies did not control for CMV-specific T-cell function. Mechanistically, the association between high viral load and death may not only result from direct effects of CMV, such as fatal pneumonia, but also from immunomodulatory effects of CMV that may result in an increased susceptibility to late bacterial and fungal infection. This phenomenon has recently been described in CMV-seronegative recipients of seropositive stem cell transplants.37 Numbers in our study were too small to draw a definitive conclusion regarding this effect.
In conclusion, late CMV disease is an important and potentially fatal complication in recipients of seropositive allografts. Depending on the patient risk profile at any individual cancer center, the incidence of late disease may be as high as 18% of patients who are alive at 3 months after transplantation. Although CMV load and CMV-specific T-cell immunity determine the risk for late CMV disease and outcome, clinical parameters present 3 months, such as prior CMV reactivation, GVHD, or low CD4 counts, are surprisingly useful in predicting a patient's long-term risk for CMV infection and disease and could define a target population for late prevention strategies.38 Although these results were obtained in recipients after myeloablative transplantation, initial results from recipients of nonmyeloablative transplants suggest that late complications of CMV are also common and that CMV reactivation during the first 3 months is a significant risk factor for late disease.39 This study shows that continued surveillance for CMV load or antigenemia or low lymphocyte counts is useful in assessing patient risk for late CMV disease and death in the long-term follow-up period. Options for the prevention of late CMV disease include extended antiviral prophylaxis or continuation of preemptive therapy based on quantitative antigenemia or CMV DNA testing.40 Because CMV disease may still develop in a substantial number of patients who have negative antigenemia findings or undetectable DNA (Figure 2), a preemptive strategy may not be effective without a weekly monitoring schedule. However, increased monitoring raises issues of cost, feasibility, and adherence, especially when patients live in remote areas. The fact that CMV viral load was independently associated with death extends results from HIV-infected subjects to the transplant population and suggests that indirect effects of CMV may be responsible for the observed effect. These data also support long-term suppressive prophylaxis given to all patients at risk rather than preemptive therapy based on virologic markers, and they provide the intriguing possibility that the prevention of CMV infection might reduce the long-term mortality rate more than expected. Nevertheless, issues of toxicity (with valganciclovir), efficacy (with valacyclovir), drug resistance, and further delay of CMV-specific immune-reconstitution remain. A randomized trial is ongoing to compare these strategies. Immunologic strategies such as adoptive transfer of donor-derived, CMV-specific T-cell clones may be available in the near future.41 42
We thank Kristen White and Patricia Woogerd for performing immunologic assays and antigenemia testing, Carol Bevan and Laurence Stensland for PCR testing, Chris Davis and Gary Schoch for data services, and the primary physicians and nurses involved in the study for their cooperation. Biotest Diagnostic Corporation (Denville, NJ) provided the monoclonal antibodies for antigenemia testing.
Prepublished online as Blood First Edition Paper, September 12, 2002; DOI 10.1182/blood-2002-03-0993.
Supported by grants CA 18029 and CA 15704 from the National Institutes of Health and grant 94-52 from the Milheim Foundation for Cancer Research (Denver, CO).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Boeckh, Program in Infectious Diseases, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109; e-mail: mboeckh@fhcrc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal