Abstract
Persistence of alemtuzumab at lympholytic concentrations after reduced-intensity conditioning allogeneic stem cell transplantations (RITs) could impair immune reconstitution and reduce donor T-cell–mediated graft-versus-leukemia/lymphoma (GVL) effects, derived from the graft or subsequent adoptive immunotherapy. We have studied the pharmacokinetics of alemtuzumab in 2 different groups: RIT (100 mg alemtuzumab in vivo over 5 days) and myeloablative allografts (20 mg alemtuzumab added in vitro to the stem cells prior to return). Alemtuzumab concentrations in RIT patients were in excess of that required to kill infused donor CD52+ cells at the time of transplantation and remained at potentially lympholytic levels (> 0.1 μg/mL) for approximately 56 days after transplantation, 26 days longer than for the myeloablative group. Total lymphocyte counts were significantly lower in the RIT group persisting beyond 6 months after transplantation (P = .005), and median absolute CD4 counts higher than 200 × 106/L were delayed until 9 months after transplantation. (Blood. 2003;102:404-406)
Introduction
Reduced-intensity conditioning allogeneic stem cell transplantations (RITs) depend on immunosuppression to facilitate donor hematopoietic and lymphoid engraftment in the context of minimal recipient myeloablation, allowing patients previously considered unfit for a conventional allograft to benefit from the putative graft-versus-leukemia/lymphoma (GVL) effect. Many varied conditioning regimens are used with excellent engraftment rates, although acute graft-versus-host disease (GVHD) occurs in up to 50% of patients in published series.1-3
We have used a RIT conditioning regimen incorporating the monoclonal antibody alemtuzumab (CAMPATH-1H)—a humanized immunoglobulin G1 (IgG1) monoclonal antibody directed against the CD52 antigen expressed on lymphoid cells and cells of monocyte lineage—to achieve in vivo T-cell depletion, which is associated with very low rates of acute and chronic GVHD and low early transplantation-related mortality.4
Current dosing of alemtuzumab is based on previous work using the homologous rat IgG2b CAMPATH-1G, which is reported to have a shorter half-life than alemtuzumab.5 Slower clearance of alemtuzumab could thus impair immune reconstitution, affect rates of viral reactivation, and limit efficacy of the donor T-cell–mediated GVL effect.
In this study we have measured the pharmacokinetics of alemtuzumab given in 2 different regimens—RIT with high dose antibody in vivo for 5 days before transplantation and conventional total body irradiation (TBI)–based T-depleted allografts with a single, lower dose added to the stem cells in vitro. The serum alemtuzumab levels, half-life, and levels at up to 28 days after transplantation were determined. The effect of alemtuzumab on the immune system was measured by analysis of absolute lymphocyte counts, CD4 and CD8 counts, rate of viral reactivation, other infective complications, and expansion of adoptive T cells transferred (where appropriate).
Study design
Studied were 10 patients in the RIT group and 5 patients in the myeloablative transplantation group (Table 1).
Patient characteristics
Patient . | Age, y/sex . | Disease . | Previous autograft? . | Disease status at transplantation . | Donor/stem cell source . | CMV serostatus, donor/recipient . |
|---|---|---|---|---|---|---|
| RIT group | ||||||
| 01 | 26/M | LG NHL (transformed) | No | VGPR | MUD/BM | Pos/Neg* |
| 02 | 29/M | HD | Yes | CR | MUD/BM | Neg/Neg |
| 03 | 52/M | LG NHL | No | CR | MUD/PBSC | Pos/Neg* |
| 04 | 56/F | HG NHL | No | CR | Sib/PBSC | Neg/Pos* |
| 05 | 42/M | LG NHL (transformed) | Yes | VGPR | Sib/PBSC (1 Ag MM) | Neg/Neg |
| 06 | 46/M | HD | Yes | CR | Sib/PBSC | Neg/Neg |
| 07 | 51/M | MM | No | PR | Sib/PBSC | Pos/Pos* |
| 08 | 44/F | LG NHL | No | CR | Sib/PBSC | Neg/Pos |
| 09 | 53/M | MM | No | PR | MUD/BM | Neg/Pos |
| 10 | 51/M | MM | No | PR | Sib/PBSC | Neg/Neg |
| Myeloablative allograft group | ||||||
| 11 | 13/F | AML | No | 2nd CR | MUD/PBSC (2 Ag MM) | Pos/Pos* |
| 12 | 26/M | AML (Previous HD) | No | 1st CR | MUD/PBSC | Neg/Neg |
| 13 | 46/M | RAEBT | No | 1st CR | MUD/PBSC | Neg/Neg |
| 14 | 40/F | AML | No | 1st CR | Sib/PBSC | Pos/Pos* |
| 15 | 27/M | AML | No | 2nd CR | MUD/PBSC | Neg/Neg |
Patient . | Age, y/sex . | Disease . | Previous autograft? . | Disease status at transplantation . | Donor/stem cell source . | CMV serostatus, donor/recipient . |
|---|---|---|---|---|---|---|
| RIT group | ||||||
| 01 | 26/M | LG NHL (transformed) | No | VGPR | MUD/BM | Pos/Neg* |
| 02 | 29/M | HD | Yes | CR | MUD/BM | Neg/Neg |
| 03 | 52/M | LG NHL | No | CR | MUD/PBSC | Pos/Neg* |
| 04 | 56/F | HG NHL | No | CR | Sib/PBSC | Neg/Pos* |
| 05 | 42/M | LG NHL (transformed) | Yes | VGPR | Sib/PBSC (1 Ag MM) | Neg/Neg |
| 06 | 46/M | HD | Yes | CR | Sib/PBSC | Neg/Neg |
| 07 | 51/M | MM | No | PR | Sib/PBSC | Pos/Pos* |
| 08 | 44/F | LG NHL | No | CR | Sib/PBSC | Neg/Pos |
| 09 | 53/M | MM | No | PR | MUD/BM | Neg/Pos |
| 10 | 51/M | MM | No | PR | Sib/PBSC | Neg/Neg |
| Myeloablative allograft group | ||||||
| 11 | 13/F | AML | No | 2nd CR | MUD/PBSC (2 Ag MM) | Pos/Pos* |
| 12 | 26/M | AML (Previous HD) | No | 1st CR | MUD/PBSC | Neg/Neg |
| 13 | 46/M | RAEBT | No | 1st CR | MUD/PBSC | Neg/Neg |
| 14 | 40/F | AML | No | 1st CR | Sib/PBSC | Pos/Pos* |
| 15 | 27/M | AML | No | 2nd CR | MUD/PBSC | Neg/Neg |
LG NHL indicates low-grade non-Hodgkin lymphoma; VGPR, very good partial remission; MUD, matched unrelated donor; BM, bone marrow; Pos, CMV seropositive; Neg, CMV seronegative; HD, Hodgkin disease; CR, complete remission; PBSC, peripheral blood stem cells; HG NHL, high-grade non-Hodgkin lymphoma; Sib, sibling; Ag, antigen; MM, multiple myeloma; PR, partial remission; AML, acute myeloid leukemia; and RAEBT, refractory anemia with excess blasts in transformation.
CMV reactivation.
Conditioning in the RIT group consisted of intravenous infusions of 20 mg/d alemtuzumab from day - 8 to - 4 (total 100 mg), 25 mg/m2 fludarabine daily from day - 7 to - 3, and 140 mg/m2 melphalan on day - 2. Cyclosporin A (CsA) was also administered from day - 1 at 3 mg/kg/d. On day 0, patients received granulocyte colony-stimulating factor (G-CSF)–mobilized unmanipulated allogeneic peripheral blood stem cells (PBSCs)6 or bone marrow.3 CsA was tailed from 2 to 3 months after transplantation.
Patients in the myeloablative group received 60 mg/kg cyclophosphamide on days - 6 and - 5 followed by TBI on days - 4 to - 1 (14.4 Gy). Fludarabine (30 mg/m2) was given from day - 9 to - 7 to the 3 patients receiving matched unrelated donor (MUD) allografts. Alemtuzumab (20 mg) was added to the stem cells at room temperature 30 minutes prior to their reinfusion. CsA was administered as for the RIT group.
Antiviral prophylaxis was with aciclovir. Weekly surveillance for cytomegalovirus (CMV) infection by quantitative polymerase chain reaction (PCR) was performed, with 2 consecutive positives triggering treatment. Antiviral and Pneumocystis carinii prophylaxis were continued until the absolute CD4+ T-cell count was higher than 200 × 106/L. Fungal prophylaxis consisted of intravenous itraconazole until neutrophil regeneration.
Results and discussion
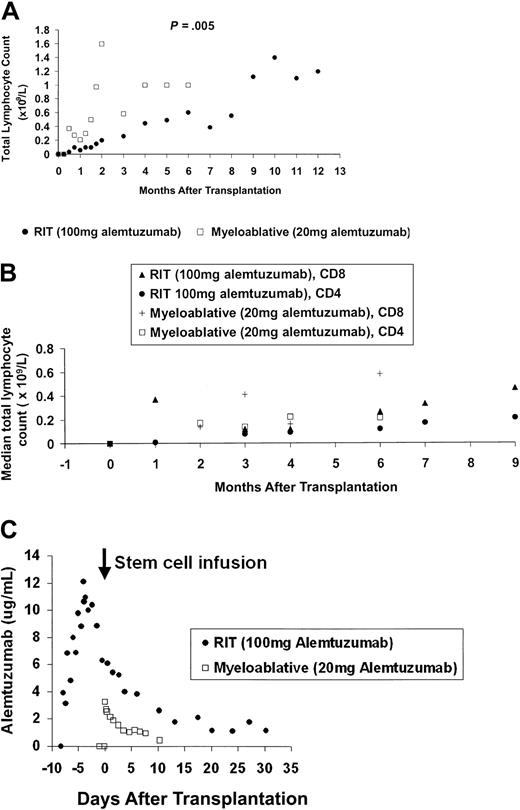
Median total lymphocyte counts lower than 0.05 × 109/L (range, 0.00-0.6 × 109/L) in the RIT group and lower than 0.01 × 109/L (range, 0.00-0.05 × 109/L) in the myeloablative allograft group occurred 24 hours after the first infusion of alemtuzumab, with values 8 weeks after transplantation of 0.15 × 109/L (range, 0.0-0.21 × 109/L) and 1.06 × 109/L (range, 0.25-2.66 × 109/L), respectively (Figure 1A) (P = .005). Significant differences in longer term lymphoid recovery are also shown, with a 5-month delay in achieving sustained total lymphocyte counts higher than 1 × 109/L for the RIT group.
Immune reconstitution and alemtuzumab pharmacokinetics. (A) Early and long-term lymphoid recovery. Median absolute total lymphocyte counts with 12 months follow-up for the high-dose in vivo (RIT) group and 6 months follow-up for the low-dose in vitro (myeloablative allograft) group. (B) Recovery of T-cell subsets. Median lymphocyte counts after transplantation until 9 months after transplantation follow-up for the high-dose in vivo (RIT) group and 6 months after transplantation for the low-dose in vitro (myeloablative allograft) group. (C) Median serum alemtuzumab levels. Assays of alemtuzumab in patient serum: serum samples for pharmacokinetic analysis were collected 24 hours prior to the first infusion of alemtuzumab, 15 minutes before and after each infusion, 4 hourly for 24 hours after the last infusion, daily for the next 7 days, and then twice weekly until at least 28 days after the last alemtuzumab infusion. Sera were stored at -70°C until analysis. Before analysis, complement was inactivated by heat incubation of the samples at 56°C for 30 minutes. Validation studies have previously confirmed that this step does not alter alemtuzumab activity. Alemtuzumab activity was measured by indirect immunofluorescence as described in detail elsewhere.7,8 Previous studies have determined the following parameters for this assay18 : limit of detection, 0.3 μg/mL; analytical range, 0.5 to 20 μg/mL; linearity, 0.999; overall precision, 13%; and bias, +9%. There was no interference by a range of normal and patient control sera, and no reactivity with F(ab′)2 fragments of alemtuzumab. The assay has been shown to be robust with respect to variations in sample treatment and storage conditions, and variations in assay procedures. All sera samples were tested in duplicate at a final dilution of 1:2. Patient weight, total leukocyte count, underlying disease, and disease status at time of transplantation did not affect alemtuzumab levels or lymphocyte depletion.
Immune reconstitution and alemtuzumab pharmacokinetics. (A) Early and long-term lymphoid recovery. Median absolute total lymphocyte counts with 12 months follow-up for the high-dose in vivo (RIT) group and 6 months follow-up for the low-dose in vitro (myeloablative allograft) group. (B) Recovery of T-cell subsets. Median lymphocyte counts after transplantation until 9 months after transplantation follow-up for the high-dose in vivo (RIT) group and 6 months after transplantation for the low-dose in vitro (myeloablative allograft) group. (C) Median serum alemtuzumab levels. Assays of alemtuzumab in patient serum: serum samples for pharmacokinetic analysis were collected 24 hours prior to the first infusion of alemtuzumab, 15 minutes before and after each infusion, 4 hourly for 24 hours after the last infusion, daily for the next 7 days, and then twice weekly until at least 28 days after the last alemtuzumab infusion. Sera were stored at -70°C until analysis. Before analysis, complement was inactivated by heat incubation of the samples at 56°C for 30 minutes. Validation studies have previously confirmed that this step does not alter alemtuzumab activity. Alemtuzumab activity was measured by indirect immunofluorescence as described in detail elsewhere.7,8 Previous studies have determined the following parameters for this assay18 : limit of detection, 0.3 μg/mL; analytical range, 0.5 to 20 μg/mL; linearity, 0.999; overall precision, 13%; and bias, +9%. There was no interference by a range of normal and patient control sera, and no reactivity with F(ab′)2 fragments of alemtuzumab. The assay has been shown to be robust with respect to variations in sample treatment and storage conditions, and variations in assay procedures. All sera samples were tested in duplicate at a final dilution of 1:2. Patient weight, total leukocyte count, underlying disease, and disease status at time of transplantation did not affect alemtuzumab levels or lymphocyte depletion.
In addition, a median absolute CD4 count higher than 200 × 106/L (range, 160-270 × 106/L) was reached at 6 months after transplantation following myeloablative allograft, but not until 9 months in the RIT group, median 210 × 106/L (range, 130-310 × 106/L) (Figure 1B).
In previous studies, alemtuzumab doses as small as 10 mg result in prolonged lymphopenia6,9 and high doses may not be required to prevent graft rejection. It has, however, been suggested that persistent elevated concentrations of alemtuzumab may delay engraftment by loss of the graft-enhancing effect of donor T cells. No graft rejection was seen in this study and median times to neutrophil and platelet recovery were 13 and 14 days, respectively.
Despite other differences between the conditioning regimens, it is likely that the differences in immune reconstitution are due principally to the persistence of alemtuzumab, as RIT conditioning using fludarabine without alemtuzumab results in more rapid immune reconstitution but higher GVHD rates.10-12
Regarding pharmacokinetics, in the RIT group the median peak level was 13.7 μg/mL (range, 7.5-16.6 μg/mL), occurring 15 minutes after the final dose (Figure 1C). At 28 days, the median level was 1.0 μg/mL and above the limit of quantitation (0.5 μg/mL) in 7 of 10 patients. The half-life from 4 to 32 days after the last infusion was 8 days, giving an estimated time to achieve levels below 0.1 μg/mL of 60 days.
In the myeloablative allograft group the median peak level was 3.2 μg/mL (range, 1.0-5.0 μg/mL), occurring at 15 minutes following the infusion of stem cells containing alemtuzumab. By day +10, levels were below the limit of quantitation in 3 patients, just above (0.8 μg/mL) in 1, and not determined in 1. The terminal half-life from 2 to 8 days after infusion was 7 days, not significantly different from the RIT group. The predicted time required to achieve antibody levels lower than 0.1 μg/mL was 30 days.
Alemtuzumab activates complement and binds human Fc receptors, but complement-mediated lysis alone is insufficient for depletion of lymphocytes in vivo13 and cell-mediated killing (ADCC) is believed more important.7 Concentrations as low as 0.1 μg/mL are sufficient to opsonize lymphocytes for cell ADCC in vitro,8 therefore ongoing depletion of CD52+ cells might occur for approximately 2 months after transplantation in the RIT group.
With respect to infection rates, 4 of 6 “at risk” donor-recipient pairs (RIT group), and 2 of 2 “at risk” pairs (myeloablative allograft group) reactivated CMV. Biopsy-proven CMV colitis and prolonged PCR positivity was seen in one RIT patient. No other documented cases of CMV disease occurred. One patient (RIT) received donor-derived CMV cytotoxic T lymphocytes (CTLs) on day +35 and cleared CMV, which is in keeping with published results with this regimen14-16 where adoptive immunotherapy as early as day +29 resulted in sustained expansion of infused cells. Despite high levels of CMV reactivation, no difference has been observed in survival when alemtuzumab was compared with alternative GVHD prophylaxis in RITs.16
Regarding GVHD, in the RIT group, 2 patients developed grade I and 1 developed grade II acute GVHD (one following further donor PBSCs at day +35). Of 8 evaluable patients, 3 developed chronic GVHD (2 limited, 1 extensive). One patient developed extensive chronic GVHD following donor leukocyte infusion.
In the myeloablative allograft group, 2 patients developed grade I acute GVHD, and both progressed to limited chronic cutaneous GVHD. One further patient developed limited chronic GVHD.
Thus, high dose in vivo alemtuzumab effectively prevents GVHD17 and allows engraftment, while circulating serum antibody levels are in excess of that required to be effective in target cell lysis and are still detectable at the time of return of CMV-specific CTLs. Ongoing dose-reduction studies will determine the optimal dose of in vivo alemtuzumab and may show more rapid immune reconstitution and perhaps improved disease-free survival.
Prepublished online as Blood First Edition Paper, March 6, 2003; DOI 10.1182/blood-2002-09-2687.
Supported by the Leukaemia Research Fund, United Kingdom (E.C.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal