We investigated whether combined signaling induced by engineered Notch ligands and hematopoietic growth factors influences hematopoietic stem-cell differentiation. We show that incubation of murine marrow precursors with Delta1ext-IgG, a Notch ligand consisting of the Delta1 extracellular domain fused to the Fc portion of human immunoglobulin G1 (IgG1), and growth factors stem cell factor (SCF), interleukin 6 (IL-6), IL-11, and Flt3-l inhibited myeloid differentiation and promoted a several-log increase in the number of precursors capable of short-term lymphoid and myeloid repopulation. Addition of IL7 promoted early T-cell development, whereas addition of granulocyte-macrophage colony-stimulating factor (GM-CSF) led to terminal myeloid differentiation. These results support a role for combinatorial effects by Notch and cytokine-induced signaling pathways in regulating hematopoietic cell fate and suggest the usefulness of Notch ligand in increasing hematopoietic precursor numbers for clinical stem-cell transplantation.
Introduction
The hematopoietic stem cell (HSC) is thought to self-renew while also producing progeny committed to multiple hematopoietic lineages, thereby generating the numerous blood cell types required during an individual's lifetime. Although hematopoietic growth factors have been shown to support differentiation and survival of HSCs, factors that enhance HSC self-renewal have yet to be identified. Combined effects of growth factors and the Notchpathway have been shown to regulate cell-fate decisions in studies of eye and neural development in invertebrates, and increasing evidence indicates they may perform a similar role in vertebrates.1-3
The Notch pathway regulates cell-fate decisions in a wide variety of cell types, often inhibiting particular differentiation programs and permitting either self-renewal or differentiation along alternate pathways.4,5 Notch receptors and Notch ligands have been found on hematopoietic precursors or marrow stromal cells.2,6-13 Retrovirus-mediated expression of activatedNotch1 enhanced self-renewal and immortalized hematopoietic precursor cells in the context of appropriate cytokines,14,15 indicating Notch's potential capacity for enhancing stem cell self-renewal in nonmutant cells. However, multiple studies have reported only a modest increase in hematopoietic precursor cell numbers following culture with Notch ligand presented on cell surfaces or with engineered Notch ligands in solution.12 16-21 Here, we show that an optimally presented engineered Notch ligand induces an extensive expansion of hematopoietic precursor cell numbers in a similar manner to retroviral-mediated expression of activated Notchconstructs. We show that Notch signaling induced by immobilized engineered Notch ligand Delta1ext-IgG, consisting of the Delta1 extracellular domain fused to the Fc domain of human immunoglobulin G1 (IgG1), induced a multiple-log increase in the number of precursor cells capable of short-term lymphoid and myeloid reconstitution and longer-term T-lymphoid reconstitution in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) recipients.
Methods
Protein purification
Delta-1ext-IgG and ControlIgG were purified from conditioned medium generated by cells from myeloma cell line NSO electroporated with constructs expressing the ligands as described. Delta1ext–IgG– and ControlIgG–conditioned media were purified as previously described.21 Briefly, medium was pumped (1 mL per minute) over a protein G column (HiTrap Protein G, Amersham Pharmacia Biotech, Piscataway, NJ) and the column was washed with 20 mM phosphate, pH 7.0. Bound protein was eluted with 100 mM glycine, pH 2.7, and neutralized with 1M Tris (tris(hydroxymethyl)aminomethane), pH 9.0. To assess purity, proteins were separated using 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie stained.
Hematopoietic cell culture and flow immunocytometry
C57BL/6J (Ly5.2) mice (8-10 weeks old) were from the Jackson Laboratory (Bar Harbor, ME). Lin−Sca-1+c-kit+ cells from bone marrow were enriched using fluorescence-activated cell sorting (FACS) as described.12 In most cases, 1000 Lin−Sca-1+c-kit+ cells were cultured with ligand in a single well of a 12-well plate (Falcon non–tissue culture–treated, Becton Dickinson, Franklin Lakes, NJ). Cells were cultured in growth medium (Iscove modified Dulbecco medium [IMDM] supplemented with 20% fetal bovine serum [FBS] and cytokines murine stem cell factor [(SCF], human Flt-3 ligand, and human interleukin-6 [IL-6], each at 100 ng/mL, and human IL-11 at 10 ng/mL; PeproTech, Rocky Hill, NJ). Cell density was maintained at about 2.5 × 105 cells/cm2 during the experiment. To maintain density, cells were transferred to larger vessels until 14 days, when on average four fifths of the culture was removed and replaced with fresh media twice per week. Ligand was immobilized on the plastic surface of the culture vessel as previously described.21 Briefly, culture wells were incubated with a solution of antihuman IgG antibody (10 μg/mL) (Sigma, St Louis, MO) in phosphate-buffered saline (PBS) for 30 minutes at 37°C, washed with PBS, blocked with Hanks with 2% bovine serum albumin (BSA) for at least 30 minutes, incubated with a solution of ligand (10 μg/mL) in Hanks with 2% BSA medium for 2 hours at 37°C, and washed extensively with PBS.
Flow analysis
Cultured cells were resuspended in PBS containing 2% FBS (PBS/FBS). All reagents for flow analysis were diluted in PBS/FBS. Cells were preincubated with antimouse CD16/CD32 (FcγRII block) for 10 minutes at 4°C and stained with phycoerythrin (PE)–conjugated monoclonal antibodies: Sca-1 (anti–Ly-6A/E), anti–c-kit (clone 2B9), anti-Thy1.2 (clone 53-2.1), anti-CD19 (clone ID3), GR-1 (anti–Ly6-G, clone RB6-8C5), anti-CD25 (PE–IL-2 receptor, α chain, PC61-IgG1; biotin-7D4-IgM), anti-CD4 (clone RM4-5), anti-CD8 (clone 53-6.7), PE-conjugated isotype matched control antibodies (Pharmingen, San Diego, CA), and F4/80 (antimacrophage, Caltag, Burlingame, CA). For simultaneous staining of CD4 and CD8, cells were incubated with biotinylated anti-CD8 and PE-conjugated anti-CD4 and stained cells were detected with Avidin-Red-670 (Gibco-BRL, Grand Island, NY). In all cases, stained cells were washed with PBS/FBS and resuspended in PBS/FBS containing 12.5 μg/mL propidium iodide to gate for dead cells.
Competitive and noncompetitive repopulation assays
Congenic C57BL/6.SJL-Ly5.1-Pep3b (Ly5.1) mice and NOD/SCID mice were bred at the Fred Hutchinson Cancer Research Center (Seattle, WA). All mice were housed in specific pathogen-free conditions and maintained on autoclaved chow ad libitum. Cultured Ly5.2 cells (1.0 to 10.0 × 106) were intravenously injected together with normal Ly5.1 bone marrow cells (1 × 105) in the tail vein of C57BL/6.SJL-Ly5.1-Pep3b recipients that had received a single dose of 10.0-Gy γ-irradiation from a linear accelerator at an exposure rate of 20 cGy per minute on the day before or the day of transplantation. Cultured Ly5.2 cells were injected intravenously into NOD/SCID recipients that had received a sublethal single dose of 3.5-Gy γ-irradiation. Peripheral blood in transplant recipients was obtained from the retro-orbital sinus. Red blood cells were removed with ammonium chloride lysis buffer and remaining nucleated cells were washed in PBS/FBS, preincubated with FcγRII block for 10 minutes at 4°C, and stained with the respective PE-conjugated lineage-specific antibodies as described in “Flow analysis.” To identify Ly5.2+ donors, cells were stained with a biotinylated monoclonal antibody specific for Ly5.2 (clone 104) and Ly5.1 (clone A20; kind gifts from Dr G. Spangrude, University of Utah, Salt Lake City) or biotinylated mouse IgG2a for 30 minutes at 4°C and stained cells were detected with Avidin-Red-670. In all cases, stained cells were washed with PBS/FBS, resuspended in PBS/FBS containing 12.5 μg/mL propidium iodide, and analyzed by BD FACSCalibur (Becton Dickinson, Mountain View, CA).
Results
Notch signaling inhibits myeloid differentiation
Recently we showed that in S20 cells and C2 myoblasts, optimal Notch signaling required immobilization of engineered Notch ligands, such as on a plastic tissue-culture plate (Figure 1A-B).22 To test whether this was also true with hematopoietic precursors, we incubated isolated Lin−Sca-1+c-kit+ cells from C57/Bl6Ly5.2 mice with plastic-bound immobilized Delta1ext-IgG (I-Deltaext-IgG), immobilized ControlIgG (I-ControlIgG), or nonimmobilized Deltaext-IgG(NI-Delta1ext-IgG) as well as growth factors SCF, IL-6, IL-11, and Flt-3l (4GF), which support self-renewal of HSC constitutively expressing activated Notch1.14Delta1ext-IgG consisted of the extracellular domain of the Notch ligand, Delta1, fused to the Fc domain of human IgG1 (Figure 1A). Control molecules consisted of a signal peptide fused to the Fc domain of human IgG1 (Figure 1A; ControlIgG) or purified human IgG1.22
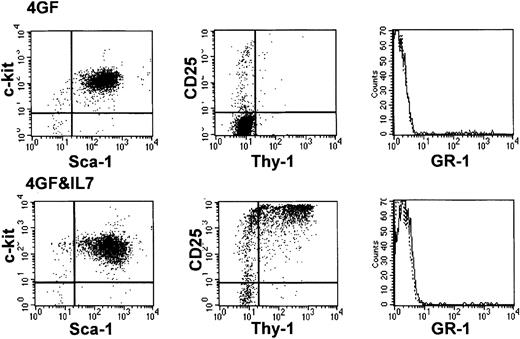
Culture of Lin−Sca-1+c-kit+hematopoietic cells with immobilized Notch ligand, Delta1ext-IgG, and hematopoietic growth factors.
(A) Schematic diagram of engineered Notch ligand consisting of the extracellular domain of Delta1 fused to the Fc portion of human IgG1 (Delta1ext-IgG). Delta1ext-IgG includes the Delta-Serrate-Lag1 (DSL) domain and epidermal growth factor (EGF) repeats. The control molecule consists of a signal peptide fused to the Fc portion of human IgG1 (ControlIgG). (B) Total number of cells generated over time was determined during culture with 4GF and I-Delta1ext-IgG (♦); 4GF, IL-7, and I-Delta1ext-IgG (●); 4GF and I-ControlIgG (▪); 4GF and NI-Delta1ext-IgG(▴). (C-E) Morphology and phenotype of cultured cells. Wright-Giemsa–stained cytospin preparations (far left panels and fluorescence histograms [right panels]) of cells cultured for 14 days with 4GF and I-Delta1ext-IgG (C), I-ControlIgG(D), or NI-Delta1ext-IgG (E). Cells were stained with monoclonal antibodies that recognize Sca-1, c-Kit, GR-1, or CD25 epitopes. Original magnification, × 400. X-axis, log fluorescence intensity; y-axis, cell number; solid line, staining with test antibody; dotted line, staining with isotype-matched control antibody.
Culture of Lin−Sca-1+c-kit+hematopoietic cells with immobilized Notch ligand, Delta1ext-IgG, and hematopoietic growth factors.
(A) Schematic diagram of engineered Notch ligand consisting of the extracellular domain of Delta1 fused to the Fc portion of human IgG1 (Delta1ext-IgG). Delta1ext-IgG includes the Delta-Serrate-Lag1 (DSL) domain and epidermal growth factor (EGF) repeats. The control molecule consists of a signal peptide fused to the Fc portion of human IgG1 (ControlIgG). (B) Total number of cells generated over time was determined during culture with 4GF and I-Delta1ext-IgG (♦); 4GF, IL-7, and I-Delta1ext-IgG (●); 4GF and I-ControlIgG (▪); 4GF and NI-Delta1ext-IgG(▴). (C-E) Morphology and phenotype of cultured cells. Wright-Giemsa–stained cytospin preparations (far left panels and fluorescence histograms [right panels]) of cells cultured for 14 days with 4GF and I-Delta1ext-IgG (C), I-ControlIgG(D), or NI-Delta1ext-IgG (E). Cells were stained with monoclonal antibodies that recognize Sca-1, c-Kit, GR-1, or CD25 epitopes. Original magnification, × 400. X-axis, log fluorescence intensity; y-axis, cell number; solid line, staining with test antibody; dotted line, staining with isotype-matched control antibody.
We found that immobilized, but not soluble, Notch ligand together with 4GF inhibited myeloid differentiation, while allowing self-renewal of precursor cells. At 14 days, a similar increase in cell number was observed in all cultures, whereas after 21 to 28 days, the increase continued only in cultures with I-Delta1ext-IgG (Figure1B). In a typical experiment, 1 × 103 sorted Lin−Sca+ c-kit+ bone marrow cells generated a total of about 3 × 1010 cells at 28 days of culture with 4GF and I-Delta1ext-IgG. At 14 days of culture, cells incubated with I-Delta1ext-IgG resembled blast cells with a “hand mirror” shape, the majority expressed Sca-1 and c-kit, and few expressed the granulocyte-associated antigen GR-1 (Figure 1C). In contrast, cells incubated with either I-ControlIgG or NI-Delta1ext-IgG were indistinguishable and contained mainly differentiated granulocytes or macrophages, consistent with the decreased expression of Sca-1 and c-kit and the increased expression of GR-1 (Figure 1D-E).
At 28 days, most cells incubated with I-Deltaext-IgGcontinued to resemble blast cells (data not shown), and 66.7% ± 17.1% (mean ± SEM) expressed Sca-1 and c-kit in 3 separate experiments (Figure 2 shows a representative experiment). In addition, 17.8% ± 7.1% expressed the low-affinity IL-2 receptor CD25, and 16.7% ± 9.9% expressed GR-1. None of the cells expressed the mature T-cell antigens CD4 or CD8 or the B-cell antigen CD19. In 4 of 6 experiments, cell numbers in cultures incubated with I-Delta1ext-IgG steadily increased for at least 42 days, when the experiments were voluntarily ended (Figure 1B).
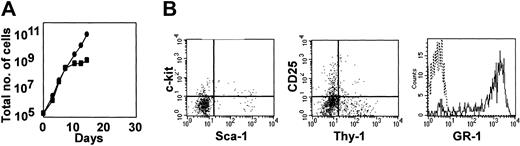
Growth and developmental potential of hematopoietic precursors after culture with I-Deltaext-IgG.
Antigen expression was determined after cells were cultured for 28 days with I-Delta1ext-IgG and 4GF (upper panel) and with I-Delta1ext-IgG, 4GF, and IL-7 (lower panel). Fewer than 0.5% of cells stained with either or both isotype control antibodies.
Growth and developmental potential of hematopoietic precursors after culture with I-Deltaext-IgG.
Antigen expression was determined after cells were cultured for 28 days with I-Delta1ext-IgG and 4GF (upper panel) and with I-Delta1ext-IgG, 4GF, and IL-7 (lower panel). Fewer than 0.5% of cells stained with either or both isotype control antibodies.
Notch signaling promotes early T-cell differentiation
Detection of the CD25 antigen in a subpopulation of cells cultured with I-Delta1ext-IgG suggested that a portion of cells had undergone early T-cell differentiation. After adding IL-7, a cytokine known to support lymphoid cells, we found further indications of early T-cell differentiation. Cell number steadily increased in cultures of Lin−Sca-1+c-kit+ cells containing 4GF, IL-7, and I-Delta1ext-IgG (Figure 2A), and most cells resembled blasts expressing both Sca-1 and c-kit, similar to cultures without IL-7 (Figure 2B; 78.7% ± 9.5% [mean ± SEM of 3 separate experiments]). However, after 21 to 28 days, most cells (72.8% ± 10.4%) expressed CD25, many coexpressed CD25 and Thy1 (Figure 2B), and cytoplasmic CD3ε mRNA was detected by reverse transcriptase–polymerase chain reaction (RT-PCR; data not shown), all indicative of early T-cell differentiation. Few cells (0.9% ± 0.1%) expressed GR-1 (Figure 2B). In contrast, cultures containing 4GF, IL-7, and I-ControlIgG were mainly nonproliferating myeloid cells by 21 to 28 days (data not shown). Cultures with 4GF plus IL-7 and I-Deltaext-IgG contained more viable cells and cell numbers increased at a faster rate than in similar cultures without IL-7 (Figure 2A), presumably reflecting survival of an IL-7–dependent lymphoid subpopulation. In each of 7 experiments, cells in cultures that included IL-7 along with 4GF continued to increase in number after 28 days for up to 42 days, when the experiments were voluntarily ended.
To show that Notch signaling was required for continued generation of Sca-1+c-kit+ precursors, Lin−Sca-1+c-kit+ cells were incubated for 28 days with 4GF, IL-7, and I-Delta1ext-IgGand then transferred to new cultures with the same cytokines but without Notch ligand. Cell number ceased to increase after 5 to 10 days (Figure 3A), and most cells were differentiated granulocytes, as indicated by morphology (data not shown), expression of GR-1, and decreased expression of Sca-1 and c-kit (Figure 3B). In contrast, cells remaining in cultures containing I-Delta1ext-IgG continued to grow and retained their blast morphology and Sca-1, c-kit, and CD25 expression for at least 2 additional weeks, at which time the experiment was ended (data not shown).
Dependence on continued presence of Notch ligand.
Number of cells (A) and antigen expression (B) were determined after Lin−Sca-1+c-kit+ cells incubated for 28 days with 4GF, IL-7, and I-Delta1ext-IgGwere transferred to new cultures and incubated with the same cytokines with (●) or without (▪) I-Deltaext-IgG. Antigen expression was determined 7 days after transferring to new cultures. Fewer than 0.5% of cells stained with either or both isotype control antibodies. Those without Deltaext-IgG are shown.
Dependence on continued presence of Notch ligand.
Number of cells (A) and antigen expression (B) were determined after Lin−Sca-1+c-kit+ cells incubated for 28 days with 4GF, IL-7, and I-Delta1ext-IgGwere transferred to new cultures and incubated with the same cytokines with (●) or without (▪) I-Deltaext-IgG. Antigen expression was determined 7 days after transferring to new cultures. Fewer than 0.5% of cells stained with either or both isotype control antibodies. Those without Deltaext-IgG are shown.
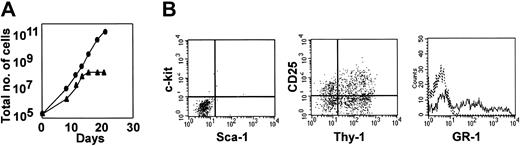
To determine the developmental potential of undifferentiated precursors that had been incubated for 28 days with 4GF and I-Delta1ext-IgG, granulocyte-macrophage colony-stimulating factor (GM-CSF) or IL-7, which support myeloid and lymphoid differentiation, respectively, were added along with 4GF and I-Delta1ext-IgG. Cell numbers steadily increased for up to about 12 days; however, after 15 days, cell numbers stopped increasing in cultures with GM-CSF, but continued to increase steadily in cultures with IL-7 (Figure 4A). The proportion of cells expressing CD25 or both CD25 and Thy-1 was also increased in cultures with IL-7, indicating early T-cell differentiation like that seen when IL-7 was present from the initiation of the culture (Figure2B), and consistent with previous demonstrations that activated Notch-1 permits or promotes T-cell differentiation.23 In contrast, most cells in cultures containing GM-CSF underwent myeloid differentiation, as indicated by morphology (data not shown) and expression of GR-1 (Figure 4B). Thus, cultures of Lin−Sca-1+c-kit+ cells incubated with I-Delta1ext-IgG and 4GF remain multipotential, able to differentiate along either the lymphoid or myeloid lineage depending on the cytokine stimulation.
Developmental potential of cells after culture with Notch ligand and cytokines.
Number of cells (A) and antigen expression (B) were determined after Lin−Sca-1+c-kit+ cells incubated for 28 days with 4GF and I-Delta1ext-IgG were transferred to new cultures containing 4GF, I-Delta1ext-IgG plus IL7 (●) or 4GF, I-Delta1ext-IgG plus GM-CSF (▴). Antigen expression was determined 7 days after transferring to cultures with additional growth factors. Those with GM-CSF are shown. In dot plots, x-axis is log fluorescence intensity after staining with antibodies to Sca-1 or Thy-1; y-axis, log fluorescence intensity after staining with antibodies to c-kit or CD25. Fewer than 0.5% of cells stained with either or both isotype control antibodies. In histograms, x-axis is log fluorescence intensity after staining with GR-1; y-axis, cell number; solid line, staining with GR-1; dotted line, staining with isotype-matched control antibody.
Developmental potential of cells after culture with Notch ligand and cytokines.
Number of cells (A) and antigen expression (B) were determined after Lin−Sca-1+c-kit+ cells incubated for 28 days with 4GF and I-Delta1ext-IgG were transferred to new cultures containing 4GF, I-Delta1ext-IgG plus IL7 (●) or 4GF, I-Delta1ext-IgG plus GM-CSF (▴). Antigen expression was determined 7 days after transferring to cultures with additional growth factors. Those with GM-CSF are shown. In dot plots, x-axis is log fluorescence intensity after staining with antibodies to Sca-1 or Thy-1; y-axis, log fluorescence intensity after staining with antibodies to c-kit or CD25. Fewer than 0.5% of cells stained with either or both isotype control antibodies. In histograms, x-axis is log fluorescence intensity after staining with GR-1; y-axis, cell number; solid line, staining with GR-1; dotted line, staining with isotype-matched control antibody.
Notch signaling increases short-term repopulating cell numbers
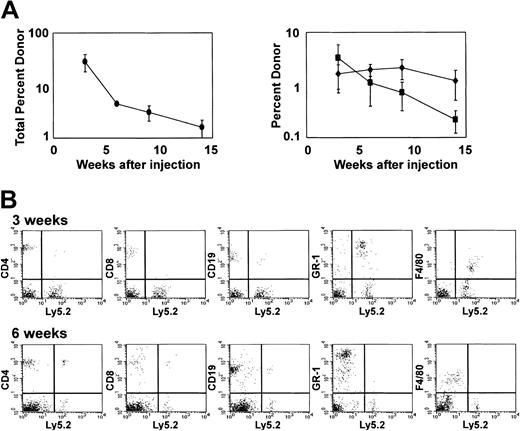
To assess whether Notch signaling affects in vivo repopulating ability, Lin−Sca-1+c-kit+ cells were cultured for 28 days with 4GF, IL-7, and I-Delta1ext-IgG in 3 separate experiments. In these experiments, 1 × 103 cells initially placed in culture generated at least 4 × 1013 cells. We injected 5 to 10 × 106 cultured cells (the progeny of ≤ 0.004 cells initially placed into culture) into each of 4 or 5 lethally irradiated (1000 cGy) congenic mice (C57BL/6.SJL-Ly5.1-Pep3b) along with 105syngeneic Ly 5.1 bone marrow cells. After 3 weeks, peripheral blood contained a mean of 28.6% ± 10.3% Ly5.2 donor cells (mean of 3 experiments; Figure 5A). This level of reconstitution at 3 weeks was comparable to that detected in peripheral blood from mice injected with 100 uncultured Lin−Sca-1+c-kit+ cells (20.8% ± 8.6%; mean of 4 experiments). In mice receiving cells cultured with I-Delta1ext-IgG, most donor cells (91%) were myeloid, expressing lineage markers GR-1 and F4/80, but a portion of cells (9%) expressed the T-cell marker CD4 or CD8 or the B-cell marker CD19 (Figure 5B). At 5 to 6 weeks after injection, recipients of cultured cells contained a reduced percentage of Ly5.2 donor cells in their peripheral blood (4.5% ± 0.3%; mean of 3 experiments), and donor cells were predominantly lymphoid rather than myeloid (Figure5B). However, at 9 weeks, most donor cells detected (80%) were lymphoid, of which nearly all (75%) were T lymphocytes. Similar or decreased reconstitution was found for cells cultured without IL-7 added to 4GF and I-Delta1ext-IgG (data not shown). Thus, Notch ligand in combination with appropriate cytokines induced at least a 4- to 5-log increase in cells capable of short-term repopulation of myeloid and lymphoid lineages.
Short-term reconstitution in competitive repopulation assays.
Competitive repopulation of lethally irradiated C57Bl/6.SJL-Ly5.1-Pep3b mice injected intravenously with 5-10 × 106 Ly5.2 cells cultured for 28 days with I-Delta1ext-IgG, 4GF, and IL7 along with 1 × 105 Ly5.1 competing bone marrow cells. (A) Mean percentage (± SEM) from 3 experiments of blood cells expressing Ly5.2 (left panel) and mean percentage (± SEM) of blood cells expressing both Ly5.2 and CD4 plus CD8 (♦) or both Ly5.2 and CD19 (▪) (right panel). (B) Peripheral blood reconstitution from one representative mouse 3 and 6 weeks after injection. The x-axis is log fluorescence intensity after staining with antibody to Ly5.2; y-axis, log fluorescence intensity after staining with lineage-specific antibody.
Short-term reconstitution in competitive repopulation assays.
Competitive repopulation of lethally irradiated C57Bl/6.SJL-Ly5.1-Pep3b mice injected intravenously with 5-10 × 106 Ly5.2 cells cultured for 28 days with I-Delta1ext-IgG, 4GF, and IL7 along with 1 × 105 Ly5.1 competing bone marrow cells. (A) Mean percentage (± SEM) from 3 experiments of blood cells expressing Ly5.2 (left panel) and mean percentage (± SEM) of blood cells expressing both Ly5.2 and CD4 plus CD8 (♦) or both Ly5.2 and CD19 (▪) (right panel). (B) Peripheral blood reconstitution from one representative mouse 3 and 6 weeks after injection. The x-axis is log fluorescence intensity after staining with antibody to Ly5.2; y-axis, log fluorescence intensity after staining with lineage-specific antibody.
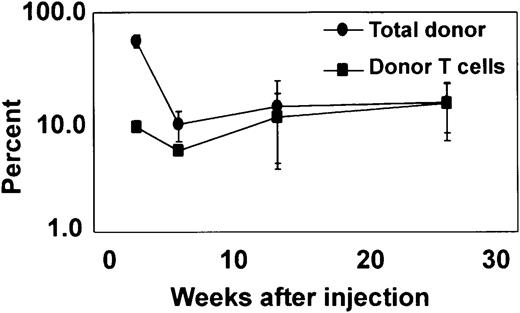
To evaluate longer-term T-cell reconstitution, in 1 experiment representative of 3 similar experiments, Lin−Sca-1+c-kit+ cells were cultured for 35 days with I-Delta1ext-IgG and 4GF plus IL-7 and 1.5 × 106 cells were injected into sublethally (350 cGy) irradiated NOD/SCID recipient mice to avoid possible competing effects of host T cells. After 3 weeks, Ly5.2 donor cells were reconstituted, consisting of myeloid cells expressing lineage markers GR-1 and F4/80 but also T cells expressing CD4 or CD8 and B cells expressing CD19 (Figure 6). After 6 weeks, fewer donor cells were detected, but the percentage of donor cells expressing T-cell markers increased, reaching a peak after 13 weeks that was maintained for at least 25 weeks (Figure 6). Donor T cells analyzed at 14 weeks expressed either CD4 or CD8 and a variety of Vβ types at expected frequencies (data not shown). These results confirm that I-Delta1ext-IgG induces a multilog increase in the number of cells capable of longer-term lymphoid in vivo reconstitution.
Long-term reconstitution of T cells in NOD/SCID recipients.
Repopulation of sublethally irradiated NOD/SCID mice injected intravenously with 1.5 × 106 Ly5.2 cells cultured for 35 days with I-Delta1ext-IgG, 4GF, and IL-7. Mean percentage (± range) from 2 mice of blood cells expressing Ly5.2 (●) and blood cells expressing Ly5.2, CD4, and CD8 (▪).
Long-term reconstitution of T cells in NOD/SCID recipients.
Repopulation of sublethally irradiated NOD/SCID mice injected intravenously with 1.5 × 106 Ly5.2 cells cultured for 35 days with I-Delta1ext-IgG, 4GF, and IL-7. Mean percentage (± range) from 2 mice of blood cells expressing Ly5.2 (●) and blood cells expressing Ly5.2, CD4, and CD8 (▪).
Discussion
These studies indicate that Notch signaling combined with appropriate cytokines regulates cell-fate choices by multipotent hematopoietic precursors. Culture of Lin−Sca-1+c-kit+ cells in combinations consisting of I-Delta1ext-IgG and 4GF with or without IL-7 inhibited myeloid differentiation and generated a 4- to 5-log increase in cells capable of short-term repopulation of lymphoid and myeloid lineages. This is the first report demonstrating that Notch ligand, in this case I-Delta1ext-IgG, induces a multilog expansion of cells with in vivo repopulating ability. Although this substantial expansion is consistent with our previous studies using retroviral-mediated expression of activated Notch, other studies in hematopoiesis have shown minimal effects with Notch ligands on repopulating cells. However, in many of those previous studies, hematopoietic precursors were cocultured with nonhematopoietic cells expressing cell-bound ligand. In those cases, ligand-presenting cells may have generated factors that interfered with the activity of Notch ligand. In other studies, engineered ligand was presented in solution, and in those cases Notch activity may have been only partially induced. This would be consistent with the present studies, where we found no effect with Delta1ext-IgG in solution, and our previous studies, where we found that Notch activation in C2 myoblasts and S20 cells required immobilization of Delta1ext-IgG.
Early T-cell differentiation was promoted in cultures containing I-Delta1ext-IgG that was further enhanced by IL-7. There was no evidence of B-cell differentiation in these cultures, in agreement with recent studies showing that constitutive Notch signaling or Notch signaling induced with the Notch ligand Delta1 inhibits B-cell differentiation presumably from a common lymphoid precursor.23 24 Longer-term T-cell reconstitution was seen in NOD/SCID recipients, but it is unknown whether it derived from longer-term reconstituting stem cells or from postthymic events with mature T cells. Although these studies do not reveal whether Notch signaling leads to early T-lymphoid differentiation by inhibiting a myeloid fate or by directing a lymphoid fate, no cytokine combination has been found to induce T-cell differentiation in vitro in the absence of fetal thymic organ culture, making it likely that Delta1 is promoting the observed lymphoid differentiation.
Terminal myeloid differentiation did occur after the addition of GM-CSF to 4GF with I-Delta1ext-IgG (Table1), indicating that Notch signaling, while able to inhibit myeloid differentiation signals induced by 4GF, did not inhibit differentiation induced by GM-CSF. Alternatively, GM-CSF signaling may inhibit Notch signaling by down-regulating Notch expression or expression of essential molecules in the Notch pathway. Thus, combinatorial signals from Notch and cytokine receptors determine whether multipotent hematopoietic precursors expand in numbers or whether they assume an alternative lymphoid or myeloid fate (Table 1).
I-Delta1ext-IgG and different cytokine combinations induce increases in the number of precursors and/or other final phenotypes
| Combination . | Result . | |||
|---|---|---|---|---|
| Delta 1ext-IgG . | 4GF plus cytokine . | Precursor cells . | Lymphoid . | Myeloid . |
| + | − | +++ | + | + |
| + | IL-7 | +++ | +++ | − |
| + | GM-CSF | − | − | +++ |
| − | − | − | − | +++ |
| − | IL-7 | − | − | +++ |
| Combination . | Result . | |||
|---|---|---|---|---|
| Delta 1ext-IgG . | 4GF plus cytokine . | Precursor cells . | Lymphoid . | Myeloid . |
| + | − | +++ | + | + |
| + | IL-7 | +++ | +++ | − |
| + | GM-CSF | − | − | +++ |
| − | − | − | − | +++ |
| − | IL-7 | − | − | +++ |
+ indicates the presence of a factor or cell type; −; its absence.
The results of this study demonstrate that the combination of Notch and cytokine-induced signaling pathways has the potential to regulate hematopoietic progenitor cell-fate decisions and indicate further that elucidating these signaling pathways in hematopoietic precursors may improve our understanding of and ability to affect stem cell self-renewal. The potential role of Notch in stem cell self-renewal is likely to involve suppression of multiple differentiation pathways by modulating expression of those proteins that can inhibit differentiation. For example, Notch signaling induces Idgene expression.25 Id proteins are dominant-negative inhibitors of E-proteins that are required for expression of proteins involved in differentiation, including B- and T-cell differentiation in hematopoiesis.26 Notch signaling has also been shown to prolong GATA-2 expression, a known inhibitor of myeloid differentiation.27 In addition, Notch signaling also could inhibit multiple differentiation programs by inducing HES-1expression, which, in association with corepressor complexes that include other regulatory molecules, such as groucho, represses transcription of target regulatory genes that generally induce differentiation and/or commitment. HES-1 is thought to inhibitE2A expression, an inducer of gene targets such asEBF and PAX5 involved in B-cell commitment.28 HES-1 has also been shown to interact with AML1 to affect differentiation programs.29 Further clarification of interacting cytokine and Notch signaling pathways may elucidate the mechanisms of stem cell self-renewal, a goal that may be more readily achieved with the ability to grow substantial numbers of cells in the presence of Delta1.
We thank G. Radich, S. Collins, B. Clurman, A. Blau, E. Giniger, and K. Ohishi for criticism of the manuscript. We also thank David Flowers and Cynthia Nourigat for superb technical assistance.
Prepublished online as Blood First Edition Paper, October 31, 2002; DOI 10.1182/blood-2002-06-1862.
Supported by grants P50HL54881 and P30DK56465 from the National Institutes of Health. I.D.B. is also supported as an American Cancer Society–F.M. Kirby Clinical Research Professor.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Irwin D. Bernstein, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D2-373, Seattle, WA 98109; e-mail:ibernste@fhcrc.org.

![Fig. 1. Culture of Lin−Sca-1+c-kit+hematopoietic cells with immobilized Notch ligand, Delta1ext-IgG, and hematopoietic growth factors. / (A) Schematic diagram of engineered Notch ligand consisting of the extracellular domain of Delta1 fused to the Fc portion of human IgG1 (Delta1ext-IgG). Delta1ext-IgG includes the Delta-Serrate-Lag1 (DSL) domain and epidermal growth factor (EGF) repeats. The control molecule consists of a signal peptide fused to the Fc portion of human IgG1 (ControlIgG). (B) Total number of cells generated over time was determined during culture with 4GF and I-Delta1ext-IgG (♦); 4GF, IL-7, and I-Delta1ext-IgG (●); 4GF and I-ControlIgG (▪); 4GF and NI-Delta1ext-IgG(▴). (C-E) Morphology and phenotype of cultured cells. Wright-Giemsa–stained cytospin preparations (far left panels and fluorescence histograms [right panels]) of cells cultured for 14 days with 4GF and I-Delta1ext-IgG (C), I-ControlIgG(D), or NI-Delta1ext-IgG (E). Cells were stained with monoclonal antibodies that recognize Sca-1, c-Kit, GR-1, or CD25 epitopes. Original magnification, × 400. X-axis, log fluorescence intensity; y-axis, cell number; solid line, staining with test antibody; dotted line, staining with isotype-matched control antibody.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/101/5/10.1182_blood-2002-06-1862/3/m_h80533940001.jpeg?Expires=1769094121&Signature=csi541fysbW12Jp2F-pP5e4tFKshQq4i8TYDgT5RE5jKNxvoB7Fa1pHVWCRw9uMlDPDuAEUeHB9gkP9QXDNGnH05igRvwaMB4jWNzLIxOvAPhpIkwgnok7V-do2K73fGr6HnyyiAvrFih1rSpiFztF4eEDoeKg3xmCggzlij2GH0-NlLD30tA8~sz0Sv3PD7VHsrkQn61fuTuMJ6SbLRV6H4pS~38cP-uBxiMQINtkYR7RvnWggNdXx2IiEjAbij9qzvdj1Sg~HxuJS~KHnohrJZbeSE3j3~S27ozHsnZjctIukkugG~fhw2gKWpw5yitSxvb9BoXtXRBMfMdhDcrw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal