Abstract
Marrow cells of myeloid lineage from 115 patients with myelodysplastic syndrome (MDS) were characterized by multidimensional flow cytometry and compared with findings in 104 patients with various disorders and 25 healthy donors. Based on phenotypic and scatter characteristics, a flow cytometric scoring system (FCSS) was developed that allowed for a simple numerical display of results. The flow cytometric scores were categorized as normal/mild (0-1), moderate (2-3), or severe (≥ 4). Most flow cytometric abnormalities were significantly (P < .05) more frequent in patients with MDS than in the control cohort. Flow cytometric scores in MDS patients were then retrospectively compared with marrow blast counts assessed by morphology, cytogenetics, hematologic parameters, and International Prognostic Scoring System (IPSS) risk categorization. The flow cytometric scores correlated inversely with leukocyte and absolute neutrophil counts (P < .01) and correlated directly with IPSS scores (P < .01) and with IPSS cytogenetic risk categories (P < .01). In 111 MDS patients who underwent allogeneic hematopoietic stem cell transplantation, flow scores correlated with posttransplantation outcome. The probabilities of posttransplantation relapse were 3%, 15%, and 33% for patients with mild, moderate, and severe FCSS scores, respectively (P < .01), and overall survival was 74%, 40%, and 36%, respectively, for the 3 groups (P < .01). In multivariate analyses, there was a significant contribution of the flow score independent of the IPSS in predicting survival and relapse (P < .01, P = .02, and P = .03, respectively). These data suggest that FCSS is useful in assessing marrows for diagnosis of MDS and in determining the prognostic outcome in patients with this disorder. (Blood. 2003;102:394-403)
Introduction
Myelodysplastic syndromes (MDSs) comprise a complex, heterogeneous group of hematopoietic stem cell disorders. Classification and prognostic indicators include objective parameters such as cytogenetic findings and number of cytopenias in addition to morphologic assessment of lineage dysplasias and quantification of myeloblasts.1 Although flow cytometry is widely used for diagnosis, characterization, and classification of various hematologic malignancies and detection of posttreatment minimal residual disease,2-5 only limited data have been presented concerning diagnostic and prognostic significance of flow cytometric immunophenotyping in patients with MDSs.6-8 With an understanding of normal antigenic expression during hematopoietic development as determined by multidimensional flow cytometry, the dysregulation of hematopoiesis observed in MDS could be characterized by deviations from the normal patterns.9 Immunophenotypic abnormalities are observed on maturing cells as well as on myeloblasts.10-13 These abnormalities often affect only subpopulations of cells that coexist with normal precursors. The characteristics and magnitude of antigenic aberrancies provide information as to the extent of dysregulation of gene products at a particular time in the differentiation of cells as well as in the disease course.
Treatment decisions in patients with MDS are generally based on disease status and the expected tempo of the disease process. Patients with advanced disease by the criteria of the French-American-British (FAB) classification14 and by the International Prognostic Scoring System (IPSS)1 are often considered for early hematopoietic stem cell transplantation (SCT) if a suitable donor is identified, whereas patients with low risk factors initially may be observed or treated with less aggressive therapeutic modalities.15 Therefore, patient management relies heavily on accurate determination of disease status at the time of evaluation. The purpose of this study was to quantify and score complex flow cytometric abnormalities in a standardized, comprehensive analysis, to correlate flow cytometry scores with conventional and established parameters, and to determine the prognostic significance of flow cytometry scores for relapse, nonrelapse mortality (NRM), and survival in patients with MDS undergoing allogeneic SCT.
Patients, materials, and methods
Study populations
Marrow aspirates from 115 patients with a diagnosis of de novo or secondary MDS based on accepted diagnostic criteria, who were referred to the Fred Hutchinson Cancer Research Center (FHCRC) for consideration of allogeneic SCT between April 1995 and December 2000, were analyzed by flow cytometry; results were retrospectively correlated with morphologic, cytogenetic, and hematologic parameters. Patient age ranged from 4 to 66 years (including 7 pediatric patients 4-16 years of age, and 108 adults > 16 years of age); 58 were female and 57 male. One hundred eleven of the MDS patients subsequently underwent SCT; for various reasons 4 patients did not undergo transplantation. Marrow aspirates were collected in sodium heparin at or near the initial examination date as part of the intake evaluation. Criteria for inclusion in the study consisted of morphologic evidence of dysplasias, abnormal peripheral blood cell counts, and clinical histories. Patients referred with an initial diagnosis of MDS who were identified as having clonal lymphoid abnormalities were excluded from the study.3 Patients with chronic myelomonocytic leukemia (CMML) with a white blood cell (WBC) count more than 12 × 109/L were also excluded. One patient who developed MDS on a background of paroxysmal nocturnal hemoglobinuria (PNH) was included in the study.
An additional 104 patients with aplastic anemia, nonhematopoietic malignancies, and Hodgkin disease who were also referred for transplantation, and potential marrow donors who had marrow aspirates obtained because of abnormal peripheral blood cell counts were studied in comparison to MDS patients as a control cohort. Flow cytometry was also performed on marrow aliquots from 25 individuals with complete blood cell counts in the normal range at the time they served as marrow donors for transplantation. Informed consent for all research studies was obtained according to the procedures approved by the institutional review and ethics board of the FHCRC.
Morphologic assessment of marrow
Wright-Giemsa—stained smears of marrow aspirates that had been characterized by flow cytometry were also assessed morphologically for dyserythropoiesis, megakaryocytic anomalies, dysgranulopoiesis, and myeloid hypolobulation and hypogranulation. Cases diagnosed as MDS were further subclassified according to the FAB classification criteria14 and the World Health Organization (WHO) system16 (for patients referred after 1999). Complete morphologic diagnoses and flow cytometric results were available in 114 of 115 patients. The FAB classification of refractory anemia (RA) was rendered in 53 patients, refractory anemia with excess blasts (RAEB) in 30, refractory anemia with excess blasts in transformation (RAEB-t) in 15, refractory anemia with ringed sideroblasts (RARS) in 2, and CMML in 2 patients; 9 patients were described as “unclassifiable.” Three patients were given the WHO classification of refractory cytopenia with multilineage dysplasia, and a specific morphologic diagnosis was not available in one patient. Correlations between morphologic classification using FAB or WHO subtypes and flow cytometry were not attempted in this study; however, blast counts determined on the basis of morphology were separately correlated with flow findings.
A differential count was performed to ascertain percentages of myeloblasts based on total nucleated cell count. In the case of hypocellular smears from aspirates, the proportion of morphologically identified myeloblasts was obtained from B-5 formalin-fixed clot sections stained with hematoxylin and eosin, and periodic acid-Schiff.
Cytogenetic studies
Conventional cytogenetic analysis was performed on direct and 24- to 48-hour unstimulated bone marrow cultures using standard techniques, and analyzed following trypsin Giemsa banding; 20 metaphases were evaluated.
IPSS scoring
Prognostic scores were calculated based on marrow blast percentage, karyotype, and the number of cytopenias according to the IPSS (for 113 of 115 patients with available cytogenetics and hematologic data).1 The IPSS was also applied to patients with secondary MDS. Marrow blast percentages were obtained by light microscopy as described in “Morphologic assessment of marrow.” Cytopenias were defined as hemoglobin value (Sysmex 1500 or 8000, Long Grove, IL) less than 10 g/dL, absolute neutrophil count (ANC) less than 1.5 × 109/L, and platelet values less than 100 × 109/L. Karyotypes were divided into subgroups of good (normal, —Y, del(5q), del(20q)), poor (3 or more abnormalities or chromosome 7 abnormalities), or intermediate (all remaining karyotypes) risk.17
Flow cytometry
Monoclonal antibodies used in this study were obtained from the indicated sources: fluorescein isothiocyanate (FITC-conjugated): CD16 (3G8) from Pharmingen International, San Jose, CA; CD15 (MMA), CD34 (8G12), CD7 (4H9), CD5 (L17F12), CD38 (HB7), HLA-DR (L243) from Becton Dickinson Immunocytometry Systems, San Jose, CA; CD33 (WM-54) from DAKO, Carpinteria, CA. Phycoerythrin (PE-conjugated): CD11b (D12), CD13 (L138), CD33 (P67.6), CD14 (MφP9), CD19 (SJ25C1), CD34 (8G12), CD56 (NCAM16.2) from Becton Dickinson. Peridinin-chlorophyll a (PerCP-conjugated) CD45 (2D1) from Becton Dickinson. A standardized panel of reagents was used to characterize populations of myeloid lineage (Table 1). The procedure for labeling cells has been described.18
Combinations of antigen specificities with the respective fluorescence markers used in the analysis
FITC . | PE . | PerCP . |
|---|---|---|
| HLA-DR | CD11b | CD45 |
| CD5 | CD19 | CD45 |
| CD38 | CD56 | CD45 |
| CD16 | CD13 | CD45 |
| CD15 | CD34 | CD45 |
| CD14 | CD33 | CD45 |
| CD7 | CD56 | CD45 |
| HLA-DR | CD34 | CD45 |
FITC . | PE . | PerCP . |
|---|---|---|
| HLA-DR | CD11b | CD45 |
| CD5 | CD19 | CD45 |
| CD38 | CD56 | CD45 |
| CD16 | CD13 | CD45 |
| CD15 | CD34 | CD45 |
| CD14 | CD33 | CD45 |
| CD7 | CD56 | CD45 |
| HLA-DR | CD34 | CD45 |
Acquisition of flow data was performed on a CytoronAbsolute (Ortho Diagnostics, Raritan, NJ) or on a FACS Calibur (Becton Dickinson Immunocytometry Systems). Data analysis was performed using Winlist software (Verity House, Topsham, ME). Daily instrument quality controls including fluorescence standardization, linearity assessment, and spectral compensation were performed to ensure identical operation from day to day.19 In a standard 3-color immunofluorescence protocol, forward (FSC) and right angle light scatter (SSC) were collected along with 3-color antibody combinations to generate 5 characteristics per cellular event. These 5 characteristics determined the coordinates for that event in a 5-dimensional space.20-22 Electronic gating on the basis of FSC and SSC excluded cellular debris and nonviable cells.23 Cell population percentages were assessed by CD45 staining and SSC, as described,18 and based on nucleated, total nonerythroid cells following NH4CL lysis. A cell differential was obtained by either directly gating on discrete populations identified by CD45 and SSC, or by back-gating using 2-color antibody combinations to identify cells of interest.18,24-26
Boolean logic gating schemes were used for gating so that the populations of interest were electronically separated for independent analysis and also for distinguishing overlapping populations electronically.22 Abnormalities within the maturing myeloid, monocytic, and myeloblast populations were determined on the basis of deviation from normal patterns as previously described.21,27-29 Cells were defined as having abnormalities when 2 analysts (D.A.W., M.R.L.) agreed that the population was at least 0.5 decades disparate from the relative intensity of antigens expressed on corresponding normal cells.
Figure 1 depicts normal maturational patterns as determined by the standardized antibody panel. In myelodysplastic marrows, the patterns of maturational abnormalities were measured as the degree of departure from normal antigen expression.9,20,21,29 The abnormalities described and illustrated in Figures 2 and 3 characterize many of the common patterns observed using this standardized panel.
Normal myeloid antigen expression and relationships in bone marrow. (A-B) SSC, FSC, and CD45 intensity in marrow cells from a healthy donor. Promyelocytes contain primary granules giving rise to the high SSC signal. Later stage neutrophils are marked by a decrease in SSC related to the appearance of secondary granules. (C-D) CD11b and HLA-DR show a characteristic relationship in normal maturing myeloid cells and monocytes that follow maturation stages. Normal monocytes retain HLA-DR throughout their development in contrast to neutrophils that rapidly lose this antigen at the promyelocyte stage. The maturation of monocytes is first identified by the rapid appearance of CD11b while maintaining intermediate levels of CD45. (E-F) CD13 is a unique antigen that appears, disappears, and reappears relatively quickly during normal myeloid development. Normal myeloblasts and promyelocytes express high levels of CD13. The antigen is lost on myelocytes and then gradually emerges again as the cells develop to the segmented neutrophil stages. CD13 and CD16 are normally expressed in a consistent “concave” pattern as shown in panel E. Normal monocytes show heterogeneous coexpression of CD13 and CD16. (G-H) Progression from the myeloblast stage to more mature myeloid cells involves acquisition of high levels of CD15 with loss of CD34 and HLA-DR, whereas mature monocytes maintain dim expression of CD15 and lack CD34. (I-J) As myeloid cells mature, there is a slight decrease in CD33 intensity. Early stages of monocyte development are accompanied by increases in both CD13 and CD33 expression. Later stages of monocyte development are defined by the coordinated increase in both CD45 and CD14. (K-L) Maturing myeloid cells and monocytes are normally negative for CD56 and CD7.
Normal myeloid antigen expression and relationships in bone marrow. (A-B) SSC, FSC, and CD45 intensity in marrow cells from a healthy donor. Promyelocytes contain primary granules giving rise to the high SSC signal. Later stage neutrophils are marked by a decrease in SSC related to the appearance of secondary granules. (C-D) CD11b and HLA-DR show a characteristic relationship in normal maturing myeloid cells and monocytes that follow maturation stages. Normal monocytes retain HLA-DR throughout their development in contrast to neutrophils that rapidly lose this antigen at the promyelocyte stage. The maturation of monocytes is first identified by the rapid appearance of CD11b while maintaining intermediate levels of CD45. (E-F) CD13 is a unique antigen that appears, disappears, and reappears relatively quickly during normal myeloid development. Normal myeloblasts and promyelocytes express high levels of CD13. The antigen is lost on myelocytes and then gradually emerges again as the cells develop to the segmented neutrophil stages. CD13 and CD16 are normally expressed in a consistent “concave” pattern as shown in panel E. Normal monocytes show heterogeneous coexpression of CD13 and CD16. (G-H) Progression from the myeloblast stage to more mature myeloid cells involves acquisition of high levels of CD15 with loss of CD34 and HLA-DR, whereas mature monocytes maintain dim expression of CD15 and lack CD34. (I-J) As myeloid cells mature, there is a slight decrease in CD33 intensity. Early stages of monocyte development are accompanied by increases in both CD13 and CD33 expression. Later stages of monocyte development are defined by the coordinated increase in both CD45 and CD14. (K-L) Maturing myeloid cells and monocytes are normally negative for CD56 and CD7.
Light scatter and CD45 abnormalities in MDS. (A-B) Normal granularity is measured as the log difference of SSC between maturing myeloid cells and the normal lymphocytes present. (C-D) A dysplastic marrow illustrates decreased granularity and shows SSC nearly a half log lower than normal, as indicated by lines. In addition, the myeloblasts show abnormally decreased CD45 expression.
Light scatter and CD45 abnormalities in MDS. (A-B) Normal granularity is measured as the log difference of SSC between maturing myeloid cells and the normal lymphocytes present. (C-D) A dysplastic marrow illustrates decreased granularity and shows SSC nearly a half log lower than normal, as indicated by lines. In addition, the myeloblasts show abnormally decreased CD45 expression.
Examples of various myeloid and monocytic aberrant antigenic patterns in MDS. (A) Abnormal retention of HLA-DR in subpopulations of maturing myeloid cells. (B) Abnormal development in a subpopulation of monocytes that lack CD11b. (C) An abnormal “convex” rather than “concave” relationship between CD13 and CD16. (D) Aberrant lack of maturation to CD16. (E-F) CD56 expression on a subpopulation of myeloid cells and a population of monocytes with bright abnormal CD56 expression. (G-H) Abnormal, dim CD34 expression on maturing myeloid cells and monocytes. (I-J) Presence of CD7 on maturing myeloid cells and monocytes.
Examples of various myeloid and monocytic aberrant antigenic patterns in MDS. (A) Abnormal retention of HLA-DR in subpopulations of maturing myeloid cells. (B) Abnormal development in a subpopulation of monocytes that lack CD11b. (C) An abnormal “convex” rather than “concave” relationship between CD13 and CD16. (D) Aberrant lack of maturation to CD16. (E-F) CD56 expression on a subpopulation of myeloid cells and a population of monocytes with bright abnormal CD56 expression. (G-H) Abnormal, dim CD34 expression on maturing myeloid cells and monocytes. (I-J) Presence of CD7 on maturing myeloid cells and monocytes.
Descriptions of MDS characteristics used for flow cytometry scoring
Abnormal granularity. Abnormal granularity was characterized by a decrease in SSC as compared with normal mature neutrophils, indicating fewer cytoplasmic granules or hypogranularity in a cell population (Figure 2).10,30,31
Abnormal decrease in CD45 expression. Coordinated increases in CD45 expression are a hallmark of normal maturation.26 Decreased intensity of CD45 in a given population in reference to the normal lymphocytes present in a sample indicates abnormal maturation as illustrated by the abnormal myeloblast population (red) in Figure 2D.
Abnormal relationships between CD11b and HLA-DR in maturing myeloid cells, monocytes, or both. A characteristic acquisition of CD11b and loss of HLA-DR mark normal neutrophil development after the myeloblast stage.27,28 Decreases in intensities of CD11b with other immunophenotypic evidence of a left shift were considered normal findings. Abnormalities in CD11b and HLA-DR relationships led to patterns different from normal as illustrated in Figure 3A-B.
Abnormal relationships between CD13 and CD16 in the maturing myeloid compartment. Abnormal relationships between these antigens include a “convex” pattern (Figure 3C), lack of CD16 expression (Figure 3D), or other bizarre antigenic patterns. (Note: the loss of CD16 may result from genetic polymorphism32 and is also present in PNH.33 )
Expression of CD56 on maturing myeloid cells or monocytes. Expression of the adhesion molecule CD56 may occur due to abnormalities in these compartments or because of early or intense granulopoiesis following a marrow insult (Figure 3E-F) (J. Cornbleet, oral personal communication, February 1996).
Lack of CD33 expression on maturing myeloid cells or monocytes. The loss of CD33 is a possible antigenic aberrancy; however, it may occur as a normal variant or as a manifestation of genetic polymorphism.34-36
Asynchronous shift to the left. Maturational asynchrony is detected by the expression of antigens that appear on immature cells in concert with antigens identified only on mature cells.27 The appearance of a shift to the left within a given set of antigenic parameters that is not observed in other parameters indicates abnormal maturation. For example, as normal myeloblasts mature, CD15 dramatically increases, whereas CD34 expression is lost. The dim expression of CD15 on maturing myeloid cells without a concomitant shift to the left by CD11b, HLA-DR, CD13, and CD16 would indicate asynchronous maturation.
Presence of CD34 on maturing myeloid cells or monocytes. CD34 is an indicator of early myeloid precursors and is not observed on normal maturing myeloid cells or monocytes. Figure 3G-H shows subpopulations of maturing myeloid cells and monocytes with dim CD34 expression.
Presence of lymphoid antigens on maturing myeloid cells or monocytes. In normal hematopoiesis, the gene products determining myeloid or lymphoid development have been selected correctly without improper expression of genes from other lineages. An exception is observed in abnormal cells that exhibit true lineage infidelity.37 Usually, the aberrant lineage expression is dimmer than what is found on normal cells, similar to cases of acute myelogenous leukemia (AML) that express CD7 or CD19 with less intensity of expression than the same antigens expressed on lymphoid cells.2,27 The expression of nonlineage antigens must be interpreted cautiously because some markers are “lineage associated” rather than lineage specific.38 For example, normal monocytes are positive for the T lymphoid antigen CD4. Figure 3I-J shows subpopulations of maturing myeloid cells and monocytes with CD7 expression.
Lack of CD13, CD33, or CD14 on monocytes. The finding indicates an aberrant phenotype or maturational arrest within the monocyte compartment.
Presence of abnormal myeloblasts. Criteria such as percentage of CD34+ cells, position by CD45 and SSC, and presence/absence of myeloid antigens were taken into consideration when defining a myeloblast. Normal myeloblasts identified by the heterogeneous expression of CD34 also exhibit HLA-DR, CD13, and CD33 at high levels, but do not express other markers of mature neutrophils such as CD11b, CD15, and CD16. Normal myeloblasts are intermediate in size by FSC but have low SSC. The progression to promyelocytes is denoted by the loss of CD34 and HLA-DR expression, acquisition of high levels of CD15, and a marked increase in SSC, but without expression of CD11b.9 Table 2 lists examples of abnormalities observed in myeloblast populations in marrows from patients with MDS. Many marrows had myeloblasts showing more than one of these abnormalities.
Myeloblast abnormalities detected by flow cytometry in 115 MDS patients
Abnormality . | N (%) . |
|---|---|
| Abnormal decrease in CD45 expression | 19 (16) |
| CD11b+ | 4 (3.5) |
| HLA-DR− | 5 (4.3) |
| Lack of CD13 or CD33 | 1 (0.9) |
| Presence of CD19, CD5, CD34, CD56, or CD7 | 26 (23) |
| Abnormal granularity | 7 (6.1) |
| Lack of CD34 | 4 (3.5) |
| Abnormal myeloid antigen intensity | 22 (19) |
| Antigenic homogeneity | 33 (29) |
Abnormality . | N (%) . |
|---|---|
| Abnormal decrease in CD45 expression | 19 (16) |
| CD11b+ | 4 (3.5) |
| HLA-DR− | 5 (4.3) |
| Lack of CD13 or CD33 | 1 (0.9) |
| Presence of CD19, CD5, CD34, CD56, or CD7 | 26 (23) |
| Abnormal granularity | 7 (6.1) |
| Lack of CD34 | 4 (3.5) |
| Abnormal myeloid antigen intensity | 22 (19) |
| Antigenic homogeneity | 33 (29) |
Increased lymphoid-to-myeloid ratio. Determination of absolute counts of cells in marrow is not possible. The relative proportion of lymphocytes to myeloid cells, however, can be used to identify abnormally low proportions of myeloid cells. An increased proportion of lymphoid cells to myeloid cells in this patient population is generally indicative of a decrease in myelopoiesis.
FCSS
A flow cytometric scoring system (FCSS) was devised as a means of condensing multiple flow cytometric abnormalities into numerical scores (Table 3). Scores were calculated based on types of abnormalities in the maturing myeloid cells and monocytes, flow cytometric blast counts, and degree of impaired myelopoiesis based on the lymphoid-to-myeloid ratio. Additional weight was given to marrows showing lineage infidelity (presence of lymphoid antigens on myeloid or monocytic cells) or marked maturational asynchrony as evidenced by CD34 expression on maturing myeloid cells or monocytes. These 2 abnormalities are believed to result from significant genetic dysregulation. Scores were calculated on marrow samples obtained at initial (pretransplantation) examination of the patient. Scores for abnormalities in maturing myeloid cells and monocytes were generated as follows:
Components of flow scoring system
Points . | Myeloid abnormailities . | Monocytic abnormalities . |
|---|---|---|
| 0 | Appropriate CD45/SSC | Appropriate CD45/SSC |
| Heterogeneous CD11b+, HLA-DR− | CD11b+, heterogeneous HLA-DR | |
| Normal relationships of CD13 and CD16 | CD13/CD16 coexpression | |
| CD33+ | CD33/CD14 coexpression | |
| CD19/CD5/CD34/CD56/CD7− | CD19/CD5/CD34/CD56/CD7− | |
| Synchronous shift to the left | ||
| 1 | One of the following is present: | One of the following is present: |
| Abnormal granularity | Abnormal granularity | |
| Abnormal decrease in CD45 expression | Abnormal CD11b or HLA-DR expression | |
| Presence of HLA-DR or lack of CD11b | Loss of CD13 or CD16 | |
| Convex or abnormal relationship between CD13 and CD16 | Presence of CD56 | |
| Expression of CD56 on subpopulation of myeloid cells | Lack of CD33 or CD14 | |
| Lack of CD33 expression (may be a normal variant) | ||
| Asynchronous shift to the left | ||
| 2 | 2-3 of the above abnormalities or presence of CD34 on myeloid cells or presence of lymphoid antigens on myeloid cells | 2-3 of the above abnormalities or presence of CD34 on monocytes or presence of lymphoid antigens on monocytes (exception is CD4) |
| 3 | 4 or more of the above abnormalities or 1 or more of the abnormalities plus presence of CD34 or lymphoid antigen expression on myeloid cells | 4 or more of the above abnormalities or 1 or more of the abnormalities plus presence of CD34 or lymphoid antigen expression on monocytes |
Points . | Myeloid abnormailities . | Monocytic abnormalities . |
|---|---|---|
| 0 | Appropriate CD45/SSC | Appropriate CD45/SSC |
| Heterogeneous CD11b+, HLA-DR− | CD11b+, heterogeneous HLA-DR | |
| Normal relationships of CD13 and CD16 | CD13/CD16 coexpression | |
| CD33+ | CD33/CD14 coexpression | |
| CD19/CD5/CD34/CD56/CD7− | CD19/CD5/CD34/CD56/CD7− | |
| Synchronous shift to the left | ||
| 1 | One of the following is present: | One of the following is present: |
| Abnormal granularity | Abnormal granularity | |
| Abnormal decrease in CD45 expression | Abnormal CD11b or HLA-DR expression | |
| Presence of HLA-DR or lack of CD11b | Loss of CD13 or CD16 | |
| Convex or abnormal relationship between CD13 and CD16 | Presence of CD56 | |
| Expression of CD56 on subpopulation of myeloid cells | Lack of CD33 or CD14 | |
| Lack of CD33 expression (may be a normal variant) | ||
| Asynchronous shift to the left | ||
| 2 | 2-3 of the above abnormalities or presence of CD34 on myeloid cells or presence of lymphoid antigens on myeloid cells | 2-3 of the above abnormalities or presence of CD34 on monocytes or presence of lymphoid antigens on monocytes (exception is CD4) |
| 3 | 4 or more of the above abnormalities or 1 or more of the abnormalities plus presence of CD34 or lymphoid antigen expression on myeloid cells | 4 or more of the above abnormalities or 1 or more of the abnormalities plus presence of CD34 or lymphoid antigen expression on monocytes |
Points = 0: Normal myeloid and monocytic flow studies in which myeloblasts, maturing myeloid cells, and monocytes were indistinguishable from normal as defined by the above criteria.
Points = 1: Only one of the abnormalities listed was present. For example, there was a single abnormality in the myeloid cells but not in the monocytic cells. (If there were single abnormalities in both myeloid and monocytic cells, 2 points were assessed).
Points = 2: Two or 3 abnormalities in either myeloid or monocytic cells or one abnormality in each lineage. (If both myeloid cells and monocytes had 2 or 3 abnormalities, a total score of 4 was generated.) A score of 2 was also given if myeloid or monocytic cells expressed CD34 or lymphoid antigens but lacked other abnormalities.
Points = 3: Either myeloid cells or monocytes had 4 or more abnormalities, or they had 1 to 3 of the single abnormalities and expressed either CD34 or lymphoid antigens.
Points = 4: Both myeloid cells and monocytes showed 2 or 3 abnormalities.
One additional point was given if myeloblasts were less than 5% but clearly abnormal. Two additional points were given for 5% to 10% abnormal myeloblasts, 3 points for 11% to 20%, and 4 points for 21% to 30% (there were no patients with more than 5% myeloblasts that had phenotypically normal myeloblasts). One point was also given if the lymphoid-to-myeloid ratio was 1.0 or greater.
The total flow score comprised the sum of all abnormalities observed for a patient at a specific time point. Total scores reflecting the degree of flow cytometric aberrancies were grouped into the following categories: 0-1 = normal/mild; 2-3 = moderate; 4-9 = severe. Patient samples were evaluated and scored by 2 authors (D.A.W., M.R.L.) blinded to additional clinical information about the patient.
Transplantation
Among the 111 patients who received allogeneic SCT, 89 were conditioned with oral busulfan (prescribed dose 1 mg/kg every 6 hours for 16 doses on days —7 through —4; busulfan doses were adjusted to achieve steady-state plasma concentrations of 800-900 ng/mL) followed by intravenous cyclophosphamide (60 mg/kg on days —3 and —2). Seven patients were conditioned with cyclophosphamide, 2 × 60 mg/kg intravenously, and fractionated total-body irradiation (TBI) for total doses of 800 to 1320 cGy; 9 received oral busulfan, 0.44 mg/kg for 16 doses, followed by 6 × 200 fractionated TBI; and the remaining 6 patients received miscellaneous regimens. Marrow (n = 82) or peripheral blood stem cells (n = 29) were infused intravenously after completion of TBI or within 36 to 48 hours of the last dose of cyclophosphamide. Cyclosporine and methotrexate were given as graft-versus-host disease (GVHD) prophylaxis. Engraftment and acute and chronic GVHD were assessed by established criteria, as described previously.39-41 GVHD was treated with corticosteroids, monoclonal antibodies, cyclosporine, mycophenolate mofetil, tacrolimus, or combinations of these agents. Details of the long-term follow-up program have been reported recently.42
Statistics
Correlation between continuously valued quantities such as flow score and hematologic parameters was evaluated using the Spearman rank correlation coefficient. Flow scores and blast counts were compared between 2 groups using 2-sided Wilcoxon rank sum tests. Trends across ordered groups were evaluated using a nonparametric test for trend.43 Proportions were compared between groups using either a χ2 test or Fisher exact test. Flow score was treated as a continuous valued diagnostic test for which the accuracy parameters sensitivity and specificity were calculated. Sensitivity was evaluated among MDS patients and is defined as the proportion of patients with a flow score above a specified cut-point for positivity (true positive rate). Specificity was evaluated among patients with a diagnosis other than MDS and defined as the proportion of subjects with a flow score below a certain criterion for positivity (also known as the true-negative rate). Sensitivity and specificity are reported for a range of cutoff values for the flow score to provide information about the trade-offs in these quantities as the cutoff is shifted. Proportion correctly classified was the proportion of subjects with true-positive or true-negative values at the specified cut-point.
Survival curves were calculated using the method of Kaplan and Meier (K-M).44 Incidence of relapse, GVHD, and NRM were evaluated using cumulative incidence methodology with death or relapse treated as competing risk events where appropriate.45 Comparisons between time to event curves were made using a log-rank test. Cox proportional hazards regression was performed to assess the relative impact of flow score and components of the IPSS score on survival, relapse, and NRM. All reported P values are 2-sided. Results were analyzed as of June 30, 2002.
Results
Myeloid and monocytic dyspoiesis in MDS compared with control groups
Among 115 MDS patients studied, 90 (78%) had abnormalities in either the myeloid or monocytic compartment or both, alone or combined with the presence of phenotypically abnormal myeloblasts that ranged from 0.6% to 58% (Table 4). In 4 patients, the disease was originally classified as MDS by the referring institutions but had progressed to more than 30% myeloblasts (thus qualifying as AML by the FAB criteria16 ) by the time they were evaluated at the FHCRC. These patients were retained in the study. The most common abnormality in marrows from MDS patients was the presence of phenotypically abnormal myeloblasts (62%). In 27 of the 115 MDS patients (23%), phenotypically abnormal myeloblasts were present at less than 5% of total nonerythroid cells. The most common phenotypic aberrancies in maturing myeloid cells and monocytes were in the abnormal relationship between CD13 and CD16 in myeloid cells (23%), asynchronous shifts to the left (23%), and presence of CD56 on maturing myeloid cells (16%) and monocytes (17%). Sixty-nine patients (69%) had more than one abnormality.
Myeloid and monocytic dyspoiesis detected by flow cytometry
. | Patient group . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Flow cytometric abnormality . | MDS (n = 115) . | Aplastic anemia (n = 26) . | Nonhematopoietic malignancies (n = 15) . | Hodgkin disease (n = 26) . | Bone marrow transplant donors with cytopenias (n = 37) . | All non-MDS subjects (n = 104) . | |||||
| Abnormal myeloblasts, no. (%) | 72 (62) | 0 (0)* | 0 (0)* | 0 (0)* | 1 (3)* | 1 (1)* | |||||
| Maturing myeloid | |||||||||||
| Abnormal granularity | 9 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0)* | |||||
| Abnormal decrease CD45 | 4 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Abnormal relationship CD13/CD16 | 27 (23) | 3 (12) | 0 (0)* | 3 (12) | 6 (16) | 12 (12)* | |||||
| Abnormal relationships | |||||||||||
| HLA-DR/CD11b | 6 (5) | 0 (0) | 0 (0) | 1 (4) | 1 (3) | 2 (2) | |||||
| Asynchronous shift to the left | 26 (23) | 0 (0)* | 0 (0)* | 0 (0)* | 0 (0)* | 0 (0)* | |||||
| Presence of CD56 | 18 (16) | 1 (4) | 1 (7) | 6 (23) | 0 (0)* | 8 (8) | |||||
| Lack of CD33 | 6 (5) | 1 (4) | 0 (0) | 0 (0) | 3 (8) | 4 (4) | |||||
| Presence of CD34 | 7 (6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0)* | |||||
| Presence of lymphoid antigens | 4 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Monocytes | |||||||||||
| Abnormal granularity | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Lack of CD13 or CD16 | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Abnormal relationship HLA-DR/CD11b | 5 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Presence of CD56 | 19 (17) | 0 (0)* | 0 (0) | 3 (12) | 0 (0)* | 3 (3)* | |||||
| Lack of CD33 or CD14 | 3 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0)* | 0 (0) | |||||
| Presence of CD34 | 14 (12) | 0 (0) | 0 (0) | 0 (0) | 0 (0)* | 0 (0)* | |||||
| Presence of lymphoid antigens | 4 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Abnormal L/M ratio | 38 (33) | 19 (73)† | 1 (7)* | 0 (0)* | 1 (3)* | 21 (20)* | |||||
| Abnormal cytogenetics | 71 (62)‡ | 0 (0)* | 0 (0)* | 1 (4)* | 0 (0)* | 1 (1)* | |||||
. | Patient group . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Flow cytometric abnormality . | MDS (n = 115) . | Aplastic anemia (n = 26) . | Nonhematopoietic malignancies (n = 15) . | Hodgkin disease (n = 26) . | Bone marrow transplant donors with cytopenias (n = 37) . | All non-MDS subjects (n = 104) . | |||||
| Abnormal myeloblasts, no. (%) | 72 (62) | 0 (0)* | 0 (0)* | 0 (0)* | 1 (3)* | 1 (1)* | |||||
| Maturing myeloid | |||||||||||
| Abnormal granularity | 9 (8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0)* | |||||
| Abnormal decrease CD45 | 4 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Abnormal relationship CD13/CD16 | 27 (23) | 3 (12) | 0 (0)* | 3 (12) | 6 (16) | 12 (12)* | |||||
| Abnormal relationships | |||||||||||
| HLA-DR/CD11b | 6 (5) | 0 (0) | 0 (0) | 1 (4) | 1 (3) | 2 (2) | |||||
| Asynchronous shift to the left | 26 (23) | 0 (0)* | 0 (0)* | 0 (0)* | 0 (0)* | 0 (0)* | |||||
| Presence of CD56 | 18 (16) | 1 (4) | 1 (7) | 6 (23) | 0 (0)* | 8 (8) | |||||
| Lack of CD33 | 6 (5) | 1 (4) | 0 (0) | 0 (0) | 3 (8) | 4 (4) | |||||
| Presence of CD34 | 7 (6) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0)* | |||||
| Presence of lymphoid antigens | 4 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Monocytes | |||||||||||
| Abnormal granularity | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Lack of CD13 or CD16 | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Abnormal relationship HLA-DR/CD11b | 5 (4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Presence of CD56 | 19 (17) | 0 (0)* | 0 (0) | 3 (12) | 0 (0)* | 3 (3)* | |||||
| Lack of CD33 or CD14 | 3 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0)* | 0 (0) | |||||
| Presence of CD34 | 14 (12) | 0 (0) | 0 (0) | 0 (0) | 0 (0)* | 0 (0)* | |||||
| Presence of lymphoid antigens | 4 (3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||||
| Abnormal L/M ratio | 38 (33) | 19 (73)† | 1 (7)* | 0 (0)* | 1 (3)* | 21 (20)* | |||||
| Abnormal cytogenetics | 71 (62)‡ | 0 (0)* | 0 (0)* | 1 (4)* | 0 (0)* | 1 (1)* | |||||
The data field shows the number with percentage in parentheses.
Number of abnormalities are significantly lower than MDS patients (P of a 2-sided Fisher exact test < .05).
Number of abnormalities is significantly higher than MDS patients (P of a 2-sided Fisher exact test < .05).
One patient missing cytogenetic data.
Flow cytometric results in patients with MDS were compared with those in 25 healthy donors. None of the 25 healthy donors had abnormal myeloid, monocytic, or myeloblast findings. The frequencies of flow cytometric myeloid and monocytic abnormalities in patients with previously diagnosed MDS were then compared with results in 104 patients who, as described, had either received chemotherapy or patients who exhibited peripheral blood cytopenias and were evaluated to rule out marrow disorders. Although some flow cytometric abnormalities were detected in this group, aberrant findings were significantly less frequent than in MDS patients (Table 4). Three of 26 patients (12%) with aplastic anemia had abnormal relationships between CD13 and CD16, one expressed CD56 on maturing myeloid cells, and one lacked CD33 expression. As expected, patients with aplastic anemia had increased lymphoid-to-myeloid ratios (present in 73%) indicating decreased myelopoiesis, as compared with 33% of MDS patients. Six of 26 patients (23%) with Hodgkin disease, and 1 of 15 patients with nonhematopoietic malignancies showed CD56 expression on maturing myeloid cells and monocytes. One patient with Hodgkin disease with abnormal CD56 expression was found to have multiple complex cytogenetic abnormalities suggestive of secondary MDS. In addition, one prospective marrow donor with granulocytopenia was found to have abnormal myeloblasts present at 4.9% of total nonerythroid cells, and the marrow was subsequently found by light microscopy to have dysplastic erythroid and megakaryocytic cells. Overall, most flow cytometric abnormalities among MDS patients differed significantly from findings in the control group (P < .05).
Flow cytometry scores based on the above findings for the 115 MDS patients were compared to the scores determined for the 104 control cohort patients. Various score levels were used as cutoffs to distinguish between these 2 groups of patients (Table 5). Fifty-five percent of MDS patients had flow scores of 3 or greater, whereas none of the control patients did; that is, a flow score of 3 or more allowed for a specificity of 100% with 55% sensitivity. A flow score of 2 or more resulted in 93% specificity with 70% sensitivity.
Sensitivity and specificity for flow cytometric scores among MDS patients and the non-MDS cohort
Cut point . | Sensitivity, % . | Specificity, % . | Correctly classified, % . |
|---|---|---|---|
| 0 or more | 100.00 | 0.00 | 52.75 |
| 1 or more | 83.48 | 59.22 | 72.02 |
| 2 or more | 70.43 | 93.20 | 81.19 |
| 3 or more | 54.78 | 100.00 | 76.15 |
| 4 or more | 41.74 | 100.00 | 69.27 |
| 5 or more | 24.35 | 100.00 | 60.09 |
| 6 or more | 14.78 | 100.00 | 55.05 |
| 7 or more | 6.96 | 100.00 | 50.92 |
| 8 or more | 3.48 | 100.00 | 49.08 |
| 9 or more | 1.74 | 100.00 | 48.17 |
| Above 9 | 0.00 | 100.00 | 47.25 |
Cut point . | Sensitivity, % . | Specificity, % . | Correctly classified, % . |
|---|---|---|---|
| 0 or more | 100.00 | 0.00 | 52.75 |
| 1 or more | 83.48 | 59.22 | 72.02 |
| 2 or more | 70.43 | 93.20 | 81.19 |
| 3 or more | 54.78 | 100.00 | 76.15 |
| 4 or more | 41.74 | 100.00 | 69.27 |
| 5 or more | 24.35 | 100.00 | 60.09 |
| 6 or more | 14.78 | 100.00 | 55.05 |
| 7 or more | 6.96 | 100.00 | 50.92 |
| 8 or more | 3.48 | 100.00 | 49.08 |
| 9 or more | 1.74 | 100.00 | 48.17 |
| Above 9 | 0.00 | 100.00 | 47.25 |
Flow cytometry scores and IPSS
In 113 pretransplantation patients with complete laboratory and cytogenetic data, the correlation between flow and IPSS scores was evaluated (Table 6). A Spearman coefficient for the 2 scores was 0.64, indicating a strong correlation between the 2 measures. Scores based on flow cytometric data significantly increased with increasing IPSS scores (P < .01). In addition, individual components of the IPSS score were compared with flow scores. As expected, the percentage of myeloblasts, as determined by flow cytometry, also increased with increasing IPSS scores (P < .01). WBC counts and ANC values were available in 114 patients (Table 7). As the flow cytometry score increased, WBC counts and ANC values decreased (P < .01), suggesting that higher flow scores correlated with ineffective granulopoiesis. Correlations with hemoglobin and platelet counts were not attempted because the majority of patients had received multiple red blood cell and platelet transfusions before evaluation. Because absolute counts of cells in marrow aspirates cannot be directly determined, the ratio of lymphoid to myeloid cells was used as a means of assessing inadequate myelopoiesis. The lymphoid-to-myeloid ratio also showed a high negative correlation with both ANC and WBC counts as indicated by significant Spearman correlation coefficients (-0.60 and -0.56, respectively; P < .001).
Comparison of IPSS and flow cytometric scores in MDS patients
IPSS score . | N . | Flow score, median, % (range) . | Myeloblasts by flow, median, % (range) . |
|---|---|---|---|
| 0 (low risk) | 13 | 1 (0-4) | 2.5 (0.2-7.0) |
| 0.5-1.0 (int-1 risk) | 52 | 2 (0-6) | 2.9 (0.0-11.0) |
| 1.5-2.0 (int-2 risk) | 31 | 4 (0-7) | 6 (0.8-39.0) |
| 2.5 or more (high risk) | 17 | 6 (3-9) | 11 (0.8-58.0) |
IPSS score . | N . | Flow score, median, % (range) . | Myeloblasts by flow, median, % (range) . |
|---|---|---|---|
| 0 (low risk) | 13 | 1 (0-4) | 2.5 (0.2-7.0) |
| 0.5-1.0 (int-1 risk) | 52 | 2 (0-6) | 2.9 (0.0-11.0) |
| 1.5-2.0 (int-2 risk) | 31 | 4 (0-7) | 6 (0.8-39.0) |
| 2.5 or more (high risk) | 17 | 6 (3-9) | 11 (0.8-58.0) |
Comparison of flow score and measures of hematopoiesis in MDS patients
Flow score . | N . | WBC count, median (range) . | ANC value, median (range) . |
|---|---|---|---|
| 0-1 (mild) | 34 | 3.7 (2.1-7.1) | 1.6 (0.2-5.3) |
| 2-3 (moderate) | 33 | 3.0 (0.2-26.4) | 1.5 (0.0-20.3) |
| 4 or more (severe) | 48 | 2.1 (0.6-50.7) | 0.5 (0.0-36) |
Flow score . | N . | WBC count, median (range) . | ANC value, median (range) . |
|---|---|---|---|
| 0-1 (mild) | 34 | 3.7 (2.1-7.1) | 1.6 (0.2-5.3) |
| 2-3 (moderate) | 33 | 3.0 (0.2-26.4) | 1.5 (0.0-20.3) |
| 4 or more (severe) | 48 | 2.1 (0.6-50.7) | 0.5 (0.0-36) |
Karyotypes were available in 114 MDS patients. There was a significant increase in flow cytometry scores with increasing cytogenetic risk by IPSS criteria (P = .02). Myelodysplastic patients with a 5q— karyotype as the sole abnormality (cytogenetic good-risk category)1,46 had the fewest overall flow cytometric abnormalities with a mean flow score of 1.4. The largest proportion of patients with severe phenotypic abnormalities (high flow scores) was in the group of patients with complex karyotypes (cytogenetic poor-risk category), who had significantly higher flow scores than patients with normal cytogenetics (P = .02). However, for a number of patients, flow scores and IPSS cytogenetic categories were disparate. Eighteen of 42 patients (42%) with normal cytogenetics (good risk) had flow scores of 4 or higher, 4 of 18 patients had abnormal myeloblasts less than 5%. Three of 4 patients with 20q— (good risk) as the sole karyotypic abnormality had flow scores of 3 or greater.
Flow cytometric analysis calculated blasts as a percentage of total nonerythroid cells (erythrocytes were lysed by NH4Cl), whereas morphologic counts used total nucleated cells as the denominator. Thus, flow cytometric myeloblast percentages were expected to be higher than those derived from morphology. However, flow cytometric myeloblast counts were often lower than the morphologic blast counts reported by the pathologist. Conceivably, hypogranular promyelocytes or other abnormally maturing myeloid cells may be included in the blast count by light microscopy. Twenty-seven patients were identified by flow cytometry as having abnormal myeloblasts at less than 5%. Nineteen of these marrows were not identified as abnormal by microscopic examination.
Flow cytometry score and outcome after transplantation
One hundred eleven patients underwent allogeneic SCT. Flow cytometry was evaluated a median of 21 days (range, 1-124 days) prior to SCT. At a median follow-up of 4 years (range, 1.1-6.5 years), 59 patients were alive. Fifty-two died at a median of 5.8 months (range, 0.4-52.5 months) after SCT. The 5-year overall survival (OS) was 49% for the entire group, 53% for patients with idiopathic MDS, 26% for patients with secondary MDS, and 50% for patients with predisposing hematologic disease (P = .08). Nineteen patients had disease recurrence for a cumulative relapse incidence of 18% (idiopathic MDS, 15%; secondary MDS, 33%; predisposing disease, 20%; P = .10). There were no significant differences with regard to NRM or acute (grades II-IV) or chronic GVHD.
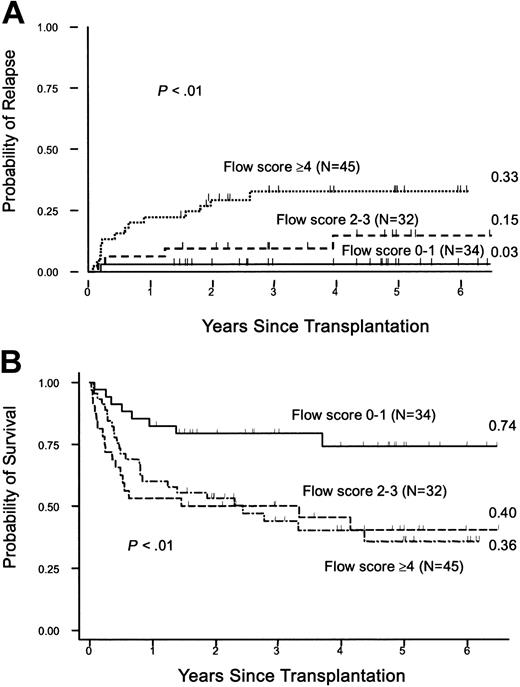
Posttransplantation outcome was analyzed on the basis of pretransplantation flow cytometry scores categorized into mild, moderate, and severe. As shown in Figure 4A, the cumulative incidences of relapse at 5 years were 3%, 15%, and 33%, respectively (P < .01). The corresponding numbers for OS were 74%, 40%, and 36%, respectively (P < .01; Figure 4B). When patients with secondary MDS and predisposing hematologic disease (n = 26) were excluded, the relapse incidences were 0.0%, 13%, and 28%, respectively, for the 3 flow score groups (P = .01). The incidence of NRM was marginally associated with flow scores (P = .06), mainly due to a significant difference between mild and moderate groups. Acute and chronic GVHD did not significantly correlate with flow scores.
Flow scores and survival. (A) Cumulative incidence of relapse by flow score. Patients were assigned flow scores based on pretransplantation marrow analysis and outcome was analyzed using various cutoff values as described in the text. Shown are the cumulative incidences of relapse (figures at right margin) for the groups with mild (0-1), moderate (2-3), and severe (≥ 4) scores, respectively. (B) Survival. Kaplan-Meier estimates of survival by flow scores. Shown are survival estimates (figures at right margin) for patients with mild, moderate, and severe flow scores.
Flow scores and survival. (A) Cumulative incidence of relapse by flow score. Patients were assigned flow scores based on pretransplantation marrow analysis and outcome was analyzed using various cutoff values as described in the text. Shown are the cumulative incidences of relapse (figures at right margin) for the groups with mild (0-1), moderate (2-3), and severe (≥ 4) scores, respectively. (B) Survival. Kaplan-Meier estimates of survival by flow scores. Shown are survival estimates (figures at right margin) for patients with mild, moderate, and severe flow scores.
Multivariate Cox regression was performed using the individual components of the IPSS (morphologic blast percentages, cytogenetics, and cytopenias) and the flow score to assess the added value of flow cytometry in predicting clinical outcome. The results of this analysis are shown in Table 8. The Cox regression models show that there was a significant contribution by the flow score beyond that provided by the IPSS in predicting survival, relapse, and NRM (P < .01, P = .02, and P = .03, respectively). Although cytogenetics remained significant for survival and relapse (P < .01 for both), morphologic blasts and cytopenias were not significant risk factors.
Multivariate Cox regression models
Factor . | Hazard ratio . | P* . | [95% Confidence interval] . |
|---|---|---|---|
| Outcome/survival Flow score | |||
| Mild | 1.0 | — | — |
| Moderate | 3.8 | < .01 | [1.6,9.3] |
| Severe | 2.4 | .07 | [0.9,6.3] |
| Overall likelihood ratio, P | < .01 | ||
| Morph blast, % | |||
| Less than 5 | 1.0 | — | — |
| 5-10 | 1.8 | .18 | [0.8,4.2] |
| 11-20 | 1.3 | .55 | [0.5,3.6] |
| 21 or more | 1.9 | .29 | [0.6,6.2] |
| Overall likelihood ratio, P | .54 | ||
| Karyotype group | |||
| Good | 1.0 | — | — |
| Intermediate | 0.8 | .68 | [0.3,2.1] |
| Poor | 2.9 | <.01 | [1.5,5.3] |
| Overall likelihood ratio, P | <.01 | ||
| Cytopenias | |||
| 0/1 | 1.0 | — | — |
| 2/3 | 1.2 | .63 | [0.6,2.3] |
| Outcome/relapse Flow score | |||
| Mild | 1.0 | — | — |
| Moderate | 6.6 | .06 | [0.7,62.0] |
| Severe | 11.8 | < .01 | [1.3,104.3] |
| Overall likelihood ratio, P | .02 | ||
| Morph blast, % Less than 5 | 1.0 | — | — |
| 5-10 | 1.1 | .94 | [0.3,4.4] |
| 11-20 | 1.5 | .56 | [0.4,6.7] |
| 21 or more | 2.6 | .27 | [0.5,12.9] |
| Overall likelihood ratio, P | .68 | ||
| Karyotype group | |||
| Good | 1.0 | — | — |
| Intermediate | 1.3 | .77 | [0.2,7.0] |
| Poor | 9.0 | < .01 | [2.8,28.8] |
| Overall likelihood ratio, P | < .01 | ||
| Cytopenias | |||
| 0/1 | 1.0 | — | — |
| 2/3 | 1.2 | .75 | [0.4,3.9] |
| Outcome/nonrelapse mortality Flow score | |||
| Mild | 1.0 | — | — |
| Moderate | 3.1 | .01 | [1.2,7.7] |
| Severe | 1.1 | .81 | [0.4,3.4] |
| Overall likelihood ratio, P | .03 | ||
| Morph blast, % Less than 5 | — | — | — |
| 5-10 | 2.2 | .13 | [0.8,5.8] |
| 11-20 | 1.6 | .45 | [0.5,5.1] |
| 21 or more | 1.5 | .63 | [0.3,8.3] |
| Overall likelihood ratio, P | .51 | ||
| Karyotype group | |||
| Good | — | — | — |
| Intermediate | 0.6 | .40 | [0.2,1.9] |
| Poor | 1.9 | .09 | [0.9,4.0] |
| Overall likelihood ratio, P | .09 | ||
| Cytopenias | |||
| 0/1 | — | — | — |
| 2/3 | 1.4 | .36 | [0.6,3.1] |
Factor . | Hazard ratio . | P* . | [95% Confidence interval] . |
|---|---|---|---|
| Outcome/survival Flow score | |||
| Mild | 1.0 | — | — |
| Moderate | 3.8 | < .01 | [1.6,9.3] |
| Severe | 2.4 | .07 | [0.9,6.3] |
| Overall likelihood ratio, P | < .01 | ||
| Morph blast, % | |||
| Less than 5 | 1.0 | — | — |
| 5-10 | 1.8 | .18 | [0.8,4.2] |
| 11-20 | 1.3 | .55 | [0.5,3.6] |
| 21 or more | 1.9 | .29 | [0.6,6.2] |
| Overall likelihood ratio, P | .54 | ||
| Karyotype group | |||
| Good | 1.0 | — | — |
| Intermediate | 0.8 | .68 | [0.3,2.1] |
| Poor | 2.9 | <.01 | [1.5,5.3] |
| Overall likelihood ratio, P | <.01 | ||
| Cytopenias | |||
| 0/1 | 1.0 | — | — |
| 2/3 | 1.2 | .63 | [0.6,2.3] |
| Outcome/relapse Flow score | |||
| Mild | 1.0 | — | — |
| Moderate | 6.6 | .06 | [0.7,62.0] |
| Severe | 11.8 | < .01 | [1.3,104.3] |
| Overall likelihood ratio, P | .02 | ||
| Morph blast, % Less than 5 | 1.0 | — | — |
| 5-10 | 1.1 | .94 | [0.3,4.4] |
| 11-20 | 1.5 | .56 | [0.4,6.7] |
| 21 or more | 2.6 | .27 | [0.5,12.9] |
| Overall likelihood ratio, P | .68 | ||
| Karyotype group | |||
| Good | 1.0 | — | — |
| Intermediate | 1.3 | .77 | [0.2,7.0] |
| Poor | 9.0 | < .01 | [2.8,28.8] |
| Overall likelihood ratio, P | < .01 | ||
| Cytopenias | |||
| 0/1 | 1.0 | — | — |
| 2/3 | 1.2 | .75 | [0.4,3.9] |
| Outcome/nonrelapse mortality Flow score | |||
| Mild | 1.0 | — | — |
| Moderate | 3.1 | .01 | [1.2,7.7] |
| Severe | 1.1 | .81 | [0.4,3.4] |
| Overall likelihood ratio, P | .03 | ||
| Morph blast, % Less than 5 | — | — | — |
| 5-10 | 2.2 | .13 | [0.8,5.8] |
| 11-20 | 1.6 | .45 | [0.5,5.1] |
| 21 or more | 1.5 | .63 | [0.3,8.3] |
| Overall likelihood ratio, P | .51 | ||
| Karyotype group | |||
| Good | — | — | — |
| Intermediate | 0.6 | .40 | [0.2,1.9] |
| Poor | 1.9 | .09 | [0.9,4.0] |
| Overall likelihood ratio, P | .09 | ||
| Cytopenias | |||
| 0/1 | — | — | — |
| 2/3 | 1.4 | .36 | [0.6,3.1] |
P value from a likelihood ratio test. — indicates not applicable.
As indicated, the percentage of myeloblasts, as determined by flow cytometry, also increased with increasing IPSS scores (P < .01). However, because blast count is included in both the flow cytometric score and IPSS score, the correlation observed may be a result of enumeration of blasts in the 2 systems. Flow cytometry can evaluate whether myeloblasts are normal or phenotypically aberrant, and adding information beyond myeloblast percentage may be of prognostic value in addition to conventional parameters. The flow score was modified in an analysis so that 2 points were given to all patients when myeloblasts were abnormal, rather than adding points on the basis of percentage of myeloblasts (to avoid having percent blasts in both the flow score and the IPSS). An additional multivariate Cox regression using the adjusted flow score showed similar results. Survival, relapse, and NRM by flow score remained significant (P < .01, P = .03, and P = .03); cytogenetics was also still significant (P < .01 for both), whereas morphologic blasts and cytopenias were not significant.
A comparison of flow cytometric score with IPSS scores shows that among the 12 patients with low risk IPSS scores, 11 had flow scores in the mild or moderate category. Only one patient within the IPSS low-risk group was severe by flow score (Table 9). None of these patients experienced a relapse after transplantation. Conversely, there were no patients with low flow scores within the IPSS high-risk group.
Cross-tabulation of IPSS category and flow score
. | Flow score category . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| IPSS category . | Mild . | Moderate . | Severe . | Total . | |||
| Low | 8 | 3 | 1 | 12 | |||
| Int-1 | 18 | 22 | 12 | 52 | |||
| Int-2 | 6 | 6 | 19 | 31 | |||
| High | 0 | 1 | 13 | 14 | |||
| Total | 32 | 32 | 45 | 109 | |||
. | Flow score category . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| IPSS category . | Mild . | Moderate . | Severe . | Total . | |||
| Low | 8 | 3 | 1 | 12 | |||
| Int-1 | 18 | 22 | 12 | 52 | |||
| Int-2 | 6 | 6 | 19 | 31 | |||
| High | 0 | 1 | 13 | 14 | |||
| Total | 32 | 32 | 45 | 109 | |||
Figure 5A shows the incidence curves for probability of relapse by IPSS category. Although there was a clear separation of high versus low IPSS category, there was less separation between the intermediate-1 and intermediate-2 categories. Within the IPSS intermediate-1 risk group, 12 of 52 patients had total flow scores indicating severe abnormalities and suggesting more advanced disease by flow cytometry than assessed by IPSS scoring alone. Figure 5B shows the probability of relapse by flow score for patients in the IPSS intermediate-1 risk group. There was a significantly higher probability for relapse in patients with severe flow scores compared to those with moderate or mild flow scores within the IPSS intermediate-1 group (P < .01). Among 31 patients in the intermediate-2 IPSS group, 6 had low flow scores (0-1), possibly indicating less severe dysmyelopoiesis. Figure 5C shows the probability of relapse by flow score within the IPSS intermediate-2 group. There was insufficient heterogeneity of flow scores within the low and high IPSS groups to construct relapse incidence curves by flow score category. The data suggest that for patients in the intermediate-1 risk by IPSS, the flow score adds discriminating power with regard to relapse. Within this group, mild, moderate, and severe risk flow score abnormalities resulted in relapse incidences of 0%, 13%, and 50%, respectively.
Cumulative incidence of relapse by IPSS score. (A) The cumulative incidences of relapse for low, intermediate-1, intermediate-2, and high IPSS groups are given at the right margin. (B) Cumulative incidence of relapse within the intermediate-1 (B) and within the intermediate-2 (C) IPSS risk group by flow score. Differences were significant for the intermediate-1 risk group (P < .01), but not for the intermediate-2 group.
Cumulative incidence of relapse by IPSS score. (A) The cumulative incidences of relapse for low, intermediate-1, intermediate-2, and high IPSS groups are given at the right margin. (B) Cumulative incidence of relapse within the intermediate-1 (B) and within the intermediate-2 (C) IPSS risk group by flow score. Differences were significant for the intermediate-1 risk group (P < .01), but not for the intermediate-2 group.
Discussion
The appearance of cell surface antigens on normal hematopoietic cells is a highly regulated and coordinated process that may be altered in hematopoietic diseases.9,20,21,26-29,47 MDSs comprise a rather heterogeneous spectrum of disorders, and several studies have shown a variety of aberrant antigen expression.6-8,10-13 In the present study we attempted to capture abnormal parameters determined by multidimensional flow cytometry into an FCSS to measure “distance from normal.” As observed with aberrant antigen expression in acute leukemias, the abnormalities detected in MDS patients were different from individual to individual prompting a scoring system based on the sum of the abnormalities rather than focusing on individual antigenic differences.2,38,47 The scoring system tested the components of myeloid maturation separately—blasts, monocytes, and neutrophils. The measure of phenotypic abnormalities on the maturing myeloid, monocytoid, and blasts showed a high correlation to IPSS scores. Moreover, in patients with MDS who underwent allogeneic SCT, the flow scores used to measure abnormal antigen expression correlated with posttransplantation outcome.
Flow cytometric analysis confirmed that a large proportion of MDS marrows had myeloid, monocytic, and myeloblast characteristics divergent from normal and significantly different from findings in patients with other marrow diseases. In the proposed flow scoring system, scores of 3 or greater completely distinguished MDS patients from controls, and flow scores of 2 or greater were significantly correlated with a diagnosis of MDS. Although no abnormalities were observed in marrows from healthy donors, single abnormalities were occasionally observed in patients with other diseases, particularly after chemotherapy. In such cases the marrow showed increased myelopoiesis, associated with single flow aberrancies. These data suggest that flow scores are useful to distinguish true myeloid dyspoiesis from chemotherapy effects, and aplastic anemia from MDS.48 Additional studies comparing results in patients with MDS to patients with other marrow diseases may further refine flow cytometric features as diagnostic parameters for MDS.
Patients with complex cytogenetic abnormalities showed extensive flow cytometric aberrancies and increased proportions of phenotypically abnormal myeloblasts. Conversely, patients with favorable karyotypes (using IPSS criteria) were more likely to have low flow scores, although several patients with favorable cytogenetics, including a normal karyotype, had marked flow cytometric abnormalities and increased flow scores. Therefore, flow cytometric analysis may be especially useful for risk assessment of MDS in patients with normal cytogenetics with minimal dysplastic changes by light microscopy and with low blast cell counts. Objective flow cytometric scoring in a larger data set may also be useful for following patients over time and in patients with syndromes overlapping with MDS such as myeloproliferative syndromes, HIV cytopenias, hypoplastic anemias, and AML with low blast cell counts.
Antigenic abnormalities observed on dysplastic maturing myeloid, monocytic, and myeloblastic cells are biologic signals of abnormal maturation. The present data suggest that myeloid abnormalities become more prominent as the disease progresses. The data also indicate that these abnormalities have prognostic significance for outcome after allogeneic SCT. The probability of posttransplantation relapse increased significantly with increasing flow scores, and the probability of survival declined. Although not surprising in patients who also had a poor-risk karyotype,41 the correlation was observed for all patients. Although karyotype remains a major prognostic factor for survival and relapse, a flow score that measures the dysplastic features of the myeloid series was shown to have a stronger impact than morphologic blast count or cytopenias in a multivariate analysis. The data also support that flow cytometric scoring may further separate individual patients with respect to risk of relapse in a larger data set that includes higher numbers of patients in each of the IPSS categories.
The flow cytometric analysis described here was restricted to the study of maturing myeloid cells, monocytes, and myeloblasts with specific, uniform combinations of myeloid and lymphoid antibodies. Although various antibody combinations are useful to study cellular immunophenotypes in MDS by flow cytometry,11,12,48 the consistent use of a precise panel of 13 antibodies generated a database that allowed for the development of a scoring system. Analyses of the erythroid and megakaryocytic compartments were not included in this analysis for several reasons. For cells of erythroid lineage, the panel of available antibodies is too limited to evaluate maturational irregularities. However, considering the fact that anemia often is the presenting and most prominent feature in patients with MDS, efforts should be directed at expanding our abilities to phenotypically characterize the erythroid compartment. In regard to megakaryocytes, difficulties are mostly technical in nature. Because of their low frequency, large size, propensity to clump, and the complicated preanalytical techniques involved, megakaryocytes are currently not amenable to routine flow cytometric analysis.49 In addition, platelet adhesion to other cells such as monocytes may lead to incorrect identification of megakaryocytes.50 Finally, although it may be possible to further refine the FCSS by incorporating additional antibodies, the methodology must remain practical if it is to be applied routinely.
In summary, the flow scoring system proposed here correlated with the established IPSS, and on its own was of prognostic significance (P < .01) in patients undergoing allogeneic SCT. The identification of myeloid or monocytic dyspoiesis and abnormal immunophenotypes of myeloblasts in patients with MDS by flow cytometry may add prognostic significance beyond that obtained by morphology and cytopenias. Future studies could conceivably combine and integrate multidimensional flow cytometric analyses with other parameters of MDS to further prognostic accuracy.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2002-09-2768.
Supported in part by Public Health Service grants CA 87948 and HL 36444. M.B. is supported by a fellowship from the Max Kade Foundation, New York, NY.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Eileen Bryant for guidance on cytogenetic data. We are grateful to Keely Ghirardelli and Richard Bennington for their excellent technical laboratory support and to Joseph Pierangeli for his careful reading of this manuscript.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal