Abstract
Here we describe the in vitro generation of a novel adherent cell fraction derived from highly enriched, mobilized CD133+ peripheral blood cells after their culture with Flt3/Flk2 ligand and interleukin-6 for 3 to 5 weeks. These cells lack markers of hematopoietic stem cells, endothelial cells, mesenchymal cells, dendritic cells, and stromal fibroblasts. However, all adherent cells expressed the adhesion molecules VE-cadherin, CD54, and CD44. They were also positive for CD164 and CD172a (signal regulatory protein-α) and for a stem cell antigen defined by the recently described antibody W7C5. Adherent cells can either spontaneously or upon stimulation with stem cell factor give rise to a transplantable, nonadherent CD133+CD34−stem cell subset. These cells do not generate in vitro hematopoietic colonies. However, their transplantation into nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice induced substantially higher long-term multilineage engraftment compared with that of freshly isolated CD34+ cells, suggesting that these cells are highly enriched in SCID-repopulating cells. In addition to cells of the myeloid lineage, nonadherent CD34− cells were able to give rise to human cells with B-, T-, and natural killer–cell phenotype. Hence, these cells possess a distinct in vivo differentiation potential compared with that of CD34+ stem cells and may therefore provide an alternative to CD34+ progenitor cells for transplantation.
Introduction
Hematopoiesis is sustained by a very small number of hematopoietic stem cells capable of self-renewal and differentiation into multiple hematopoietic lineages.1 The ability to maintain or expand a population of hematopoietic stem cells in vitro without inducing their differentiation is crucial for clinical applications such as gene therapy, the destruction of tumor cells, and the expansion of stem cells and progenitor cells.2,3During the last decade, considerable progress has been made in the isolation and characterization of primitive hematopoietic cell populations in mice and humans.4-8 The sialomucin CD34 is commonly used as a marker to characterize and isolate human stem cells and progenitor cells because surface CD34 is highly expressed by primitive cells but is down-regulated as these cells differentiate into more mature cells.9 Recent results, however, have indicated the existence of a murine CD34− cell population that has a marked capacity for long-term repopulation and may be a more primitive precursor of CD34+ cells.10-12 Other reports have described the existence of human CD34−hematopoietic cell populations that can engraft in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice13 or fetal sheep after primary or secondary transplantation.14 Sato et al15 demonstrated that CD34 expression on murine hematopoietic stem cells is a reversible process that reflects their activation state. By using a murine bone marrow stromal cell line, Nakamura et al16 were able to expand cell fractions that did not express CD34 and lineage markers (CD34−Lin− cells) and to convert them to CD34+ cells; these results demonstrate that CD34− cells can be precursors of CD34+ cells.
Another important stem cell marker, CD133 (previously designated AC133), is predominantly expressed on CD34brighthematopoietic stem cells and progenitor cells.17,18Because this pentaspan molecule is expressed by CD34− and immature CD34+ stem cell subsets, it appears to be a more specific stem cell marker than CD34.19
Our study was originally designed to elucidate the proliferation and differentiation capacity of mobilized CD133+ peripheral blood cells. When we attempted to expand the population of CD133+ hematopoietic stem cells in the presence of Flt3/Flk2 ligand (FL) and interleukin-6 (IL-6), a novel population of adherent cells emerged that lacked markers of hematopoietic stem cells, endothelial cells, mesenchymal cells, dendritic cells, and stromal fibroblasts. Here we show that this population is a distinct adherent cell fraction that either spontaneously or upon stimulation with stem cell factor (SCF) gives rise to nonadherent transplantable CD34− stem cells. These cells represent a novel class of adult transplantable CD34− stem cells distinct from CD34+ progenitor cells.
Patients, materials, and methods
Isolation of CD133+ cells
We obtained mobilized peripheral blood cells from healthy donors in accordance with guidelines of the local ethics committee (University of Tübingen). Enrichment of CD133+ cells from light-density mononuclear cells was performed by magnet-activated cell sorting (MACS) using the AC133 Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the positively selected CD133+ population was evaluated by a FACSCalibur flow cytometer (Becton Dickinson, Heidelberg, Germany).
Generation of adherent cells from CD133+ cells
One million enriched CD133+ cells were cultured in 1 mL expansion medium, consisting of RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 100 ng/mL FL and IL-6 (R&D Systems, Wiesbaden-Nordenstadt, Germany). On day 7 of culture, half of the expanded population was transferred to another flask containing fresh RPMI-1640 medium supplemented with 10% FBS without cytokines and cultured at 37°C in an atmosphere of 5% CO2 for 2 to 3 weeks. Nonadherent (NA) cells were transferred to another flask and cultured for another 3 to 4 weeks until adherent cells were morphologically homogeneous and no NA cells were present in the cell culture medium. For time-lapse studies, cells were grown in 25-cm2 culture flasks, whereas for laser scanning microscopy, cells were cultured in 2- or 4-well Lab-Tek cell culture chambers (Nunc, Wiesbaden, Germany).
For phenotypic characterization by flow cytometry, adherent cells were trypsinized, washed twice with phosphate-buffered saline (PBS), and incubated with mouse antibodies to human antigens CD34, CD133, CD117, CD90, KDR, CD31, CD140b, CD83, CD1a, CD164, CD44, CD49d, CD50, CD54, CD106 (vascular cell adhesion molecule–1), VE-cadherin, W7C5 antigen, and CD172a (signal regulatory protein-α [SIRP-α]). Antibodies W7C5, 103B2 (CD164), and SE5A5 (CD172a) were generated as described.20-22 Antibodies against VE-cadherin were purchased from DAKO (Hamburg, Germany), against CD106 from R&D Systems, against CD133 from Miltenyi Biotec, and against KDR from Sigma-Aldrich (Munich, Germany). The other antibodies were purchased from BD Pharmingen (Heilderberg, Germany).
Transfection of the highly enriched CD133+ cells
Eighteen hours before transfection, highly enriched CD133+ cells (5 × 106 cells/5 mL expansion medium) were plated in 25-cm2 tissue culture flasks and incubated at 37°C in a 5% CO2 atmosphere. The next day, the cell monolayer was transfected with a freshly prepared liposome solution (55°C) containing HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–NaCl and 1 μg/μL Clonfectin (BD Biosciences Clontech, Heidelberg, Germany). To prepare a plasmid DNA solution, we mixed 5 μL pEGFP-N2 vector DNA with 100 μL serum-free medium. Eight micrograms of Clonfectin solution was mixed with 100 μL serum-free medium, combined with the plasmid DNA solution, and incubated at room temperature for 30 minutes. Plasmid/Clonfectin solution was solved in 1.8 mL serum-free medium and applied to the cells. Cells were incubated for 3.5 hours in a 5% CO2 incubator, after which the transfection solution was removed and the cells were washed twice with PBS (37°C) before the fresh expansion medium was added. In the next step, transfected cells were further cultured for 3 to 5 weeks to generate adherent cells.
Generation of NA cells from adherent cells
We replaced FBS-supplemented RPMI-1640 medium with serum-free, SCF-supplemented (100 ng/mL) medium (StemSpan; Cell Technologies, St. Katharinen, Germany) and incubated the cultures at 37°C in 5% CO2. NA cells appeared as early as 14 hours. To obtain a larger number of NA cells, we harvested them after 72 to 96 hours of culture. Cell phenotype was analyzed by flow cytometry.
CFC and CAFC assay
Human colony-forming cells (CFCs) were assayed under standard conditions in methylcellulose. We performed cobblestone area–forming cell (CAFC) assay23 to measure the number of progenitors within populations of enriched CD133+ cells, CD34+ cells, and NA CD34− cells. Serial cell dilutions (32-1000 cells per well) were used. The percentage of wells with at least one phase-dark hematopoietic clone beneath the stromal layer was determined each week for 6 weeks, and frequencies of CAFCs were calculated by likelihood maximization.
Transplantation into mice
NOD/SCID mice obtained from Jackson Laboratories (Bar Harbor, ME) were handled under sterile conditions and maintained in single-cage ventilated microisolators at the University Children's Hospital. Total-body irradiation (268 cGy) of 6-week-old mice was performed in a137Cs source (Gammamaster Plus; Gammacell, Leiden, The Netherlands). We gave the first group of mice one intravenous injection of 5000 NA CD34− cells, which we obtained by treating adherent cells with recombinant human SCF (100 ng/mL; R&D Systems) for 72 hours; we gave the second group 5000 highly enriched, mobilized CD34+ cells from peripheral blood; and we gave the third group 5000 freshly isolated CD34+ cells that had subsequently been treated with SCF for 72 hours. Control mice received injections of PBS (300 μL per mouse).
To determine the frequency of SCID-repopulating cells (SRCs), we transplanted each of the following concentrations of NA CD34− cells into 10 NOD/SCID mice: 1250, 2500, 5000, and 10 000 cells. We humanely killed them 8 to 10 weeks later. Bone marrow cells from the femurs and tibiae of each mouse were flushed into RPMI-1640 containing 10% FBS. To rule out that NA CD34− cells before transplantation into NOD/SCID mice contain CD3+, CD19+, or CD56+cells, we stained them with antibodies against these antigens and subsequently analyzed them by flow cytometry. A detailed analysis showed in all cases that no cells were positive for these antigens.
Flow cytometric analysis of murine bone marrow and peripheral blood
We lysed red blood cells in samples of bone marrow and peripheral blood by adding 8.3% ammonium chloride; we washed the remaining cells with PBS containing 5% FBS. Approximately 106 cells were incubated for 30 minutes at 4°C with mouse antihuman antibodies against the following antigens: CD2, CD3, CD4, CD5, CD7, CD8, CD19, CD34 (human progenitor cell antigen–2), CD45, CD56 (neural cell adhesion molecule 16.2), CD71, CD133/2, T-cell receptor (TCR)αβ, TCR-γδ, human leukocyte antigen (HLA)–DR, and glycophorin-A. All antibodies except those specific for CD71, glycophorin-A (Immunotech, Hamburg, Germany), and CD133 (Miltenyi Biotec) were purchased from Becton Dickinson. Cells were washed twice in PBS and analyzed by FACSCalibur (Becton Dickinson). In control experiments, cells were incubated with mouse immunoglobulin G conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin-chlorophyll protein (PerCP), or allophycocyanin (APC).
RT-PCR analysis
We analyzed RNA extracted from murine bone marrow by reverse transcriptase–polymerase chain reaction (RT-PCR). Primers (BIG Biotech, Freiburg, Germany) were complementary to human CD34 and CD133 gene regions that contained no homology with corresponding mouse genes. Primers for the human CD34 gene were as follows: sense, 5′-GTCTTGACAACAACGGTACTGC-3′; antisense, 5′-CAAGACCAGCAGTAGACACTGA-3′ (amplicon length: 647 nucleotides). Primers for the human CD133 gene were as follows: sense, 5′-TGAACACACACCAGTTTACAGG-3′; antisense, 5′-ACGCAGGTTTCTCTATGATGGC-3′ (amplicon length: 296 nucleotides). Amplicons were analyzed in a 2% agarose gel.
PCR-ELISA analysis of human cell engraftment
Human DNA in bone marrow of recipient mice was detected by using semiquantitative PCR–enzyme-linked immunosorbent assay (ELISA) (Roche Diagnostics, Mannheim, Germany), as described.24
We used primers (5′-AAGGATACCACAATAAGCTGC-3′, 5′-GTGCCAGTCTCCACAAACC-3′) and the oligonucleotide probe (5′-GCTAAAGGTCAAGATATTCAGTGAGAC-3′–biotin) that are specific for human cartilage-specific CART-1 mRNA. PCR was performed under standard conditions. For semiquantitation, we serially diluted human DNA in murine DNA.
Results
In vitro generation of adherent cells from highly enriched CD133+ cells
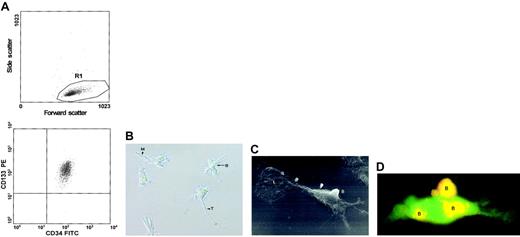
We used MACS to positively select mobilized peripheral blood CD133+ cells (Figure 1A) that were subsequently cultured in the presence of defined cytokine combinations. Although 99.6% ± 0.3% of the cells expressed CD34, only 3% ± 1% of the cells also expressed the novel stem cell marker CD164.20 Interestingly, we discovered that after 3 to 5 weeks of culture with 100 ng/mL FL and 100 ng/mL IL-6, these cells gave rise to a novel population of cells that adhered to the plastic surface of the flasks. The adherent cells had morphologic features different from those of endothelial cells, stromal fibroblasts, dendritic cells, and mesenchymal cells.
Morphologic characterization of adherent cells.
(A) CD133+ cells mobilized from peripheral blood were enriched by MACS. Flow cytometry showed that the purity of the positively selected CD133+ cells was generally greater than 99.5%. (B) Inverse microscopic image of typical 6-week-old adherent cells (original magnification, × 200). B indicates bud; M, magnupodium; T, tenupodium. (C) Raster electron micrograph of a 6-week-old adherent cell (original magnification, × 2000). B indicates a bud on the cell surface; L, a spoonlike lobopodium at the terminus of the cell (upper left corner of the micrograph). (D) A 6-week-old adherent cell that emerged from a population of EGFP-expressing CD133+ cells is counterstained with PE-conjugated mouse antihuman CD133 antibody. Yellow fluorescence caused by the overlay of green (EGFP) and red (CD133) fluorescence indicates double staining of the buds (indicated by B).
Morphologic characterization of adherent cells.
(A) CD133+ cells mobilized from peripheral blood were enriched by MACS. Flow cytometry showed that the purity of the positively selected CD133+ cells was generally greater than 99.5%. (B) Inverse microscopic image of typical 6-week-old adherent cells (original magnification, × 200). B indicates bud; M, magnupodium; T, tenupodium. (C) Raster electron micrograph of a 6-week-old adherent cell (original magnification, × 2000). B indicates a bud on the cell surface; L, a spoonlike lobopodium at the terminus of the cell (upper left corner of the micrograph). (D) A 6-week-old adherent cell that emerged from a population of EGFP-expressing CD133+ cells is counterstained with PE-conjugated mouse antihuman CD133 antibody. Yellow fluorescence caused by the overlay of green (EGFP) and red (CD133) fluorescence indicates double staining of the buds (indicated by B).
Morphologic and phenotypic characterization of the adherent cells
To further explore the morphology and phenotype of the adherent cells, we performed raster electron microscopy, time-lapse studies, and flow cytometry. All adherent cells developed 2 types of pseudopodia: tenupodia (long, thin pseudopodia) and magnupodia (short, thick pseudopodia) (Figure 1B). Although the morphology of the adherent cells was rather homogeneous, their sizes ranged from 15 to 55 μm. Most strikingly, these cells displayed buds that protruded 9 μm from the cell surface and lobopodia (spoonlike projections), which have not been previously observed on human cells (Figure 1C). To determine whether these cells possessed a hematopoietic phenotype, we incubated them with anti-CD34 monoclonal antibody conjugated to FITC and anti-CD133 monoclonal antibody conjugated to PE. Fluorescence microscopy showed that none of the adherent cells expressed surface CD34. However, parts of these cells expressed CD133. To analyze the pattern of CD133 in greater detail, we transfected highly purified CD133+ cells with an expression vector encoding enhanced green fluorescent protein (EGFP). Adherent cells obtained after 5 weeks from the transfected population were incubated with the PE-conjugated monoclonal anti-CD133 antibody. Fluorescence microscopy of individual cells revealed that CD133 was expressed exclusively on the buds of the adherent CD34− cells (Figure 1D).
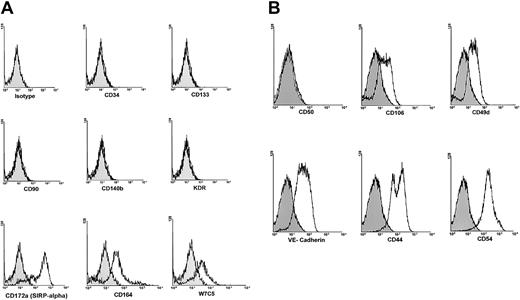
Flow cytometric analysis of 6- to 8-week-old adherent cells showed the absence of markers of hematopoietic stem cells (CD133, CD34), endothelial cells (CD140b, KDR, CD31), mesenchymal cells (CD140b, CD90), dendritic cells (CD83, CD1a), and stromal fibroblasts (CD90). However, all adherent cells expressed considerable levels of VE-cadherin and CD106 and high levels of adhesion molecules CD54 and CD44. They also expressed the more recently described antigens CD164,20 CD172a (SIRP-α),21 and the stem cell antigen defined by the antibody W7C522 (Figure2).
Phenotypic characterization of adherent cells.
Six-week-old adherent cells were treated with trypsin, harvested, washed once with PBS, incubated with mouse monoclonal antibodies to various human antigens, washed twice with PBS, and analyzed by flow cytometry. (A) The cells were negative for CD34, CD133, CD90, CD140b, and KDR. However, they expressed high levels of CD172a, CD164, and the antigen detected by the monoclonal antibody W7C5. (B) Adherent cells were negative for CD50, but expressed considerable levels of VE-cadherin and CD106. In addition, these cells expressed moderate levels of CD49d and high levels of CD44 and CD54 adhesion molecules. Filled histograms show isotype control IgG staining profile, whereas open histograms show specific antibody staining profile.
Phenotypic characterization of adherent cells.
Six-week-old adherent cells were treated with trypsin, harvested, washed once with PBS, incubated with mouse monoclonal antibodies to various human antigens, washed twice with PBS, and analyzed by flow cytometry. (A) The cells were negative for CD34, CD133, CD90, CD140b, and KDR. However, they expressed high levels of CD172a, CD164, and the antigen detected by the monoclonal antibody W7C5. (B) Adherent cells were negative for CD50, but expressed considerable levels of VE-cadherin and CD106. In addition, these cells expressed moderate levels of CD49d and high levels of CD44 and CD54 adhesion molecules. Filled histograms show isotype control IgG staining profile, whereas open histograms show specific antibody staining profile.
To assess the frequency of the initiating cells for the adherent cells within the highly enriched CD133+ cell population, we subjected this population to limiting dilution analysis (n = 3). The cells within this population were cultured for 10 days in the presence of FL and IL-6 (100 ng/mL each). Approximately 1 of every 25 CD133+ cells (95% confidence interval [CI], 1 of 20 to 1 of 34 CD133+ cells) gave rise to an adherent cell with a morphology and phenotype like those described in the paragraphs above.
Phenotypic characterization of the nonadherent CD34−cells
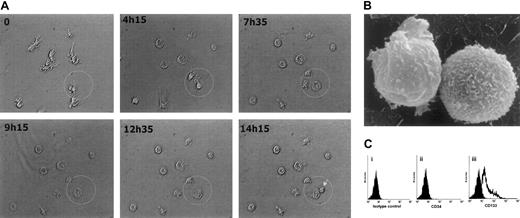
To obtain a homogeneous population of adherent cells, we extensively removed the NA cells during a period of 3 to 5 weeks. Unexpectedly, we again detected NA round cells in the medium after 5 weeks of culture. Evidence of spontaneously generated NA cells suggested that adherent cells themselves can produce cytokines that promote entry into the cell cycle. Results of ELISAs (n = 3) showed that these cells produced substantial amounts of IL-6 (80 ± 11.5 pg per 1 mL supernatant) and SCF (5.6 ± 0.8 pg per 1 mL supernatant). To observe morphologic changes in the adherent cells during culture and to determine whether these cells can divide and give rise to NA cells, we monitored the cells in a special incubation chamber (37°C in an atmosphere of 5% CO2) by time-lapse digital photomicrography. Because of the lack of the ligands for CD164 and CD172a, which are highly expressed by adherent cells, we determined whether stimulation by SCF could promote the proliferation of these cells. When cultured in the presence of SCF, the adherent cells retracted their tenupodia and magnupodia and became large, round cells with lobopodia (Figure 3A); this change allowed them to move through the local microenvironment. In the next step, these large adherent cells divided and gave rise to NA cells. After 14 hours in culture with SCF, only 1 of 10 large cells divided. The NA cells had a diameter of approximately 20 μm (Figure 3B). This finding strongly suggests that NA cells emerge directly from the adherent cells.
Emergence and characterization of nonadherent cells.
(A) Time-lapse images of cells in a special incubation chamber were acquired by a digital camera and analyzed by Analysisdocu software (Soft Imaging Systems, Leidenfelden, Germany). At hour 0 of culture, the cell morphology was characterized by pseudopodia and buds. After 4 hours, the cells began to retract their pseudopodia and become more spherical. Cells that were destined to divide then began to enlarge. After 14 hours and 15 minutes of culture, a cell division was observed (arrow). (B) Raster electron micrograph (original magnification, × 2000) shows that NA cells are spherical and lack pseudopodia. (C) A representative flow cytometric analysis of surface CD34 and CD133 expression on NA cells (n = 6) derived by stimulation of 6-week-old adherent cells with SCF for 72 hours. Cells were incubated with an isotype-matched control mouse antibody (i). No NA cells expressed CD34 (ii), but a subset expressed CD133 (iii).
Emergence and characterization of nonadherent cells.
(A) Time-lapse images of cells in a special incubation chamber were acquired by a digital camera and analyzed by Analysisdocu software (Soft Imaging Systems, Leidenfelden, Germany). At hour 0 of culture, the cell morphology was characterized by pseudopodia and buds. After 4 hours, the cells began to retract their pseudopodia and become more spherical. Cells that were destined to divide then began to enlarge. After 14 hours and 15 minutes of culture, a cell division was observed (arrow). (B) Raster electron micrograph (original magnification, × 2000) shows that NA cells are spherical and lack pseudopodia. (C) A representative flow cytometric analysis of surface CD34 and CD133 expression on NA cells (n = 6) derived by stimulation of 6-week-old adherent cells with SCF for 72 hours. Cells were incubated with an isotype-matched control mouse antibody (i). No NA cells expressed CD34 (ii), but a subset expressed CD133 (iii).
Flow cytometric analysis showed that none of the NA cells expressed surface CD34 (Figure 3Cii), whereas 19% ± 9% of NA cells expressed CD133 (Figure 3Ciii).
The apparent ability of the adherent cells to give rise to a CD133+CD34− stem cell subset suggests that the latter represent a very early stage of human hematopoiesis. In addition to CD133, the NA CD34− cells (n = 3) expressed CD45 (47% ± 15% expressed CD45RO and 30% ± 10% expressed CD45RA), HLA class I (47% ± 15%), CD49d (53% ± 16%), and CXCR4 (40% ± 12%). For the sake of brevity, these NA cells that contain the CD133+CD34− stem cell subset are referred to as NA CD34− cells for the remainder of this study. To determine the capacity of the adherent cells to generate NA CD34− cells, we cultured them for 3 weeks in the presence or absence of SCF. We observed that cultures supplemented with SCF contained approximately twice as many NA CD34− cells as cultures without SCF; this finding suggests that SCF enhances recruitment of the adherent cells into the cell cycle (Table1).
Capacity of the adherent cells to generate nonadherent CD34− cells
| . | Number of NA cells generated per 5000 adherent cells . | ||
|---|---|---|---|
| Week 1 . | Week 2 . | Week 3 . | |
| Unstimulated adherent cells | 180 ± 74 | 100 ± 68 | 40 ± 18 |
| Adherent cells stimulated with SCF | 396 ± 118 | 270 ± 91 | 100 ± 35 |
| . | Number of NA cells generated per 5000 adherent cells . | ||
|---|---|---|---|
| Week 1 . | Week 2 . | Week 3 . | |
| Unstimulated adherent cells | 180 ± 74 | 100 ± 68 | 40 ± 18 |
| Adherent cells stimulated with SCF | 396 ± 118 | 270 ± 91 | 100 ± 35 |
Six-week-old adherent cells were transferred to 96-well microtiter plates (5000 cells per well) in triplicate and were cultured for 3 additional weeks in serum-free medium in the presence or absence of 100 ng/mL SCF. The number of NA cells generated each week was recorded. Data represent the means ± standard deviations of 3 experiments.
Functional characterization of NA CD34− cells
Human Lin−CD34− stem cells show low activity in CFC assays and in long-term culture-initiating cell assays,13 but are capable of initiating multilineage human hematopoiesis in either preimmune fetal sheep25 or NOD/SCID mice.26 In our study, NA CD34− cells that arose from adherent cells after stimulation with SCF for 72 hours generated no hematopoietic colonies. Unlike the highly enriched CD133+ or CD34+ cells, the NA CD34− cells generated no CAFCs at week 2 or week 6 (Table2).
Frequency of clonogenic progenitors in populations of highly enriched CD133+ cells, highly enriched CD34+ cells, and nonadherent CD34− cells
| Cell fraction . | No. CFCs per 1000 cells, mean ± SEM . | Frequency of CAFCs . | |
|---|---|---|---|
| Week 2 . | Week 6 . | ||
| CD133+ | 187 ± 35; n = 6 | 1 among 205; n = 5 | 1 among 154; n = 5 |
| CD34+ | 225 ± 65; n = 8 | 1 among 321; n = 5 | 1 among 178; n = 5 |
| NA CD34− | ND; n = 4 | ND; n = 4 | ND; n = 4 |
| Cell fraction . | No. CFCs per 1000 cells, mean ± SEM . | Frequency of CAFCs . | |
|---|---|---|---|
| Week 2 . | Week 6 . | ||
| CD133+ | 187 ± 35; n = 6 | 1 among 205; n = 5 | 1 among 154; n = 5 |
| CD34+ | 225 ± 65; n = 8 | 1 among 321; n = 5 | 1 among 178; n = 5 |
| NA CD34− | ND; n = 4 | ND; n = 4 | ND; n = 4 |
Human CFCs were assayed in methylcellulose by standard methods. Progenitors were quantified by CAFC assays with serial cell dilutions (32-1000 cells per well). The percentage of wells containing at least one phase-dark hematopoietic clone beneath the stromal layer was determined each week for 6 weeks, and the frequency of CAFCs was calculated by likelihood maximization.
SEM indicates standard error of the mean; ND, not detected.
To determine whether these cells have in vivo repopulating potential and to compare their repopulating potential with that of mobilized peripheral blood CD34+ cells, we transplanted into the sublethally irradiated NOD/SCID mice either 5000 NA CD34− cells derived from adherent cells after stimulation with SCF for 72 hours or 5000 highly enriched, mobilized CD34+ hematopoietic cells from peripheral blood. Surprisingly, 8 weeks after transplantation, the level of engraftment in recipients of NA CD34− cells was substantially greater than in recipients of highly enriched CD34+ cells. Stimulation of the freshly isolated CD34+ cells with SCF for 72 hours enhanced engraftment only marginally (Figure4A). This finding indicates that NA CD34− cells possess a substantially greater engraftment potential than CD34+ progenitor cells.
Engraftment potential of CD34+ cells and nonadherent CD34− cells.
(A) Eight weeks after intravenous injection of NOD/SCID mice with 5000 NA CD34− cells or 5000 CD34+ cells, bone marrow cells were analyzed by flow cytometry with antibody to human CD45, without gating. Graph shows the percentage engraftment in 10 recipients of NA CD34− cells produced in vitro by stimulation of adherent cells with SCF for 72 hours (▪), 8 recipients of freshly isolated CD34+ cells (■), 6 recipients of freshly isolated CD34+ cells that had been stimulated in vitro with SCF for 72 hours (●), and 6 mice that received only injections of PBS (○). (B) Effect of cell dose on engraftment potential of NA CD34− cells in NOD/SCID mice. The percentage of human cells in the bone marrow of 40 recipients was determined 8 to 10 weeks after transplantation by flow cytometric analysis of human CD45 expression. (C) Analysis of DNA isolated from the bone marrow cells of recipients in panel B. The levels of human DNA were compared with the values of human-mouse control mixtures. The results are given as the means ± standard deviations of 3 PCR-ELISA measurements.
Engraftment potential of CD34+ cells and nonadherent CD34− cells.
(A) Eight weeks after intravenous injection of NOD/SCID mice with 5000 NA CD34− cells or 5000 CD34+ cells, bone marrow cells were analyzed by flow cytometry with antibody to human CD45, without gating. Graph shows the percentage engraftment in 10 recipients of NA CD34− cells produced in vitro by stimulation of adherent cells with SCF for 72 hours (▪), 8 recipients of freshly isolated CD34+ cells (■), 6 recipients of freshly isolated CD34+ cells that had been stimulated in vitro with SCF for 72 hours (●), and 6 mice that received only injections of PBS (○). (B) Effect of cell dose on engraftment potential of NA CD34− cells in NOD/SCID mice. The percentage of human cells in the bone marrow of 40 recipients was determined 8 to 10 weeks after transplantation by flow cytometric analysis of human CD45 expression. (C) Analysis of DNA isolated from the bone marrow cells of recipients in panel B. The levels of human DNA were compared with the values of human-mouse control mixtures. The results are given as the means ± standard deviations of 3 PCR-ELISA measurements.
NA CD34− cell population contains SCID-repopulating cells
To determine whether NA CD34− cells have SCID-repopulating activity and to determine the frequency of SRCs, we transplanted varying concentrations of NA CD34− cells into sublethally irradiated NOD/SCID mice. It should be noted that transplant-recipient mice received no injections of cytokines (eg, IL-3, SCF, and granulocyte-macrophage colony-stimulating factor) to increase the level of engraftment of human cells. Nonetheless, transplanted NA CD34− cells successfully engrafted and generated cells of human myeloid and lymphoid lineages. Mouse bone marrow was analyzed for the presence of human cells 8 to 10 weeks after transplantation. The levels of human cell engraftment in 40 NOD/SCID mice were quantified by flow cytometry and DNA analysis (Figure 4B-C).
Human cells engrafted in 36 of 40 recipient mice, a finding indicating that NA CD34− cells contain a substantial number of SRCs. The level of human cell engraftment in the bone marrow of these mice was 1% to 4% (5 × 105 to 2 × 106 human cells). Limiting dilution analysis showed one repopulating unit among 853 NA CD34− cells (95% CI, 1 among 719 to 1 among 2120).
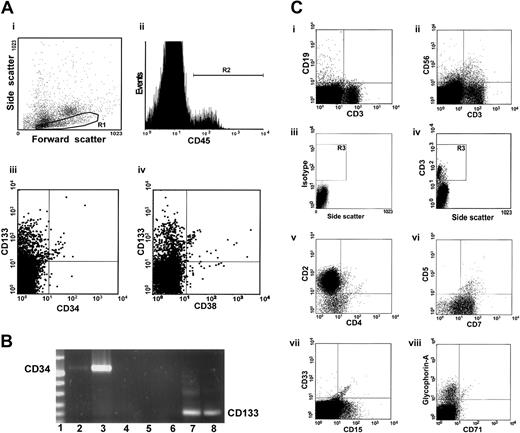
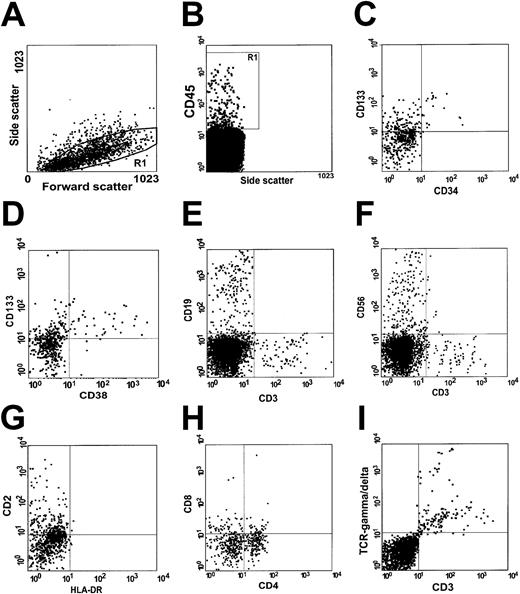
To examine the capacity of the NA CD34− cells to differentiate and proliferate, we performed flow cytometry to analyze engraftment in NOD/SCID mice in which 10 000 NA CD34−cells were transplanted. Cells with the properties of low, medium, or high forward scatter were gated and further analyzed (Figure5Ai region R1). Four percent of cells in the bone marrow of a representative mouse were human cells expressing CD45, a human-specific panleukocyte marker (Figure 5Aii gated region R2). In addition to multilineage engraftment, we observed the primitive CD133+CD34−CD38− stem cell fraction in the bone marrow of this mouse. A few differentiated CD133+CD34+ cells were also detected (Figure 5Aiii-iv). Analysis using RT-PCR detected larger quantities of human mRNA for CD133 than for CD34 in bone marrow cells of mice in which the transplanted cells engrafted (Figure 5B), a finding consistent with those of the flow cytometric analysis. Interestingly, in addition to human CD19+ cells (Figure 5Ci), cells with NK (CD56+), NKT (CD56+CD3+) (Figure 5Cii), and T-cell phenotype (CD3+) (Figure 5Ci-ii) were also identified. Multicolor analysis of the cells within gate R3 (Figure 5Civ) revealed that the majority of the CD3+ cells coexpressed CD2 and CD7. Only a small proportion of these cells expressed CD4 and CD5 (Figure 5Cv-vi). These cells were, however, negative for CD8 (data not shown). The emergence of human cells with T- and NK-cell phenotype indicates a unique repopulation capacity of the NA CD34− cells because highly purified primitive Lin−CD34+CD38− cells that engraft in NOD/SCID recipients never give rise to T cells.27 In addition to the CD33+ and CD15+ cells (Figure 5Cg), more differentiated glycophorin-A+ erythroid cells that lack CD45 and CD71 were identified (Figure 5Ch); this finding indicates that the NA CD34− cells contain progenitor cells that can give rise to the cells with both myeloid and lymphoid phenotypes.
Multilineage differentiation of human nonadherent CD34− cells in the bone marrow of NOD/SCID mice.
(A) Bone marrow cells from a mouse given 10 000 NA CD34−cells were incubated with antibodies to various human antigens and analyzed by flow cytometry. Cells with low, medium, and high forward scatter (Ai, region R1) were gated and further analyzed. Panel Aii shows a histogram of CD45 (panleukocyte marker) expression. Panels Aiii and Aiv show expression of CD133 and CD34 (hematopoietic stem cell markers) and CD38 (a differentiation marker) within the CD45+ cell population. Most cells had a primitive CD133+CD34−CD38− phenotype. (B) RT-PCR analysis of human CD133 and CD34 mRNA in bone marrow cells of NOD/SCID mice that received NA CD34− cells. Lane 1 contains DNA molecular-weight markers. We used human CD34-specific primers to generate cDNA from bone marrow cells of a representative mouse recipient (lane 2), from highly enriched human CD34+cells from mobilized peripheral blood (positive control; lane 3), and from bone marrow cells of a representative mouse that received an injection of only PBS (lane 4). Lane 5 contains a sample of distilled water that was used in RT-PCR. We used human CD133-specific primers to generate cDNA from bone marrow cells of a representative mouse that received an injection of only PBS (lane 6); from highly enriched, mobilized CD34+ cells from human peripheral blood (lane 7); and from bone marrow cells of a representative mouse recipient (lane 8). (C) The human cells were tested for expression of the lineage-specific markers CD19 (a human pan–B-cell marker; Ci), CD56 (an NK-cell marker; Cvi), and CD3 (a human pan–T-cell marker; Ci-ii,Civ). Subpopulations of human cells with T-cell phenotype were analyzed on the basis of expression of CD2, CD4, CD5, and CD7 (Cv-vi) on CD3+ cells within gate R3 (R1+R2; Civ), CD33 and CD15 (myeloid markers; Avii), and glycophorin-A (an erythroid cell marker; Cviii).
Multilineage differentiation of human nonadherent CD34− cells in the bone marrow of NOD/SCID mice.
(A) Bone marrow cells from a mouse given 10 000 NA CD34−cells were incubated with antibodies to various human antigens and analyzed by flow cytometry. Cells with low, medium, and high forward scatter (Ai, region R1) were gated and further analyzed. Panel Aii shows a histogram of CD45 (panleukocyte marker) expression. Panels Aiii and Aiv show expression of CD133 and CD34 (hematopoietic stem cell markers) and CD38 (a differentiation marker) within the CD45+ cell population. Most cells had a primitive CD133+CD34−CD38− phenotype. (B) RT-PCR analysis of human CD133 and CD34 mRNA in bone marrow cells of NOD/SCID mice that received NA CD34− cells. Lane 1 contains DNA molecular-weight markers. We used human CD34-specific primers to generate cDNA from bone marrow cells of a representative mouse recipient (lane 2), from highly enriched human CD34+cells from mobilized peripheral blood (positive control; lane 3), and from bone marrow cells of a representative mouse that received an injection of only PBS (lane 4). Lane 5 contains a sample of distilled water that was used in RT-PCR. We used human CD133-specific primers to generate cDNA from bone marrow cells of a representative mouse that received an injection of only PBS (lane 6); from highly enriched, mobilized CD34+ cells from human peripheral blood (lane 7); and from bone marrow cells of a representative mouse recipient (lane 8). (C) The human cells were tested for expression of the lineage-specific markers CD19 (a human pan–B-cell marker; Ci), CD56 (an NK-cell marker; Cvi), and CD3 (a human pan–T-cell marker; Ci-ii,Civ). Subpopulations of human cells with T-cell phenotype were analyzed on the basis of expression of CD2, CD4, CD5, and CD7 (Cv-vi) on CD3+ cells within gate R3 (R1+R2; Civ), CD33 and CD15 (myeloid markers; Avii), and glycophorin-A (an erythroid cell marker; Cviii).
Flow cytometric analysis of the peripheral blood of this representative mouse revealed that 0.9% of the cells within gate R1 (Figure6A) expressed CD45, a human-specific panleukocyte marker (Figure 6B). Further analysis of CD45+cells showed not only the primitive CD133+CD34− stem cell subset (Figure 6C-D), but also circulating cells with B-cell (Figure 6E), NK-cell (Figure6F), and T-cell phenotype (Figure 6E-F). Most of the CD3+cells expressed CD2 (Figure 6G), and a proportion of them additionally expressed CD4, CD8, or both (Figure 6H). Remarkably, the majority of CD3+ cells in the peripheral blood of this NOD/SCID mouse expressed TCR-γδ (Figure 6I), and very few of them expressed TCR-αβ (data not shown).
Circulating human cells in the peripheral blood of NOD/SCID mice.
Peripheral blood cells from the same mouse were gated as described above (A) and tested for expression of human CD45, a human-specific panleukocyte marker (B). Human primitive CD133+CD34−CD38− (C) and CD133+CD34+CD38+ (D) stem cells were detected. CD19 (a human pan–B-cell marker; E), CD56 (an NK-cell marker; F), and CD3 (a human pan–T-cell marker; E-F) were also expressed. Human cells with a T-cell phenotype expressed CD2 (G) as well as CD4 and CD8 (H), and the majority of them expressed TCRγδ (I).
Circulating human cells in the peripheral blood of NOD/SCID mice.
Peripheral blood cells from the same mouse were gated as described above (A) and tested for expression of human CD45, a human-specific panleukocyte marker (B). Human primitive CD133+CD34−CD38− (C) and CD133+CD34+CD38+ (D) stem cells were detected. CD19 (a human pan–B-cell marker; E), CD56 (an NK-cell marker; F), and CD3 (a human pan–T-cell marker; E-F) were also expressed. Human cells with a T-cell phenotype expressed CD2 (G) as well as CD4 and CD8 (H), and the majority of them expressed TCRγδ (I).
A considerable number of CD133+CD34+ cells were also identified, a finding suggesting that CD133 is a marker of more primitive stem cells and that expression of CD133 developmentally precedes that of CD34.
Discussion
The present study provides direct evidence of the existence of a novel class of adherent cells that lack CD34. However, they express considerable levels of VE-cadherin and CD106 and high levels of adhesion molecules CD54 and CD44. In addition, these cells express high levels of the sialomucin CD16420 (a marker expressed on both CD34+ and CD34− stem cells), CD172a,21 and a stem cell antigen defined by the antibody W7C5.22 Adherent cells possess a distinct morphology characterized by tenupodia, magnupodia, and, in particular, lobopodia and buds; these buds express CD133, which is detectable by fluorescence microscopy. Moreover, they do not express markers of primary bone marrow stromal fibroblasts (CD90), endothelial cells (KDR, CD31), dendritic cells (CD83, CD1a), or mesenchymal cells (CD90, CD140b). Because we were able to generate the adherent cells from CD133+ cells of bone marrow, cord blood, and even fetal liver, we assume that these cells might be involved at early stages of human hematopoiesis (unpublished observations, January 2000).
We show here that these adherent cells can either spontaneously or after stimulation with SCF give rise to transplantable NA CD34− cells that contain a CD133+CD34− stem cell subset. Transplantation of 5000 NA CD34− cells resulted in successful engraftment in NOD/SCID mice, whereas the same number of highly enriched CD34+ cells failed to engraft. This finding is in line with those of Zanjani et al,14 who showed that transplantation of 1 to 4 × 103 CD34+ cells was not sufficient for engraftment in the fetal sheep model. However, when the number of transplanted CD34+ cells was increased to 64 × 103 cells, successful engraftment occurred in 80% of the sheep. Our finding is also consistent with the finding that highly enriched populations of CD34+ cells contain a significantly lower number of SRCs, and, consequently, the level of engraftment is lower in NOD/SCID mice that receive CD34+cells than in those that receive the more primitive CD34+CD38− cell fraction.27 28The fact that most of our transplanted CD34+ cells were CD38+ could explain why the levels of engraftment associated with these cells were significantly lower than those associated with NA CD34− cells.
Transplantation of various concentrations of NA CD34−cells into NOD/SCID mice showed that these cells contained a substantial number of SRCs. Limiting dilution analysis showed that there was 1 repopulating unit among 853 NA CD34− cells (95% CI, 1 among 719 to 1 among 2120). This frequency was as high as that of cord blood CD34+CD38− cell fractions27,28 and 147-fold higher than that of a newly discovered Lin−CD34− cell fraction.13 This frequency of one repopulating unit among 853 NA CD34− cells represents a level of enrichment that is 1090-fold greater than that of unseparated cord blood, 3517-fold greater than that of unseparated adult bone marrow, 7034-fold greater than that of mobilized peripheral blood cells from healthy donors,29 and 117-fold greater than that of enriched, mobilized CD34+ cells from peripheral blood.30Moreover, this frequency in our study represents a level of enrichment that is 375-fold greater than that of the fetal stem cell fraction in cord blood31 and 6.4-fold greater than that of cord blood CD34+ cells.30 Taken together, our findings indicate that the population of NA CD34− cells that contains the CD133+CD34− stem cell subset had a number of SRCs that was greater than that of CD34− or CD34+ stem cell subsets reported so far. In this study, we provide evidence that NA CD34− cells not only are highly enriched in SRCs with long-term engraftment capacity, but also can give rise to human cells with a T-cell phenotype in the bone marrow and peripheral blood of transplanted NOD/SCID mice. This suggests a unique repopulation capacity of the NA CD34− cells because highly purified primitive Lin−CD34+CD38−cells that engraft in NOD/SCID recipients never give rise to T cells.27 The majority of the bone marrow CD3+ cells expressed CD2 and CD7, and a proportion of them expressed CD4 and CD5. Interestingly, virtually none of them expressed CD8. According to our results, the phenotype of these cells in the bone marrow of NOD/SCID mice is CD45+CD3+CD2+CD7+ CD4+CD5+CD8−, which indicates a primitive prethymic phenotype. The majority of the human cells with a T-cell phenotype in the mouse peripheral blood expressed CD2, whereas most of them expressed either CD4 or CD8 and only a proportion were CD4+CD8+. Interestingly, the majority of CD3+ cells in the peripheral blood expressed TCRγδ and very few of them expressed TCRαβ, indicating a selective clonal expansion of these cells. Future transplantation studies with NA CD34− cells should clarify the migration capacity of these cells into the thymus, their developmental pathways into different T-cell subpopulations, and finally their migration into peripheral blood. The fact that NA CD34− cells can give rise to cells with B-, T-, NK-, and NKT-cell phenotype and myeloid cells suggests that this novel cell fraction is highly enriched in progenitors for both lymphoid and myeloid lineages.
We showed in this study that in vitro culture of highly purified CD133+ cells in the presence of FL and IL-6 for 3 to 5 weeks induces a complete down-regulation of CD34 expression. Interestingly, CD133 remains on protoplasmic protrusions and, in this instance, is detectable by fluorescence microscopy but not by flow cytometry. These adherent cells were able, however, either spontaneously or after stimulation with SCF, to give rise again to a subset of NA CD133+CD34− stem cells, suggesting that CD133 appears on the surface of hematopoietic stem cells before CD34. The evidence that these cells are able to initiate a full human graft in NOD/SCID mice suggests that expression of CD34 is not a prerequisite to the acquisition of repopulating activity by these cells. This result is also consistent with the finding that hematopoiesis in CD34 knockout mice is identical to that in wild-type mice.32 33 The presence of a considerable number of engrafted human CD133+CD34+ cells in the bone marrow and peripheral blood of recipient mice demonstrates the potential of cells within the CD133+CD34−subset to further differentiate into cells that express CD34 after in vivo repopulation. Therefore, CD133 appears to be a more appropriate marker by which to characterize rare CD34− stem cell subsets.
The fact that the adherent cells can give rise to a CD133+CD34− stem cell subset suggests that these cells appear at a very early stage of human hematopoiesis. Thus, adherent cells may be preferable targets for gene transfer, an approach that can be used to further study the mechanisms of self-renewal and the differentiation potential of hematopoietic stem cells. The identification of NA CD133+CD34− stem cells that can initiate multilineage hematopoiesis in NOD/SCID mice and particularly give rise to human cells with T- and NK-cell phenotype may have important implications for stem cell selection procedures and clinical transplantation protocols. These cells may therefore provide a novel alternative to CD34+ progenitor cells for transplantation.
We thank Dr D. Schroeter from the Deutsches Krebsforschungszentrum in Heidelberg for the raster electron microscopy images and M. Schneidereit from Soft Imaging Systems-SIS (Leinfelden, Germany) for technical support. We are very grateful to Prof. L. Kanz for critical reading of the manuscript and Dr Julia C. Jones and Dr Sharon Naron for excellent editorial assistance. We also thank B. Spring, A. Marxer, C. Bäuerle, A. Dorner, U. Junker, and O. Bartuli for excellent technical assistance.
Prepublished online as Blood First Edition Paper, September 19, 2002; DOI 10.1182/blood-2002-03-0711.
Supported by grants from the Wilhelm-Sander Siftung (S.K.) and Deutsche Knochenmark Spenderdatei (S.K. and R.H.), the German José Carreras Leukemia Foundation (R.H.), the Deutsche Forschungsgemeinschaft (SFB510 projects A1 and C4; H.-J.B., J.T.W., and P.G.S.), and the Fortüne Research Program 545 of the University of Tübingen (K.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Selim Kuçi, Department of Hematology and Oncology, University Children's Hospital, Hoppe-Seyler-Str 1, D-72076 Tübingen, Germany; e-mail:smkuci@med.uni-tuebingen.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal