Acadesine, 5-aminoimidazole-4-carboxamide (AICA) riboside, induced apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) cells in all samples tested (n = 70). The half-maximal effective concentration (EC50) for B-CLL cells was 380 ± 60 μM (n = 5). The caspase inhibitor Z-VAD.fmk completely blocked acadesine-induced apoptosis, which involved the activation of caspase-3, -8, and -9 and cytochrome c release. Incubation of B-CLL cells with acadesine induced the phosphorylation of adenosine monophosphate-activated protein kinase (AMPK), indicating that it is activated by acadesine. Nitrobenzylthioinosine (NBTI), a nucleoside transport inhibitor, 5-iodotubercidin, an inhibitor of adenosine kinase, and adenosine completely inhibited acadesine-induced apoptosis and AMPK phosphorylation, demonstrating that incorporation of acadesine into the cell and its subsequent phosphorylation to AICA ribotide (ZMP) are necessary to induce apoptosis. Inhibitors of protein kinase A and mitogen-activated protein kinases did not protect from acadesine-induced apoptosis in B-CLL cells. Moreover, acadesine had no effect on p53 levels or phosphorylation, suggesting a p53-independent mechanism in apoptosis triggering. Normal B lymphocytes were as sensitive as B-CLL cells to acadesine-induced apoptosis. However, T cells from patients with B-CLL were only slightly affected by acadesine at doses up to 4 mM. AMPK phosphorylation did not occur in T cells treated with acadesine. Intracellular levels of ZMP were higher in B-CLL cells than in T cells when both were treated with 0.5 mM acadesine, suggesting that ZMP accumulation is necessary to activate AMPK and induce apoptosis. These results suggest a new pathway involving AMPK in the control of apoptosis in B-CLL cells and raise the possibility of using acadesine in B-CLL treatment.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is characterized by the accumulation of long-lived B lymphocytes.1,2 Most of the circulating cells appear to be nondividing and the clonal excess of B cells is mainly caused by defects that prevent programmed cell death rather than by alterations in cell cycle regulation.3 Glucocorticoids and other chemotherapeutic agents used clinically, including the nucleoside analogues 2-chloro-2′-deoxyadenosine and fludarabine, induce apoptosis in B-CLL lymphocytes,4-8 suggesting that apoptosis is the mechanism of their therapeutic action. Several signaling pathways regulate apoptosis induced by chemotherapy. Thus, we recently demonstrated that phosphatidylinositol 3-kinase and protein kinase C play important roles in the survival of B-CLL cells. Furthermore, inhibition of these kinases increases glucocorticoid- and fludarabine-induced apoptosis ex vivo in the presence of survival factors.9
The precursor of nucleotide biosynthesis acadesine or 5-aminoimidazole-4-carboxamide (AICA) riboside has various effects in several types of eukaryotic cells. These effects include inhibition of growth and depletion of pyrimidine nucleotide pools in fibroblasts,10,11 accelerated repletion of purine nucleotide pools in heart,12 reduction of endurance in skeletal muscle,13 inhibition of fatty acid, sterol synthesis, and gluconeogenesis in hepatocytes, and increase in glucose uptake in muscle.14 Acadesine is phosphorylated to AICA ribotide (ZMP), which mimics 5′-adenosine monophosphate (AMP) and activates both AMP-activated protein kinase (AMPK) and AMPK kinase (AMPKK).14,15 The effects on glucose and lipid metabolism are mediated through activation of the AMPK cascade.14
Previous studies report that acadesine inhibits glucocorticoid-induced apoptosis in quiescent thymocytes,16 apoptosis caused by serum deprivation in fibroblasts overproducing fructose 2,6-bisphosphate,17 and ceramide-induced apoptosis in primary astrocytes.18 To contribute to the understanding of glucocorticoid-induced apoptosis in B-CLL cells, we attempted to block apoptosis with acadesine and, surprisingly, we found that acadesine induced apoptosis in B-CLL cells, whereas T cells from these patients were not affected. Here we study the mechanism of acadesine-induced apoptosis and propose a new pathway involving AMPK and AMPKK in the control of apoptosis in B-CLL cells.
Patients, materials, and methods
Patients with B-CLL and cell isolation
Seventy samples from patients with B-CLL who had not received treatment in the previous 6 months were studied. B-CLL was diagnosed according to standard clinical and laboratory criteria. Cells were obtained from the Hospital Clinic, Barcelona, Spain. Written informed consent was obtained from all patients in accordance with Hospital Clinic Ethical Committee. Mononuclear cells from peripheral blood samples were isolated by centrifugation on a Ficoll-Hypaque (Seromed, Berlin, Germany) gradient and cryopreserved in liquid nitrogen in the presence of 10% dimethyl sulfoxide (DMSO).
T-cell isolation
Blood samples from 4 healthy donors were obtained from the Hospital Clinic. Mononuclear cells were separated on a Ficoll-Hypaque gradient. After separation, T cells were isolated using 2-amino-ethylisothiouronium bromide hydrobromide (AET)–activated sheep red blood cells (SRBCs). 2 mL SRBC were washed in PBS and resuspended thoroughly in 10 mL of freshly made 2% (wt/vol) AET pH 8.0. The suspension was incubated at 37°C for 30 minutes, washed 5 times in saline solution, and resuspended in RPMI 1640 culture medium (Biological Industries, Beit Haemek, Israel) containing 40% heat-inactivated fetal calf serum (Gibco BRL, Paisley, United Kingdom), to obtain a solution of 1% AET-treated SRBCs. T cells were isolated from 3 mL of the mononuclear cell suspension by mixing with an equal volume of the 1% AET-treated SRBC solution previously prepared. The mixed solution was incubated at 37°C for 10 minutes, centrifuged at 300g for 5 minutes, and maintained for 12 hours at 4°C. The pellet was gently resuspended and SBRC-bound T cells were purified in a second Ficoll-Hypaque gradient and washed. SRBCs were then lysed by 5 minutes of incubation with 0.15 M NH4Cl, washed in saline buffer, and centrifuged at 500g.
Reagents
Acadesine (5-aminoimidazole-4-carboxamide [AICA] riboside) was obtained from Toronto Research Chemicals (North York, ON, Canada). Adenosine, nitrobenzylthioinosine (NBTI), doxorubicin, oligomycin, DMSO, and propidium iodide (PI) were from Sigma Chemicals (St Louis, MO). 5-Iodotubercidin was from Biomol Research Labs (Plymouth Meeting, PA). Annexin V–fluorescein isothiocyanate (FITC) was from Bender MedSystems (Vienna, Austria). Z-VAD.fmk (benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone) was from Bachem (Bubendorf, Switzerland). SB 220025, U0126, and H-89 were from Calbiochem (Darmstadt, Germany). SP 600125 was from Tocris (Bristol, United Kingdom).
Cell culture
Lymphocytes were cultured immediately after thawing or isolation at a concentration of 2 to 5 × 106 cells/mL in RPMI 1640 culture medium supplemented with 10% heat-inactivated fetal calf serum, 1% glutamine, and 1% penicillin-streptomycin at 37°C in a humidified atmosphere containing 5% carbon dioxide.
Analysis of apoptosis by flow cytometry
Apoptosis was measured by annexin V binding. Exposure of phosphatidylserine was quantified by surface annexin V staining as previously described.19 To analyze apoptosis in T cells from the samples, 500 000 cells were incubated for 24 hours with the indicated factors. Cells were then washed in PBS, and incubated in 50 μL annexin-binding buffer with allophycocyanin (APC)–conjugated anti-CD3 and phycoerythrin (PE)–conjugated anti-CD19 for 10 minutes in the dark. Cells were then diluted with annexin-binding buffer to a volume of 250 μL and incubated with 0.5 μL annexin V–FITC for 15 minutes in the dark. Then, 250 μL annexin-binding buffer and 5 μL PI were added just before flow cytometric analysis. Data were analyzed using Cell Quest software (Becton Dickinson, Mountain View, CA).
Western blot analysis
Cells were lysed with Laemmli sample buffer or radioimmunoprecipitation assay (RIPA) extraction buffer and Western blot analysis was performed as described previously20 using the following antibodies: polyclonal antiphospho-AMPK (Thr172), polyclonal antiphospho-p53 (Ser15) polyclonal anticleaved caspase-9 (Cell Signaling Technology, Beverly, MA), monoclonal anti-p53 Ab-5 (Neomarkers, Fremont, CA), polyclonal anticaspase-3 (Transduction Laboratories, Lexington, KY), and monoclonal anticaspase-8 (Cell Diagnostica, Münster, Germany). Antibody binding was detected using a secondary antibody conjugated to horseradish peroxidase and the enhanced chemiluminescence (ECL) detection system (Amersham, Buckinghamshire, United Kingdom).
Cytochrome c release measurements
Release of cytochrome c from mitochondria to cytosol was measured by Western blot as previously described21with some modifications. Cells (3 × 107) were harvested, washed once, and gently lysed in 150 μL ice-cold lysis buffer (250 mM sucrose, 1 mM EDTA [ethylenediaminetetraacetic acid], 0.1% digitonin, 25 mM Tris [tris(hydroxymethyl)aminomethane], pH 6.8, 1 mM dithiothreitol, 1 μg/mL leupeptin, 1 μg/mL pepstatin, 1 μg/mL aprotinin, 1 mM benzamidine, and 0.1 mM phenylmethylsulfonyl fluoride). Lysates were centrifuged at 12 000g at 4°C for 3 minutes to obtain the fractions. Mitochondrial and cytosolic fractions were lysed with sample buffer and electrophoresed on a 15% polyacrylamide gel and then analyzed by Western blot using monoclonal antibodies against cytochrome c, clone 7H8.2C12 (Pharmingen, San Diego, CA), or cytochrome oxidase subunit II (Molecular Probes, Eugene, OR).
Analysis of intracellular nucleotides by HPLC
Cells (2 × 107) were used for nucleotide analysis. After incubation, cells were harvested by centrifugation, washed twice with PBS, and again centrifuged. Supernatant was removed, and the nucleotides were extracted from cell pellet with 300 μL ice-cold 10% perchloric acid. The extract was left on ice for 15 minutes, mixed, and subsequently centrifuged at 12 000g for 5 minutes at 4°C. The supernatant was separated from the protein pellet, neutralized with 5.0 M potassium carbonate, and filtered with Ultrafree-MC Centrifugal Filter Units (NMWL 10 000; Millipore, Bedford, MA) by centrifugation at 12 000g for 30 minutes at 4°C. Neutralized extracts were frozen and stored at −80°C until high-performance liquid chromatography (HPLC) analysis was performed. Samples were centrifuged at 12 000g for 10 minutes to eliminate salts just before analysis. Nucleotide analyses were carried out as follows. A 40-μL sample was injected onto a Spherisorb 5 SAX anion exchange column (2.5 × 4.6 mm; Traces, Teknokroma, Spain) using a gradient of 5 mM NH4H2PO4, pH 2.5 (buffer A) and 500 mM NH4H2PO4, pH 3.9 (buffer B) at a flow rate of 1 mL/min. A linear gradient was developed over 40 minutes at 0% buffer B to 100% buffer B. An LKB Model 2152 high-pressure liquid chromatograph (Bromma, Sweden) equipped with a Kontron 432 detector (Amersham, Uppsala, Sweden) was used. The various peaks in the extracts were identified by comparison for retention times with known external standards and the relative absorbance at 260 nm. The following nucleotides were routinely quantified in the extracts: adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), ZMP, guanosine triphosphate (GTP), guanosine diphosphate (GDP), and guanosine monophosphate (GMP). The results were expressed as nmol/107 cells.
Results
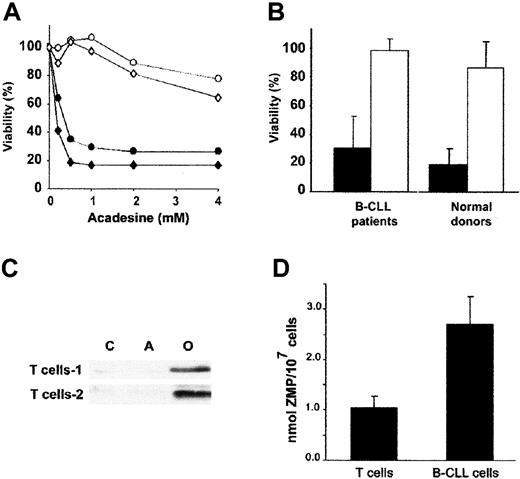
Acadesine induces apoptosis in B-CLL cells
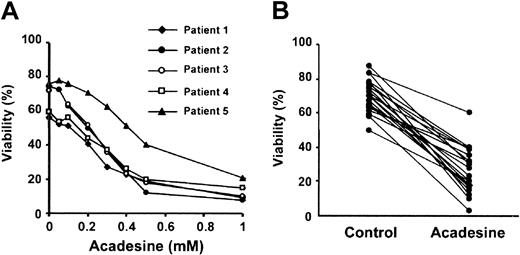
Because acadesine (1-2 mM) blocks glucocorticoid-induced apoptosis of quiescent thymocytes,16 we examined whether glucocorticoid-induced apoptosis in B-CLL cells was also blocked by acadesine. We studied the effect of several doses of acadesine, ranging from 50 μM to 1 mM, on the viability of B-CLL cells. Unexpectedly, acadesine induced apoptosis in a dose-dependent manner (Figure1A) and the half-maximal effective concentration (EC50) was 380 ± 60 μM (n = 5). All the patients were sensitive to acadesine and the viability after incubation with 0.5 mM acadesine for 24 hours decreased from 68% ± 12 % to 26% ± 12% (n = 70). Figure 1B shows the effect of 0.5 mM acadesine on the viability of B-CLL cells from 20 representative patients. The effect of acadesine on the viability of B-CLL cells was the same when cells were isolated and treated in cell culture or treated in the whole blood sample and then isolated, thus indicating that blood components do not affect acadesine-induced apoptosis (data not shown).
Cytotoxic effect of acadesine on B-CLL cells.
(A) Dose-response of acadesine on B-CLL cells. Cells from 5 patients were incubated for 24 hours with various doses of acadesine as indicated. (B) Cells from 70 patients were incubated for 24 hours with or without 0.5 mM acadesine. Viability was measured by analysis of phosphatidylserine exposure and PI uptake as described in “Patients, materials, and methods” and it is expressed as the percentage of nonapoptotic cells. The figure shows results from 20 representative patients.
Cytotoxic effect of acadesine on B-CLL cells.
(A) Dose-response of acadesine on B-CLL cells. Cells from 5 patients were incubated for 24 hours with various doses of acadesine as indicated. (B) Cells from 70 patients were incubated for 24 hours with or without 0.5 mM acadesine. Viability was measured by analysis of phosphatidylserine exposure and PI uptake as described in “Patients, materials, and methods” and it is expressed as the percentage of nonapoptotic cells. The figure shows results from 20 representative patients.
Acadesine induced caspase activation and cytochrome crelease from mitochondria
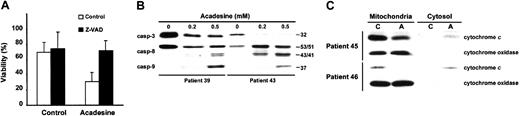
We examined the role of caspases and the mitochondrial pathway in acadesine-induced apoptosis. When B-CLL cells were incubated with acadesine in the presence of the caspase inhibitor Z-VAD.fmk (200 μM) for 24 hours, apoptosis was completely blocked (Figure2A). Furthermore, acadesine induced proteolysis and activation of caspase-3 as measured by the disappearance of procaspase-3 (Figure 2B). To study the mechanism leading to activation of caspase-3, we analyzed the effect of acadesine on the activation of caspase-8 and -9. Acadesine induced processing of caspase-8 and -9 in B-CLL cells, as shown by the appearance of their intermediate cleavage products (43/41 kDa and 37 kDa, respectively).
Acadesine induces caspase processing and mitochondrial cytochrome
c release. (A) Z-VAD.fmk protects cells from acadesine-induced apoptosis. B-CLL cells were preincubated for 1 hour with 200 μM Z-VAD.fmk and 0.5 mM acadesine was added for 24 hours. Viability was measured by analysis of phosphatidylserine exposure and PI uptake as described in “Patients, materials, and methods.” Data are shown as the mean value ± SD of 4 patients. (B) Analysis of the processing of caspase-3, -8, and -9. B-CLL cells were treated with 0.2 or 0.5 mM acadesine for 24 hours. Cells were lysed and analyzed by Western blot as described in “Patients, materials, and methods.” The migration positions of the precursor forms of caspase-3 (32 kDa) and caspase-8 (53/51 kDa) and the cleavage products of caspase-8 (43/41 kDa) and caspase-9 (37 kDa) are indicated. (C) Analysis of cytochrome c release. B-CLL cells were treated with 0.5 mM acadesine for 24 hours and mitochondrial and cytosolic extracts were analyzed by Western blot. Cytochrome oxidase subunit II was analyzed as a control for mitochondrial protein loading. C indicates control; A, acadesine.
Acadesine induces caspase processing and mitochondrial cytochrome
c release. (A) Z-VAD.fmk protects cells from acadesine-induced apoptosis. B-CLL cells were preincubated for 1 hour with 200 μM Z-VAD.fmk and 0.5 mM acadesine was added for 24 hours. Viability was measured by analysis of phosphatidylserine exposure and PI uptake as described in “Patients, materials, and methods.” Data are shown as the mean value ± SD of 4 patients. (B) Analysis of the processing of caspase-3, -8, and -9. B-CLL cells were treated with 0.2 or 0.5 mM acadesine for 24 hours. Cells were lysed and analyzed by Western blot as described in “Patients, materials, and methods.” The migration positions of the precursor forms of caspase-3 (32 kDa) and caspase-8 (53/51 kDa) and the cleavage products of caspase-8 (43/41 kDa) and caspase-9 (37 kDa) are indicated. (C) Analysis of cytochrome c release. B-CLL cells were treated with 0.5 mM acadesine for 24 hours and mitochondrial and cytosolic extracts were analyzed by Western blot. Cytochrome oxidase subunit II was analyzed as a control for mitochondrial protein loading. C indicates control; A, acadesine.
To analyze the involvement of cytochrome c release in acadesine-induced apoptosis, cytosolic and mitochondrial fractions were obtained and the presence of cytochrome c was analyzed by Western blot. As shown in Figure 2C, acadesine produced a decrease of cytochrome c in the mitochondrial fraction and an increase in the cytosolic fraction of B-CLL cells, demonstrating that acadesine induced cytochrome c release.
Uptake and phosphorylation of acadesine are required to induce apoptosis and activate AMPK
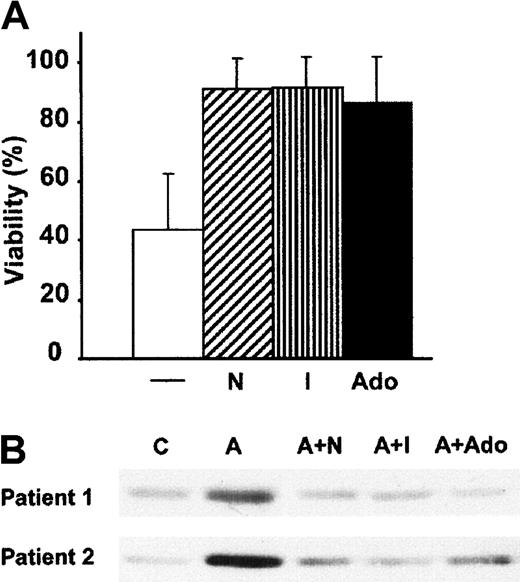
Next, we analyzed whether the apoptotic effect of acadesine was dependent on acadesine uptake. We found that 10 nM NBTI, a nucleoside transport inhibitor,22 completely inhibited acadesine-induced apoptosis in B-CLL cells (Figure3A). This result indicates that the acadesine uptake through NBTI-sensitive nucleoside transporters is necessary for its apoptotic effect. Inside the cell, acadesine is phosphorylated probably by adenosine kinase11 and then metabolized through different pathways. When B-CLL cells were incubated with 0.2 μM 5-iodotubercidin, an inhibitor of adenosine kinase,23 the apoptotic effect of 0.5 mM acadesine was blocked (Figure 3A). The intracellular accumulation of acadesine can increase the release of adenosine into the extracellular space,24 and it has been reported that adenosine induces apoptosis of B-CLL cells.25 However, addition of adenosine at equimolar concentrations did not induce apoptosis in B-CLL cells, but, on the contrary, protected them from acadesine-induced apoptosis (Figure 3A). HPLC analysis showed that NBTI, 5-iodotubercidine, and adenosine blocked intracellular ZMP accumulation in B-CLL cells treated with 0.5 mM acadesine for 3 hours (data not shown).
NBTI, 5-iodotubercidin, and adenosine protect from acadesine-induced apoptosis and AMPK phosphorylation.
(A) B-CLL cells were incubated for 24 hours with 0.5 mM acadesine alone (−) or combined with 10 nM NBTI (N), 0.2 μM 5-iodotubercidin (I), or 0.5 mM adenosine (Ado). Viability was measured as described in “Patients, materials, and methods” and expressed as the percentage of the viability of nontreated cells. Data are shown as the mean value ± SD of 10 patients. (B) B-CLL cells were incubated with 0.5 mM acadesine (indicated as “A”) in the presence or absence of 10 nM NBTI, 0.2 μM 5-iodotubercidine, and 0.5 mM adenosine for 3 hours, and whole-cell extracts were analyzed by Western blot with an antibody against phospho-AMPK (Thr172).
NBTI, 5-iodotubercidin, and adenosine protect from acadesine-induced apoptosis and AMPK phosphorylation.
(A) B-CLL cells were incubated for 24 hours with 0.5 mM acadesine alone (−) or combined with 10 nM NBTI (N), 0.2 μM 5-iodotubercidin (I), or 0.5 mM adenosine (Ado). Viability was measured as described in “Patients, materials, and methods” and expressed as the percentage of the viability of nontreated cells. Data are shown as the mean value ± SD of 10 patients. (B) B-CLL cells were incubated with 0.5 mM acadesine (indicated as “A”) in the presence or absence of 10 nM NBTI, 0.2 μM 5-iodotubercidine, and 0.5 mM adenosine for 3 hours, and whole-cell extracts were analyzed by Western blot with an antibody against phospho-AMPK (Thr172).
ZMP activates AMPK by direct activation via an allosteric AMP site and by promoting its phosphorylation at Thr172.15,26 Thus, we assessed whether AMPK was activated in acadesine-treated B-CLL cells. AMPK is activated by phosphorylation at Thr172 by the upstream AMPKK raising the activity of AMPK at least 50-fold.27Therefore, we analyzed the activation of AMPK using a specific antibody against the phosphorylated form of AMPK (Thr172). As seen in Figure 3B, treatment of B-CLL cells with 0.5 mM acadesine for 3 hours induced AMPK phosphorylation. This phosphorylation was maintained at least during 18 hours (data not shown). NBTI, 5-iodotubercidin, and adenosine inhibited acadesine-induced AMPK phosphorylation (Figure 3B).
Acadesine-induced apoptosis is independent of PKA, ERK, JNK, and p38 MAPK
Because protein kinase A (PKA) and mitogen-activated protein kinases (MAPKs) have been involved in the apoptosis of B-CLL cells,28 29 we studied their implication in acadesine-induced apoptosis using selective inhibitors.
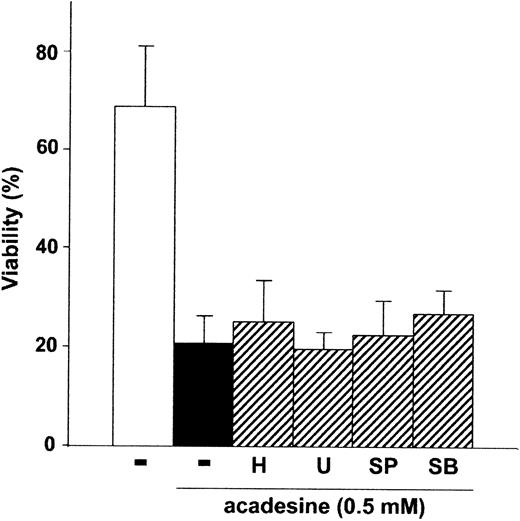
We analyzed the effect of the PKA inhibitor H-8930 in acadesine-induced apoptosis. As shown in Figure4, H-89 did not affect acadesine-induced apoptosis. To analyze the effect of ERK and JNK in acadesine-induced apoptosis, we used the MEK inhibitor U012630 and the JNK inhibitor SP600125.31 As shown in Figure 4, neither U0126 nor SP600125 protected B-CLL cells from acadesine-induced apoptosis. Moreover, none of these kinase inhibitors blocked AMPK phosphorylation induced by acadesine in B-CLL cells (data not shown).
Acadesine-induced apoptosis is independent of PKA, ERK, JNK, and p38 MAPK.
Cells from B-CLL patients were untreated or treated with 0.5 mM acadesine in the absence or presence of 5 μM H-89 (H), 10 μM SP 600125 (SP), 10 μM U0126 (U), or 10 μM SB 220025 (SB), for 24 hours. All inhibitors were preincubated for 1 hour before the addition of acadesine. Viability was measured as described in “Patients, materials, and methods.” Data are shown as the mean value ± SD of 5 patients.
Acadesine-induced apoptosis is independent of PKA, ERK, JNK, and p38 MAPK.
Cells from B-CLL patients were untreated or treated with 0.5 mM acadesine in the absence or presence of 5 μM H-89 (H), 10 μM SP 600125 (SP), 10 μM U0126 (U), or 10 μM SB 220025 (SB), for 24 hours. All inhibitors were preincubated for 1 hour before the addition of acadesine. Viability was measured as described in “Patients, materials, and methods.” Data are shown as the mean value ± SD of 5 patients.
Next, we studied the effect of two p38 MAPK inhibitors, SB203580 and PD169316, in acadesine-induced apoptosis. SB203580 and PD169316 protected B-CLL cells from acadesine-induced apoptosis and AMPK phosphorylation, at doses used to inhibit p38 MAPK. However, intracellular accumulation of ZMP was also abrogated by these p38 MAPK inhibitors (data not shown), as it has been recently reported.32 These results indicate that both inhibitors were interfering with acadesine uptake or phosphorylation to ZMP. Next, we analyzed the effect of SB220025, a p38 MAPK-inhibitor that does not interfere with nucleoside transport33 or ZMP accumulation (data not shown). As shown in Figure 4, SB220025 did not protect B-CLL cells from acadesine-induced apoptosis thus indicating that p38 MAPK is not implicated in its apoptotic effect.
Taken together, these results indicate that PKA, ERK, JNK, and p38 MAPK are not involved in acadesine-induced apoptosis in B-CLL cells.
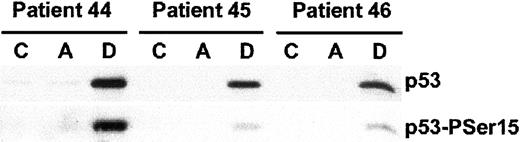
Acadesine does not induce p53 phosphorylation or accumulation
The p53 tumor suppressor protein plays a major role in the cellular response to a wide range of chemotherapeutic drugs.34 DNA damage induces p53 phosphorylation at several residues including Ser15,35 which in turn promotes both the accumulation and functional activation of p53. Very recently, it has been described that acadesine may regulate cell growth via p53 phosphorylation at Ser15, suggesting that AMPK is involved in regulating p53 function.36 To study the role of p53 in acadesine-induced apoptosis we analyzed the effect of this compound on p53 protein levels and p53 phosphorylation at Ser15. When B-CLL cells were incubated with 0.5 mM acadesine there was no effect on p53 levels or p53 phosphorylation (Figure 5). As a control of p53 activation we used doxorubicin (0.8 μM), which induced p53 protein accumulation and phosphorylation. These data demonstrate that acadesine induces apoptosis of B-CLL cells in a p53-independent manner.
Acadesine does not induce p53 accumulation or phosphorylation.
Cells from B-CLL patients were untreated (C), or treated with 0.5 mM acadesine (A) or 0.8 μM doxorubicin (D) for 24 hours. Cells were lysed and analyzed by Western blot as described in “Patients, materials, and methods.” Total levels of p53 and p53 phosphorylated at Ser15 were analyzed. Results of 3 representative patients of 5 analyzed are shown.
Acadesine does not induce p53 accumulation or phosphorylation.
Cells from B-CLL patients were untreated (C), or treated with 0.5 mM acadesine (A) or 0.8 μM doxorubicin (D) for 24 hours. Cells were lysed and analyzed by Western blot as described in “Patients, materials, and methods.” Total levels of p53 and p53 phosphorylated at Ser15 were analyzed. Results of 3 representative patients of 5 analyzed are shown.
Differential effect of acadesine on B and T cells
To analyze whether the induction of apoptosis in B-CLL cells was selective to B population, we analyzed the number of apoptotic T cells (CD3+ cells) in 4 B-CLL blood samples treated with several doses of acadesine. Figure 6A shows the results corresponding to 2 representative patients. T cells from B-CLL patients were resistant to acadesine-induced apoptosis at doses up to 1 mM. Higher concentrations of acadesine (2-4 mM) only slightly affected the viability of T cells. Furthermore, when lymphocytes from B-CLL patients (n = 18) and healthy donors (n = 4) were incubated with 0.5 mM acadesine for 24 hours, viability was markedly reduced in B cells but not in T cells (Figure 6B). These results indicate that B cells are much more sensitive than T cells to acadesine-induced apoptosis.
Differential effect of acadesine on B and T cells.
(A) Dose-response of the cytotoxic effect of acadesine on B and T cells from B-CLL patients. Cells were incubated with a range of doses of acadesine (up to 4 mM) for 24 hours. Viability was measured as nonapoptotic CD3+/CD19− T cells (○,⋄) or CD3−/CD19+ B cells (●,♦) as described in “Patients, materials, and methods.” Two representative patients of 4 analyzed are shown. Viability is expressed as the percentage of the viability of nontreated cells. (B) Comparison between the induction of apoptosis in B and T cells (black bars and white bars, respectively) from B-CLL patients and healthy donors. Cells from 18 patients and 4 healthy donors were incubated with 0.5 mM acadesine for 24 hours. Viability is expressed as the mean value ± SD. (C) T cells from healthy donors were cultured with or without 0.5 mM acadesine for 3 hours and whole cell extracts were analyzed by Western blot with an antibody against phospho-AMPK (Thr172). As a control for AMPK activation, cells were incubated with 10 μM oligomycin for 3 hours in glucose-free medium. (D) Cells were cultured with or without 0.5 mM acadesine for 3 hours and intracellular levels of ZMP were measured by HPLC, as described in “Patients, materials, and methods.” Mean values ± SD from 5 B-CLL samples and 3 T-cell samples are shown.
Differential effect of acadesine on B and T cells.
(A) Dose-response of the cytotoxic effect of acadesine on B and T cells from B-CLL patients. Cells were incubated with a range of doses of acadesine (up to 4 mM) for 24 hours. Viability was measured as nonapoptotic CD3+/CD19− T cells (○,⋄) or CD3−/CD19+ B cells (●,♦) as described in “Patients, materials, and methods.” Two representative patients of 4 analyzed are shown. Viability is expressed as the percentage of the viability of nontreated cells. (B) Comparison between the induction of apoptosis in B and T cells (black bars and white bars, respectively) from B-CLL patients and healthy donors. Cells from 18 patients and 4 healthy donors were incubated with 0.5 mM acadesine for 24 hours. Viability is expressed as the mean value ± SD. (C) T cells from healthy donors were cultured with or without 0.5 mM acadesine for 3 hours and whole cell extracts were analyzed by Western blot with an antibody against phospho-AMPK (Thr172). As a control for AMPK activation, cells were incubated with 10 μM oligomycin for 3 hours in glucose-free medium. (D) Cells were cultured with or without 0.5 mM acadesine for 3 hours and intracellular levels of ZMP were measured by HPLC, as described in “Patients, materials, and methods.” Mean values ± SD from 5 B-CLL samples and 3 T-cell samples are shown.
Then, we analyzed AMPK phosphorylation in T cells obtained from healthy donors. AMPK is not phosphorylated in normal T cells treated with 0.5 mM acadesine for 3 hours (Figure 6C). As a control of AMPK phosphorylation, T cells were treated with 10 μM oligomycin in glucose-free medium for 3 hours, a condition that activates AMPK.37 Next, we measured intracellular ZMP levels by HPLC in B-CLL cells and T cells treated with 0.5 mM acadesine. As shown in Figure 6D, ZMP reached 2.7 ± 0.5 nmol/107 cells for B-CLL cells (n = 6), and 1.1 ± 0.2 nmol/107 cells for T cells (n = 3).
Discussion
The present study shows that acadesine induces apoptosis in B-CLL cells. These results were unexpected, because acadesine inhibits glucocorticoid-induced apoptosis in quiescent thymocytes,16 apoptosis caused by serum deprivation in fibroblasts overproducing fructose 2,6-bisphosphate,17 and ceramide-induced apoptosis in primary astrocytes.18 In agreement with our results, recently it has been reported that acadesine causes apoptosis in 2 neuroblastoma cell lines38and a hepatoma cell line.39 These results indicate that acadesine can either induce or inhibit apoptosis depending on the cell type.
Mitochondria-mediated apoptosis occurs in response to a wide range of death stimuli, including chemotherapeutic agents.40 In the mitochondrial pathway, cytochrome c and other apoptogenic proteins are released from the mitochondrial intermembrane space to the cytosol. Once released, cytochrome c binds to Apaf-1 and induces activation of caspase-9.41 Our results demonstrate that acadesine induces release of cytochrome c from mitochondria triggering caspase-9 activation and apoptosis in B-CLL cells. The activation of caspase-8 could be explained by a positive loop involving caspase-9, caspase-3, and caspase-6, as described in other cells,42 although the involvement of caspases upstream the mitochondria cannot be discarded.
The transporters of acadesine into the cell have not been studied previously. One inhibitor of nucleoside transport, NBTI, blocked acadesine-induced apoptosis, demonstrating that uptake of acadesine through NBTI-sensitive transporters is a necessary step for its apoptotic effect. There are at least 7 different systems responsible for nucleoside transport in mammalian cells: 2 equilibrative and 5 concentrative Na+-dependent transporters. One equilibrative (es) and one concentrative (N5) transport system can be inhibited by nanomolar concentrations of NBTI.22Interestingly, this concentrative transporter is expressed in NB4 leukemia cells43 and human B-cell lines.44Nucleoside transport activity was found in B-CLL cells45and, recently, the expression of the 5 cloned nucleoside transporters in B-CLL cells has been studied.46 Further studies are necessary to characterize the acadesine transporters in B and T cells. This might be therapeutically relevant because a more active nucleoside transport in B cells than in T cells could explain the differential sensitivity to acadesine.
The necessary transport of acadesine into the cell rules out the possibility that this compound induces apoptosis through binding to purine receptors, which induce death in various cell types.47 The intracellular accumulation of acadesine leads to increased release of adenosine into the extracellular space.24 Adenosine can induce apoptosis in several cells including B-CLL cells.25 However, we rule out extracellular adenosine accumulation as the mechanism of acadesine-induced apoptosis, because addition of equimolar concentrations of adenosine to B-CLL cells did not induce apoptosis. Indeed, it protected them from acadesine-induced apoptosis. The effect of adenosine could be explained by competition with acadesine transport, but also by competition with an intracellular metabolizing enzyme or target.
An initial step in the metabolism of acadesine is its conversion into ZMP by a nucleoside kinase, probably adenosine kinase.11This step seems to be essential for acadesine-induced apoptosis because inhibition of adenosine kinase by 5-iodotubercidin protects the cells. Whether accumulation of ZMP, or a metabolite derived from ZMP, is the cause of acadesine-induced apoptosis is unknown. ZMP activates AMPK and AMPKK.15,26 Once activated, AMPKK phosphorylates AMPK in Thr172 raising its activity at least 50-fold.27 Our data show that acadesine activates AMPK and AMPKK in B-CLL cells at a concentration that induces apoptosis. Of note, inhibition of ZMP accumulation blocks both activation of AMPK and apoptosis in B-CLL cells. These results suggest a new proapoptotic signaling pathway involving AMPK.
PKA and MAP kinases have been involved in the apoptosis of B-CLL cells.28,29 Moreover, JNK has been involved in acadesine-induced apoptosis in a hepatoma cell line.39However, our results using selective inhibitors indicate that PKA, ERK, JNK, and p38 are not implicated in acadesine-induced apoptosis in B-CLL cells.
Recently, Inamura et al reported that acadesine treatment suppressed cell growth in HepG2 cells, associated with p53 phosphorylation at Ser15 and its accumulation, suggesting that AMPK might have a role in the regulation of p53.36 Our data demonstrate that in B-CLL cells the activation of AMPK and AMPKK induced by acadesine does not affect p53 phosphorylation or accumulation, indicating that acadesine-induced apoptosis is p53-independent, an important difference with respect to other nucleoside analogues including fludarabine, which induces p53 stabilization.48
One important point is the selectivity and the concentration of acadesine necessary to induce apoptosis. We have studied the effects of acadesine in different cell lines including EHEB, Jurkat, JVM-2, MCF-7, HEK-293, HeLa, and HUH7 (data not shown). All these cell lines were less sensitive than B-CLL cells. More interestingly, here we show that T cells from B-CLL patients are resistant to acadesine-induced apoptosis, which may be useful in the therapy of B-CLL. The finding that ZMP levels in T cells treated with acadesine are lower than those in B-CLL cells, and are not sufficient to activate AMPK, may explain the resistance of T cells to acadesine-induced apoptosis.
Fludarabine and other nucleosides are highly effective in the treatment of B-CLL, either alone or in combination with other agents.2 However, these nucleosides induce apoptosis of T cells48,49 leading to immunosuppression.50,51Thus, the differential effect of acadesine in B and T lymphocytes is of great interest. Acadesine is well tolerated by healthy individuals when given intravenously, achieving concentrations in plasma (200 μM)52 in the range of those producing apoptosis in B-CLL cells. Moreover, clinical studies in patients undergoing coronary artery bypass graft (CABG) surgery demonstrate that treatment with acadesine before and during surgery can reduce early cardiac death and myocardial infarction.53 In conclusion, present results suggest a new pathway involving AMPK in the control of apoptosis in B-CLL cells and raise the possibility of using acadesine or other AMPK activators in B-CLL treatment.
The authors thank Dr Gabriel Pons, Dr Marçal Pastor-Anglada, Dr Esther Castaño, Dr Maria Piqué, Daniel Iglesias, and Llorenç Coll for helpful discussions and suggestions; Dr Mireia Dalmau for the technical support on flow cytometry analysis; and Maria Reixach and Teresa Barajas from the Serveis Cientı́fico-Tècnics at the Universitat de Barcelona for the technical support on HPLC analysis. We also thank R. Rycroft for language assistance.
Prepublished online as Blood First Edition Paper, January 9, 2003; DOI 10.1182/blood-2002-07-2339.
Supported by grant SAF2001-3026 from the Ministerio de Ciencia y Tecnologı́a and Fondo Europeo de Desarrollo Regional (FEDER) (J.G.). C.C. is recipient of a research fellowship from the Fundación Ramón Areces, and A.F.S. and M.B are recipients of a research fellowship from the Ministerio de Ciencia y Tecnologı́a.
C.C. and J.M.L. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Joan Gil, Departament de Ciències Fisiològiques II, Campus de Bellvitge, Universitat de Barcelona, c/ Feixa Llarga s/n, E-08907 L'Hospitalet de Llobregat, Spain; e-mail:joangil@bellvitge.bvg.ub.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal