Abstract
Exosomes are small membrane vesicles secreted by cells upon fusion of multivesicular endosomes with the cell surface. The mechanisms underlying the specific sorting of proteins in exosomal membranes are far from being unraveled. We demonstrate here, using different cells, that some molecules are released in the extracellular medium via their association with lipid raft domains of the exosomal membrane. Various typical raft-associated molecules could be detected by immunoblot in exosomes and Triton X-100-insoluble fractions isolated from exosomes of different origins. Partial localization of major histocompatibility complex (MHC) class II molecules with detergent-resistant fractions isolated from Daudi-secreted exosomes was demonstrated by immunoblot and confirmed by electron microscopy colocalization of MHC class II molecules and ganglioside GM1. Moreover, we found that exosome-associated Lyn (1) had a lower molecular weight compared with Lyn detected in cell-isolated detergent-resistant domains, (2) was absent from the Triton X-100-insoluble fraction isolated from exosomes, and (3) had lost its partitioning capacity in Triton X-114. Exosomal Lyn is probably cleaved by a caspase-3-like activity contained in secreted vesicles. All together, the data highlight the presence of lipid microdomains in exosomal membranes and suggest their participation in vesicle formation and structure, as well as the direct implication of exosomes in regulatory mechanisms. (Blood. 2003;102:4336-4344)
Introduction
Exosomes were first described in studies of reticulocyte maturation about 20 years ago1,2 and more recently found to be released by other cells.3-7 Exosomes correspond to internal vesicles of multivesicular bodies (MVBs) and are released in the extracellular medium upon fusion of MVBs with the plasma membrane. Exosome secretion was shown to be responsible for the loss of transferrin receptors (TfRs) during reticulocyte maturation.8 On the other hand, exosomes secreted by antigen-presenting cells (APCs) contain few TfRs, but are enriched with major histocompatibility complex (MHC) class II molecules,3,4 which has led to investigations of their possible implication in intercellular communication during the immune response.9,10 The molecular basis of protein sorting during exosome formation is not fully understood. However, the recently identified machinery involved in MVB formation is likely to be relevant for the biogenesis of exosomes.10 A first step involves segregation of proteins at the limiting membrane of the endosome. The second step is inward budding with selected cargo into internal vesicles. At least 3 protein complexes, named endosomal sorting complex responsible for transport 1, 2, and 3 (ESCRT-1, -2, and -3), were shown to be subsequently involved in these steps.11-14 Among these steps, ubiquitination and deubiquitination of selected cargo by proteins of these complexes seem to play a key role,15-17 although other proteins follow the same pathway in a ubiquitin-independent manner.18 Finally, lipid metabolism19 and phosphoinositide signaling20-22 are certainly also involved in the MVB biogenesis.
The study of the exosomal process in reticulocytes highlighted several points. Sorting by cytosolic machinery is not an obligatory process or at least not the sole one, since (1) lipids incorporated into the external leaflet of the plasma membrane are very efficiently sorted and secreted in association with exosomes,23 (2) acetylcholinesterase (AChE), a glycosylphosphatidylinositol (GPI)-anchored protein in red cells, is similarly lost by approximately 50% by reticulocytes through exosome release during differentiation in erythrocytes,24 and (3) exoplasmic cross-linking of membrane proteins (TfR, AChE) by antibodies favors their sorting into exosomes.23 Segregation and association of specific lipids and proteins in the plasma membrane to form microdomains is now largely accepted. These membrane subdomains, termed lipid rafts, can be isolated owing to their insolubility in nonionic detergents and low buoyant density on sucrose density gradients.25 These low-density, Triton-insoluble (LDTI) fractions are enriched in cholesterol and glycosphingolipids, and contain different acylated proteins such as GPI-anchored proteins and tyrosine kinases of the Src family.26
Our previous studies on reticulocyte exosomes gave us reason to suspect the presence of rafts in secreted vesicles: (1) the cholesterol-phospholipid ratio was found to be similar to that noted in plasma membrane,27 considered a cholesterol-rich membrane compared with intracellular compartments; (2) the ganglioside GM1 is found in exosomes28 ; (3) GPI-anchored proteins such as CD55, CD58, and CD59 are selectively sorted in exosomes during reticulocyte maturation,28 possibly explaining the phenotype change in red blood cells during their final differentiation stage29 ; (4) Lyn, a protein kinase of the Src family, was found associated with exosomes secreted by reticulocytes and erythroleukemic cells30 ; and (5) the heterotrimeric G protein α-subunit (Gαi2) is present in reticulocyte exosomes.31 The finding that these molecules—considered to be typical lipid raft markers—are present in reticulocyte exosomes suggests that lipid raft domains may participate in molecule segregation during reticulocyte exosome formation. In fact, raft domain involvement in molecule sorting in exosomes (ie, internal vesicles of MVBs) may occur in various cells since electron microscopy studies using perfringolysin, a cholesterol-binding toxin, recently showed the presence, in B lymphocytes, of cholesterol in intralumenal vesicles of MVBs and in exosomes present in the intercellular space.32
We now present data showing that GM1, Lyn, flotillin-1, and stomatin, which were previously shown to be present in detergent-resistant domains in several cell types,25,33,34 are also present in LDTI fractions isolated on sucrose gradient from reticulocytes, K562, and Daudi cells. Importantly, these molecules are further sorted as raft components in exosomes. Moreover, our results suggest that these raft domains may support the sorting of other proteins, such as MHC class II molecules, that we found partially associated with detergent-resistant domains isolated from Daudi exosomes. Finally, we provide evidence that, after sorting in exosomal membranes, raft-associated Lyn is probably processed by a caspase-3-like protease in the exosomal cytosol.
Materials and methods
Cells
Reticulocyte production in Sprague-Dawley rats was induced by phenylhydrazine, as previously described.35 Human reticulocytes (always exceeding 6%) were obtained from patients during routine follow-up visits to the Hôpital St Eloi (Montpellier, France). The lymphoid B-cell line Daudi and human erythroleukemia cell line K562 were maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS), streptomycin (50 μg/mL), and penicillin (50 U/mL).
Antibodies
Rat monoclonal antibody (MoAb) anti-heat shock cognate protein 70 (anti-hsc70) (SPA-815), and mouse MoAb antihuman TfR (H68.4) were from Stressgen Biotechnologies (Victoria, BC, Canada) and Zymed Laboratories (South San Francisco, CA), respectively. Mouse MoAb antihuman leukocyte antigen-DRα (HLA-DRα) chain (clone TAL.1B5) was obtained from Dako (Hamburg, Germany). Mouse MoAb raised against human HLA-DR (clone BL2) was from Immunotech (Marseilles, France). Mouse MoAb anti-flotillin-1 (clone 18) was from BD Pharmingen (San Diego, CA); rabbit anti-Lyn, goat anti-tsg101, and rabbit anti-caspase-3 (H-277) were from Santa Cruz Biotechnology (Santa Cruz, CA). Peroxidase-conjugated goat antirat immunoglobulin G (IgG), peroxidase-conjugated donkey antimouse IgG, and donkey antigoat IgG were obtained from Jackson ImmunoResearch Laboratories (Bar Harbor, ME). Peroxidase-conjugated donkey antirabbit IgG and fluorescein isothiocyanate (FITC)-conjugated donkey antimouse IgG were from Rockland Immunochemicals (Gilbertsville, PA). Mouse antihuman stomatin (glutamic acid/alanine-rich protein 50 [GARP-50]) was a generous gift from Rainer Prohaska (University of Vienna, Austria).
Reagents
Biotinylated cholera toxin-B subunit and Triton X-114 were from Sigma (St Louis, MO); peroxidase-conjugated streptavidin was from Amersham (Arlington Heights, IL). Phycoerythrin (PE)-conjugated streptavidin and the EnzChek caspase-3 assay kit were obtained from Molecular Probes (Leiden, The Netherlands). Protein concentrations in samples were determined by the bicinchoninic acid (BCA) method (Pierce, Rockford IL).
Exosome isolation
Exosomes were isolated by differential centrifugation as described previously.35 For further exosome purification, pellets were resuspended in 4 mL 2.55 M sucrose, 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)/NaOH, pH 7.4. A linear sucrose gradient (2.25 to 0 M sucrose, 20 mM HEPES/NaOH, pH 7.4) was layered on the top of exosome suspension in a Beckman SW41 tube (Beckman Coulter, Fullerton, CA). Gradients were centrifuged for 15 hours at 30 000 rpm, after which 500 μL fractions were collected from the top of the tube. After trichloroacetic acid (TCA) precipitation, fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot.
Isolation of LDTI membranes
Cells. LDTI fractions were isolated as previously described.25 Briefly, K562 or Daudi cells (1 × 109) were washed in phosphate-buffered saline (PBS) (pH 7.3) and lysed in 2 mL ice-cold MBS lysis buffer (25 mM Mes, 150 mM NaCl, pH 6.5) containing 1% Triton X-100 for 30 minutes on ice and homogenized with 10 strokes of a dounce homogenizer. The lysate was adjusted to 40% (wt/vol) sucrose (6 mL), placed in the bottom of an ultracentrifuge tube (Beckman SW40), and overlaid with 4 mL 30% sucrose and 2.5 mL MBS. Ultracentrifugation was performed at 39 000 rpm for 16 hours at 4°C. Then, 500 μL fractions were collected from the top of the gradient. For red cells, LDTI membranes were isolated from ghosts prepared from a 250 μL packed cell volume of rat reticulocytes (exceeding 70%)35 with the same protocol scaled down by means of a Beckman SW55 rotor. For Western blot analysis, fractions were precipitated with TCA before SDS-PAGE; for dot blot analysis, 100 μL of each fraction was dotted onto nitrocellulose filters; for bead adsorption, LDTI fractions were pooled, mixed with a 5-fold volume of MBS, and centrifuged at 200 000g for 1 hour at 4°C. Pellets were then resuspended in PBS.
Exosomes. Exosomes were isolated from 1 L culture supernatant and washed once with MBS. The pellet was then resupended in 1 mL ice-cold MBS 1%, Triton X-100, at 4°C overnight and homogenized with 10 strokes of a dounce homogenizer. The lysate was adjusted to 40% (wt/vol) sucrose (2 mL), placed in the bottom of a tube (SW55, Beckman), and overlaid with 2 mL 30% sucrose and 1 mL MBS. Ultracentrifugation was performed at 39 000 rpm for 16 hours at 4°C. Fraction collection and analysis were carried out as described.
FACS analysis of exosomes and LDTI fractions
Magnetic beads with covalently bound antimouse IgG (Dynal, Oslo, Norway) were incubated with a mouse anti-HLA-DR antibody (clone BL2) as described by the manufacturer. Exosomes or LDTI fractions were incubated at room temperature for 2 hours with these activated magnetic beads. The beads were then separated magnetically and washed 3 times with PBS, 0.1% bovine serum albumin (BSA).
In parallel, exosomes or LDTI membranes were incubated with 25 μL of 4-μm-diameter aldehyde/sulfate latex beads (Interfacial Dynamics, Portland, OR) for 2 hours at room temperature in a 30 to 100 μL final volume; 50 μg BSA was then added to each sample, and the incubation was prolonged for 15 minutes. This was followed by overnight incubation in 1 mL PBS with gentle shaking. After the reaction was stopped with glycine, membrane-coated beads were then washed 3 times and resuspended in fluorescence-activated cell sorter (FACS) wash (1% FCS in PBS).
Membrane-coated latex or magnetic beads were incubated for 45 minutes with anti-HLA-DR antibody or biotin-labeled cholera toxin B subunit, followed by incubation with FITC-conjugated antibody or PE-conjugated streptavidin, and analyzed on a FACSCalibur flow cytometer (BD Biosciences, Le Pont de Claix, France), with a 488-nm argon laser and standard band pass filters for FL-1 (530/30 nm) and FL-2 (585/42 nm).
Triton X-114 partition
First, 50 μL aliquots of purified exosomes or LDTI membranes were diluted in 200 μL 1% Triton X-114 in 10 mM Tris (tris(hydroxymethyl)aminomethane)/HCl, 0.15 M NaCl, pH 7.4. The samples were incubated on ice for 5 minutes and then overlaid onto a 6% sucrose (wt/vol), 0.06% (vol/vol) Triton X-114, 10 mM Tris/HCl, 0.15 M NaCl, pH 7.4 cushion (300 μL); incubated for 3 minutes at 30°C; and centrifuged for 3 minutes at 3000g. The top aqueous phases were transferred to new tubes and re-extracted with 0.5% Triton X-114, left for 5 minutes on ice, and then overlaid onto the same sucrose cushion, incubated for 3 minutes at 30°C, and centrifuged for 3 minutes at 3000g. The aqueous phase was re-extracted with 2% Triton X-114 as described. The final aqueous phase was about 150 μL, and the detergent phase was made up to the same volume by adding 10 mM Tris/HCl, 0.15 M NaCl, pH 7.4.
Western blot analysis
Samples were separated by SDS-PAGE with the use of 10%, 12%, or 15% polyacrylamide gels, and the proteins were electrophoretically transferred to polyvinylidene difluoride (PVDF) membrane (Immobilon-P; Millipore, Billerica, MA). Membranes were blocked and incubated with primary antibodies and peroxidase-conjugated secondary antibodies. The corresponding bands were detected by means of an enhanced chemiluminescence detection kit from Amersham. Western blot quantification was carried out by densitometry scanning with ImageQuaNT (Molecular Dynamics, Piscataway, NJ) image analysis software.
Dot blot analysis
Density gradient fractions were dotted onto nitrocellulose filters (Schleicher & Schuell, DÜren, Germany) by means of a dot blot apparatus (Bio-Rad, Hercules, CA). Membranes were then treated as described for Western blot or by overlay with the horseradish peroxidase (HRP)-streptavidin and enhanced chemiluminescence (ECL) method.
Caspase-3
Caspase-3 activity was measured in purified exosomes with the EnzChek Caspase-3 assay kit (Molecular Probes) according to the manufacturer's instructions. Fluorescence was detected by means of a spectrofluorometer with excitation/emission at 496/520 nm.
Exosomes (3 mg protein) from K562, Daudi cells, and (0.6 mg protein) reticulocytes, were solubilized with RIPA buffer, and caspase-3 immunoprecipitation was carried out with the use of 2 μg rabbit anti-caspase-3 antibody (Santa Cruz Biotechnology) recognizing p11, p17, and p20 subunits and the full-length precursor of caspase, and 60 μL of a 50% slurry of protein A-sepharose (Amersham). As control, Jurkat human T-lymphoblastoid cells (107 cells), cultured in the absence or presence of 5 μM actinomycin D for 7 hours, were lysed with the use of RIPA buffer, and caspase-3 was immunoprecipitated as described. After washing, samples were loaded on 15% SDS-PAGE, and Western blotting was carried out with the use of the same anti-caspase-3 antibody.
Inhibition of caspase-3 activity during exosome formation was carried out with the use of z-DEVD-fluoromethyl ketone (Z-DEVD-FMK), a cell-permeable inhibitor (VWR International, Poole, Dorset, United Kingdom). First, 106 cells were cultured in 1 mL medium for 24 hours in the presence of 20 μM Z-DEVD-FMK or 0.2% dimethyl sulfoxide (DMSO); the supernant was discarded; and cells were incubated in the absence or presence of inhibitor for 24 hours longer. Exosomes were then collected and analyzed for Lyn by Western blotting.
Electron microscopy
Cells and exosome-coated beads were fixed with a mixture of 2% paraformaldehyde and 0.125% glutaraldehyde in 0.2 M phosphate buffer, pH 7.4 (PB) for 2 hours at room temperature. Fixed cells were processed for ultrathin cryosectioning as described previously.36 After washing with PB and PB containing 50 mM glycine, cells were embedded in 7.5% gelatin. Small blocks were infiltrated with 2.3 M sucrose at 4°C for 2 hours and then frozen in liquid nitrogen. Ultrathin cryosections were prepared with a Leica (Vienna, Austria) ultracut FCS, retrieved with a mixture of 2% methylcellulose and 2.3 M sucrose (vol/vol), and immunogold labeled. After thawing, sections were indirectly immunogold labeled with FITC or biotin-coupled cholera toxin B subunit (Sigma), followed by a rabbit anti-FITC antibody (Molecular Probes) or a rabbit antibiotin (Biovalley, Marne La Vallee, France). Rabbit antibodies were detected with protein A coupled to 10- or 15-nm gold particles (Department of Cell Biology, Utrecht University, The Netherlands). The sections were contrasted and embedded in a mixture of methylcellulose and uranyl acetate and viewed under a CM120 Philips electron microscope (Eindhoven, The Netherlands).
After removal of sucrose by ultracentrifugation in PBS, membrane fractions were loaded on formvar-carbon-coated grids for 30 minutes and immunogold labeled and constrasted as described.
Results
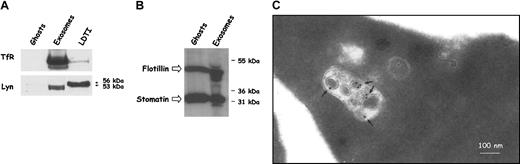
To verify the presence of lipid rafts on the reticulocyte cell surface, we isolated these subdomains from rat reticulocyte ghost membranes on the basis of their insolubility in Triton X-100 and low buoyant density in sucrose gradients. Acetylcholinesterase activity was present in the fractions corresponding to a density of approximately 15% to 20% (wt/vol) sucrose (not shown). The addition of 60 mM n-octyl-β-d-glucopyranoside during membrane solubilization markedly decreased the percentage of AChE activity present in the low-density fractions. The Src tyrosine kinase Lyn and flotillin-1 revealed by Western blot, and the ganglioside GM1 detected by dot blot with the use of peroxidase-cholera toxin B, were found in the same fractions with a similar effect of n-octyl-β-d-glucopyranoside on their distribution (not shown). Interestingly, it was recently shown that flotillin-1 and stomatin, another raft protein, are differently sorted within vesicles released from erythrocytes after treatment with Ca2+ and ionophore A23187.37 We were thus very interested in whether these proteins were present in reticulocyte exosomes. First we compared the selective enrichment of TfR and Lyn in 3 subcellular fractions: ghosts, exosomes, and LDTI membranes of rat reticulocytes (Figure 1A). As already shown,35 TfR was found to be highly enriched in exosomes as compared with the plasma membrane, and in agreement with the literature, traces of TfR were found in the LDTI fraction. As expected, Lyn was highly enriched in LDTI membranes as compared with the plasma membrane, but was also present in high amount in reticulocyte exosomes. Surprisingly, however, Lyn was detected in exosomes as a doublet of lower molecular weight as compared with the Lyn doublet (53 to 56 kDa) detected in total cell LDTI membranes.
Presence of raft markers in reticulocyte exosomes. (A) Equivalent protein amounts of ghosts, exosomes, and LDTI membranes from rat reticulocytes (exceeding 70%) were loaded on 10% SDS-PAGE and analyzed by Western blotting for Lyn and the TfR. (B) Because of GARP-50 antibody specificity, human red cells (6% reticulocytes) were subcultured in vitro. Red cell ghosts and exosomes collected from the medium were analyzed by Western blotting for human stomatin (GARP-50) and then for flotillin. (C) Rat reticulocytes (40 μL packed cell volume) were incubated with (50 μg/mL) biotinylated B-subunit of cholera toxin (bCTXB) for 3 hours at 4°C and chased for 60 minutes at 37°C. Cells were then fixed in 2% paraformaldehyde and processed as described in “Materials and methods.”
Presence of raft markers in reticulocyte exosomes. (A) Equivalent protein amounts of ghosts, exosomes, and LDTI membranes from rat reticulocytes (exceeding 70%) were loaded on 10% SDS-PAGE and analyzed by Western blotting for Lyn and the TfR. (B) Because of GARP-50 antibody specificity, human red cells (6% reticulocytes) were subcultured in vitro. Red cell ghosts and exosomes collected from the medium were analyzed by Western blotting for human stomatin (GARP-50) and then for flotillin. (C) Rat reticulocytes (40 μL packed cell volume) were incubated with (50 μg/mL) biotinylated B-subunit of cholera toxin (bCTXB) for 3 hours at 4°C and chased for 60 minutes at 37°C. Cells were then fixed in 2% paraformaldehyde and processed as described in “Materials and methods.”
As shown in Figure 1B, flotillin-1 and stomatin were detected in secreted vesicles, pointing out another difference with vesicles shed from the erythrocyte plasma membrane after Ca2+ treatment, which are devoid of flotillin-1.37 In agreement with the endosomal processing of exosomes, flotillin-1 was described as a constituent of lipid subdomains present at the plasma membrane38 as well as in recycling endosomes.39 To further investigate the sorting step at the MVB-formation level, we incubated reticulocytes with the biotinylated B subunit of cholera toxin (bCTXB) and analyzed the distribution of bCTXB by electron microscopy. Consistent with GM1 sorting in MVB, bCTXB was found predominantly on the internal vesicles and not on the limiting membrane of the multivesicular structures (Figure 1C).
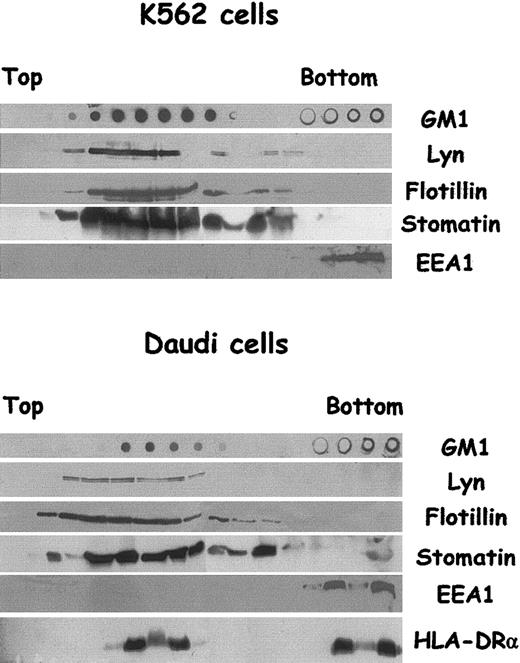
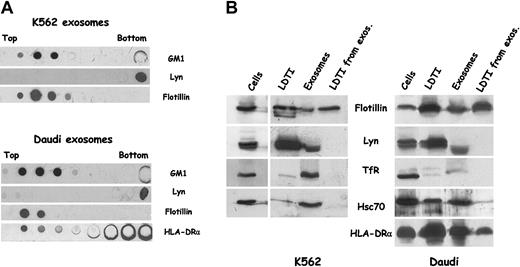
We have shown that K562 cells, an erythroleukemic cell line, secrete exosomes with characteristics similar to those of reticulocyte exosomes; for example, TfR, hsc70, and AChE are present.30 Interestingly, K562 cells were also able to sort N-Rh-PE (N-(lissamine rhodamine B sulfonyl)dioleoylphosphatidylethanolamine) incorporated on the plasma membrane, leading to lipid-labeled exosome secretion, suggesting that lipid sorting may be a general feature of exosome processing. To investigate this hypothesis, we used cells from another lineage, such as B-lymphoblastoid Daudi cells, known to secrete exosomes containing MHC class II molecules.40 We thus first examined whether the 4 raft markers GM1, Lyn, flotillin-1, and stomatin were present in LDTI fractions isolated from K562 and Daudi cells. The cells were lysed in Triton X-100 and fractionated as described to isolate raft and nonraft proteins. Figure 2 shows that the distribution of the 4 raft markers in sucrose density gradient was similar for both kinds of cells. GM1, Lyn, flotillin-1, and stomatin were detected mainly in low-density fractions, with only traces of Lyn and flotillin-1 in the bottom 40% sucrose fractions.
Characterization of LDTI membranes isolated from K562 and Daudi cells. K562 and Daudi cells were lysed in Triton X-100 and subjected to sucrose gradient centrifugation. Aliquots of fractions collected from the top of the gradient were analyzed by Western blotting with the use of antibodies against Lyn, flotillin-1, early endosomal antigen 1(EEA1), and MHC class II (α chain) molecules, and by dot blot for GM1 content with the use of peroxidase-coupled cholera toxin B subunits. Threefold lower amounts of the 4 bottom gradient fractions were loaded on SDS-PAGE.
Characterization of LDTI membranes isolated from K562 and Daudi cells. K562 and Daudi cells were lysed in Triton X-100 and subjected to sucrose gradient centrifugation. Aliquots of fractions collected from the top of the gradient were analyzed by Western blotting with the use of antibodies against Lyn, flotillin-1, early endosomal antigen 1(EEA1), and MHC class II (α chain) molecules, and by dot blot for GM1 content with the use of peroxidase-coupled cholera toxin B subunits. Threefold lower amounts of the 4 bottom gradient fractions were loaded on SDS-PAGE.
On the contrary, EEA1 and TfR (not shown), which were taken as nonraft protein markers, were detected only in the heavier sucrose fractions. The distribution of MHC class II molecules over the gradient was investigated for Daudi cells. In agreement with previous studies on B cells,41 HLA-DRα chain was partly found to be localized in low-density sucrose fractions. Taking into account the volume of each fraction loaded onto SDS-PAGE, we roughly estimated, by quantitative densitometry of the blots, that approximately 20% of HLA-DRα chain was present in LDTI fractions. The addition of 60 mM n-octyl-β-d-glucopyranoside during cell lysis almost abolished the detection of the various raft markers in low-density fractions (not shown).
We thus wondered if the same raft markers were present in exosomes secreted by K562 or Daudi cells. Exosomes collected from culture media of Daudi cells by differential centrifugation were fractionated by flotation on sucrose gradient and analyzed for the presence of the various proteins by Western blot, and for the presence of GM1 by CTXB overlay (Figure 3A).
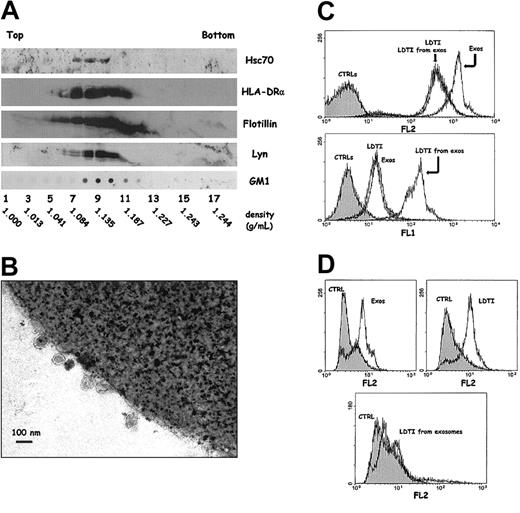
Analysis of exosomes secreted by Daudi cells. (A) Exosomes were obtained by differential centrifugation as described in “Materials and methods” and deposited on a linear sucrose gradient. Fractions were collected and analyzed by Western blot and dot blot for the indicated markers. Indicated densities (grams per milliliters) were obtained for each fraction by refractometry. (B) Alternatively, exosomes were immunoadsorbed on the surface of magnetic beads coated with anti-HLA DR (clone BL2) and processed for electron microscopy (EM) as described in “Materials and methods.” (C) FACS analysis of beads coated with different membrane or subdomains from Daudi cells. Latex beads were coated with exosomes (Exos), or with LDTI membranes isolated from cells (LDTI) or from secreted vesicles (LDTI from exos) as described in “Materials and methods.” Membrane-coated beads were then analyzed for the presence of the ganglioside GM1 through bCTXB binding and PE-streptavidin detection (FL-2, upper panel), and for the presence of MHC class II molecules through anti-HLA-DR antibody binding and FITC-antimouse IgG detection (FL-1, lower panel). CTRLs are beads incubated with PE-streptavidin but without bCTXB (FL-2, upper panel) and beads incubated with FITC-antimouse IgG but without anti-HLA-DR (FL-1, lower panel). (D) The same membrane fractions were immunoadsorbed on magnetic beads through the MHC class II molecules as described in “Materials and methods” and analyzed for the presence of GM1 as in panel C. CTRL represent beads incubated with PE-streptavidin without bCTXB.
Analysis of exosomes secreted by Daudi cells. (A) Exosomes were obtained by differential centrifugation as described in “Materials and methods” and deposited on a linear sucrose gradient. Fractions were collected and analyzed by Western blot and dot blot for the indicated markers. Indicated densities (grams per milliliters) were obtained for each fraction by refractometry. (B) Alternatively, exosomes were immunoadsorbed on the surface of magnetic beads coated with anti-HLA DR (clone BL2) and processed for electron microscopy (EM) as described in “Materials and methods.” (C) FACS analysis of beads coated with different membrane or subdomains from Daudi cells. Latex beads were coated with exosomes (Exos), or with LDTI membranes isolated from cells (LDTI) or from secreted vesicles (LDTI from exos) as described in “Materials and methods.” Membrane-coated beads were then analyzed for the presence of the ganglioside GM1 through bCTXB binding and PE-streptavidin detection (FL-2, upper panel), and for the presence of MHC class II molecules through anti-HLA-DR antibody binding and FITC-antimouse IgG detection (FL-1, lower panel). CTRLs are beads incubated with PE-streptavidin but without bCTXB (FL-2, upper panel) and beads incubated with FITC-antimouse IgG but without anti-HLA-DR (FL-1, lower panel). (D) The same membrane fractions were immunoadsorbed on magnetic beads through the MHC class II molecules as described in “Materials and methods” and analyzed for the presence of GM1 as in panel C. CTRL represent beads incubated with PE-streptavidin without bCTXB.
As already described for exosomes secreted by the human B-cell line RN,3 MHC class II molecules were distributed mainly in fractions between 1.08 and 1.18 g/mL. The hsc70, which is a major component of reticulocyte exosomes, was also found in vesicles secreted by Daudi cells. The 3 raft markers (flotillin-1, Lyn, and GM1) were found to be distributed with perfect overlapping with fractions containing MHC class II and hsc70 molecules. Immunoadsorption of exosomes on magnetic beads through the HLA-DRα chain was first carried out to control the size and heterogeneity of the vesicles. As shown in Figure 3B, the size of the exosomes was very homogenous (about 60 to 80 nm). Adsorption of membrane fractions also allows the cytofluorometric detection of molecules. We previously demonstrated the enrichment of different GPI-anchored proteins on reticulocyte exosomes using this approach.28 We thus isolated LDTI membranes from K562 and Daudi cells and from K562 and Daudi exosomes. These membrane fractions and exosomes from the 2 cell types were adsorbed on the surface of latex beads, as previously described.42 We then analyzed the presence of GM1 on the different immobilized fractions using biotinylated cholera toxin B subunit and phycoerythrin-streptavidin, and colabeling with an anti-HLA-DR antibody for Daudi fractions. The 3 fractions isolated from K562 cells (not shown) and from Daudi cells adsorbed on latex beads were labeled with phycoerythrin through CTXB (Figure 3C, upper panel). MHC class II molecules were also detected on the surface of the 3 Daudi membrane-coated beads (lower panel).
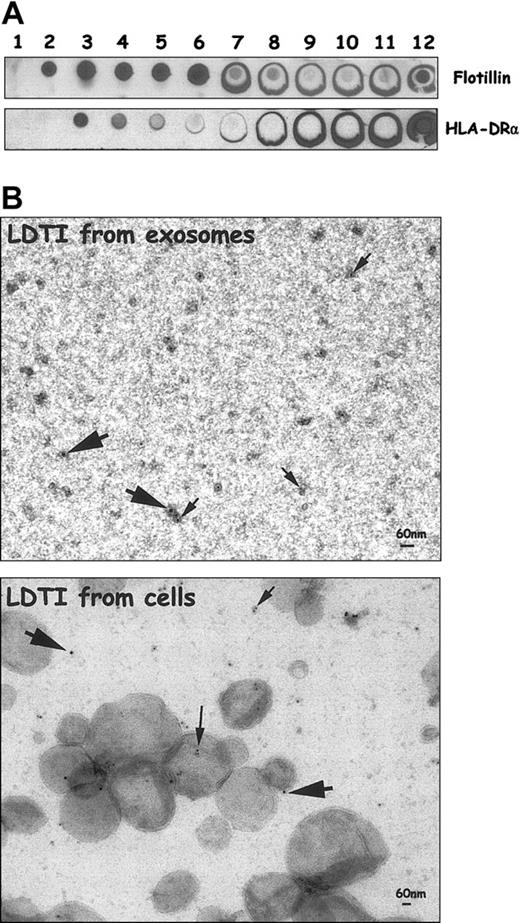
However, to assess colocalization of the 2 markers on the same membrane portion, fractions were immunoadsorbed on magnetic beads through HLA-DRα chain as described and were analyzed for bCTXB and PE-streptavidin binding. As shown in Figure 3D, GM1 was detected on the 3 immunoadsorbed membrane fractions, although more faintly on the surface of exosomal LDTI membranes (lower panel). These experiments, although not quantitative, highly suggested the presence of MHC class II molecules in LDTI membranes isolated from exosomes. To confirm this point, an electron microscopy study was carried out on the LDTI membrane fraction prepared from exosomes. LDTI membrane fractions from Daudi exosomes were thus isolated on sucrose density gradient and first analyzed by dot blot to characterize a fraction enriched with MHC class II molecules and flotillin-1 (Figure 4A).
EM colocalization of MHC class II molecules and GM1 in exosomal LDTI domains. (A) Daudi exosomes were lysed in 1% Triton X-100 and subjected to sucrose gradient centrifugation. Aliquots of fractions collected from the top of the gradient were analyzed by dot blot for the distribution of flotillin-1 and HLA-DRα. (B) Fraction no. 3 of the sucrose gradient was used for the EM study (upper panel). Detergent-resistant membrane isolated from Daudi cells was also analyzed for GM1 and MHC class II molecule colocalization (lower panel). Large arrows indicate gold particles (protein A gold 15) detecting anti-HLA-DRα; small arrows, gold particles (protein A gold 10) detecting bCTXB.
EM colocalization of MHC class II molecules and GM1 in exosomal LDTI domains. (A) Daudi exosomes were lysed in 1% Triton X-100 and subjected to sucrose gradient centrifugation. Aliquots of fractions collected from the top of the gradient were analyzed by dot blot for the distribution of flotillin-1 and HLA-DRα. (B) Fraction no. 3 of the sucrose gradient was used for the EM study (upper panel). Detergent-resistant membrane isolated from Daudi cells was also analyzed for GM1 and MHC class II molecule colocalization (lower panel). Large arrows indicate gold particles (protein A gold 15) detecting anti-HLA-DRα; small arrows, gold particles (protein A gold 10) detecting bCTXB.
Fraction no. 3 was then pelleted and used for EM colocalization of GM1 and MHC class II molecules. As shown in Figure 4B (upper panel), gold particles (Au10) detecting bCTXB (small arrows) were found on almost all membrane fragments present in the preparation. Some of these fragments were also immunogold labeled (Au15) with anti-HLA-DRα (large arrows), confirming the presence of MHC class II molecules in raft domains of exosomes. It should be noted that LDTI membranes isolated from cells (Figure 4, lower panel) appeared as larger vesicles (200 to 500 nm) as compared with membrane fragments obtained from exosomes (about 30 to 60 nm). This may be due to the fact that, as already proposed, raft subdomains present on the cell surface are assembled together during membrane solubilization with Triton X-100 to form large membrane vesicles. In contrast, the amount of raft domain on the surface of exosomes (60 to 80 nm diameter) is not sufficient to form such large vesicles. In agreement with this, small membrane fragments labeled with bCTXB or anti-HLA-DRα were also observed in LDTI membranes isolated from cells.
To further study the involvement of raft domains in molecule sorting toward exosomes, we more carefully analyzed the enrichment of some components in LDTI membranes isolated from K562 and Daudi exosomes (Figure 5). In both cell types, the whole flotillin-1 pool was distributed in low-density fractions, overlapping with most of the ganglioside GM1 detected in the sucrose gradient (Figure 5A).
Comparison of protein enrichment in the different membrane subdomains. (A) Dot blot characterization of exosomal LDTI membranes. K562 and Daudi exosomes were lysed in 1% Triton X-100 and subjected to sucrose gradient centrifugation. The distribution of the 3 raft markers (GM1, Lyn, and flotillin-1) in the gradients was assessed by dot blot. Aliquots of the fractions were analyzed for HLA-DRα distribution in the gradient from Daudi exosomes. (B) Known protein amounts of exosomes, cells, and LDTI membranes isolated from cells or exosomes were loaded on SDS-PAGE to get roughly comparable amounts of flotillin and were analyzed by Western blot for the indicated proteins.
Comparison of protein enrichment in the different membrane subdomains. (A) Dot blot characterization of exosomal LDTI membranes. K562 and Daudi exosomes were lysed in 1% Triton X-100 and subjected to sucrose gradient centrifugation. The distribution of the 3 raft markers (GM1, Lyn, and flotillin-1) in the gradients was assessed by dot blot. Aliquots of the fractions were analyzed for HLA-DRα distribution in the gradient from Daudi exosomes. (B) Known protein amounts of exosomes, cells, and LDTI membranes isolated from cells or exosomes were loaded on SDS-PAGE to get roughly comparable amounts of flotillin and were analyzed by Western blot for the indicated proteins.
On the contrary, Lyn was distributed only in the detergent-solubilized high-density fraction of K562 and Daudi exosome preparations. As previously (Figure 4A), MHC class II molecules were found to be partly distributed in LDTI fractions. We estimated that about 10% to 20% of the total HLA-DRα chain detected on the gradient was associated with low-density fractions. Western blot analysis of known amounts of different subcellular fractions from K562 and Daudi cells was carried out to confirm these dot blot data (Figure 5B).
In both cell types, flotillin-1 was enriched in fractions corresponding to purified raft domains, confirming results obtained earlier in this study. The other raft protein marker, Lyn, was found in high amounts in LDTI fractions isolated from both kinds of cells, confirming data obtained on the different sucrose gradient distribution. As described earlier for reticulocyte exosomes (Figure 1A), the tyrosine kinase in K562 and Daudi exosomes was detected as a lower-molecular weight (lower-MW) doublet, and was absent from LDTI fractions isolated from both K562 and Daudi exosomes, confirming previous data (Figure 5A). As expected, TfR was excluded from LDTI membranes and poorly enriched in Daudi exosomes,40 contrary to what was found in K562 secreted vesicles.30 The hsc70 had the same pattern as TfR in K562 fractions, but was found in all Daudi fractions except exosomal LDTI membranes. Finally, the presence of MHC class II molecules was confirmed in all Daudi fractions, including exosomal LDTI membranes.
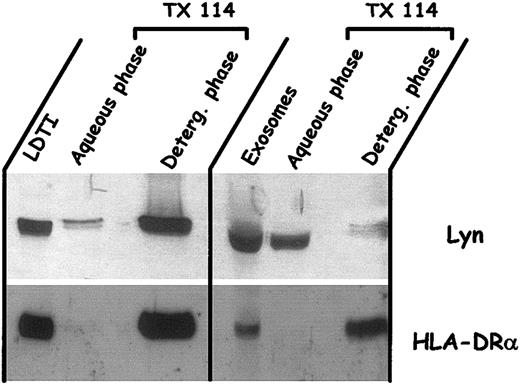
The lower MW of the Lyn doublet found in exosomes, suggesting proteolytic cleavage, together with its absence of purified exosomal LDTI membrane, was highly reminiscent of the cleavage of Lyn that occurs in apoptotic cells.43 Indeed, it was demonstrated that activated caspase-3 induced Lyn cleavage after Asp18 at the N-terminal domain, which is critical for the membrane association of Lyn through double acylation. This proteolytic cleavage induces the relocation of the protein, which becomes cytosolic. We thus compared the hydrophobic characteristics of Lyn present in LDTI membranes isolated from Daudi cells and Lyn present in Daudi exosomes using Triton X-114 partitioning.
As shown in Figure 6, Lyn was recovered mainly in the detergent phase after Triton X-114 extraction of Daudi LDTI membranes. On the contrary, Lyn, with a lower MW in exosomes, was recovered almost exclusively in the aqueous phase after Triton X-114 extraction. Note that a small amount of Lyn was detected in the detergent phase, but with a higher MW, corresponding to the native form. As a control, the HLA-DRα chain present in both cell LDTI membrane and exosomes was distributed exclusively in the detergent phase after extraction. This confirmed that Lyn cleavage in exosomes induced a loss of membrane association and relocation in the lumen of vesicles. To explain this relocation in exosomes, and only in exosomes, this suggested that proteolytic cleavage of Lyn occurred in exosomes. We thus looked for caspase-3 activity in secreted vesicles. Exosomes from the 3 kinds of cells were consequently solubilized and assayed for caspase-3-like activity.
Partition in Triton X-114 of Lyn and HLA-DRα present in cell LDTI membrane or secreted within exosomes. Exosomes and LDTI membranes isolated from Daudi cells were extracted by Triton X-114 as described in “Materials and methods.” Aliquots (50 μL) of the aqueous and detergent phases and of the starting fractions were mixed with Laemmli buffer, loaded on SDS-PAGE, and analyzed for the presence of Lyn and HLA-DRα by Western blot.
Partition in Triton X-114 of Lyn and HLA-DRα present in cell LDTI membrane or secreted within exosomes. Exosomes and LDTI membranes isolated from Daudi cells were extracted by Triton X-114 as described in “Materials and methods.” Aliquots (50 μL) of the aqueous and detergent phases and of the starting fractions were mixed with Laemmli buffer, loaded on SDS-PAGE, and analyzed for the presence of Lyn and HLA-DRα by Western blot.
As shown in Figure 7A, low DEVDase activity was detected in the 3 types of exosomes, with much higher activity (about 10-fold more) in reticulocyte exosomes. This activity was dependent on the incubation time and was negligible when the vesicles were not solubilized (not shown). Although very faint, a 19-kDa band potentially corresponding to the activated form of caspase-3 was detected by Western blot after immunoprecipitation from the 3 types of exosomes, consistent with the presence of low caspase-3 activity in the vesicles (Figure 7B). Finally, the presence of a caspase-3 inhibitor during Daudi cell culture led to recovery of exosomal Lyn with an unchanged molecular weight (Figure 7C).
Evidence of caspase-3-like activity in exosomes secreted by reticulocytes, K562, and Daudi cells. (A) Left panel: Reticulocyte exosomes were solubilized, and various protein amounts were assayed for caspase-3-like activity (1-hour incubation) with the use of Z-DEVD-rhodamine 110. Data shown (means of duplicate experiments) represent total DEVDase activities (□) or activities measured in the presence (▨) of the inhibitor (Ac-DEVD-aldehyde [Ac-DEVD-CHO]). Right panel: Similar protein amounts (250 μg) of exosome from K562 cells and Daudi cells were assayed for caspase-3-like activity (1-hour incubation) as described. (B) Caspase-3 was immunoprecipitated by means of rabbit anti-caspase-3 from exosomes of the 3 cell types: Daudi (3 mg protein), K562 (3 mg protein), and reticulocytes (0.6 mg protein). Controls were carried out with the use of Jurkat cells incubated or not for 7 hours with 5 μM actinomycin D to activate caspase-3. The p20 subunit was revealed by Western blot with the use of the same anti-caspase-3 antibody. (C) Daudi cells were cultured in the presence of Z-DEVD-FMK (20 μM) or DMSO (0.2%), as described in “Materials and methods.” Exosomes were collected from the medium and analyzed for the presence of Lyn by Western blot. Cell lysate and exosomes from Daudi cells were also loaded on the gel as migration controls.
Evidence of caspase-3-like activity in exosomes secreted by reticulocytes, K562, and Daudi cells. (A) Left panel: Reticulocyte exosomes were solubilized, and various protein amounts were assayed for caspase-3-like activity (1-hour incubation) with the use of Z-DEVD-rhodamine 110. Data shown (means of duplicate experiments) represent total DEVDase activities (□) or activities measured in the presence (▨) of the inhibitor (Ac-DEVD-aldehyde [Ac-DEVD-CHO]). Right panel: Similar protein amounts (250 μg) of exosome from K562 cells and Daudi cells were assayed for caspase-3-like activity (1-hour incubation) as described. (B) Caspase-3 was immunoprecipitated by means of rabbit anti-caspase-3 from exosomes of the 3 cell types: Daudi (3 mg protein), K562 (3 mg protein), and reticulocytes (0.6 mg protein). Controls were carried out with the use of Jurkat cells incubated or not for 7 hours with 5 μM actinomycin D to activate caspase-3. The p20 subunit was revealed by Western blot with the use of the same anti-caspase-3 antibody. (C) Daudi cells were cultured in the presence of Z-DEVD-FMK (20 μM) or DMSO (0.2%), as described in “Materials and methods.” Exosomes were collected from the medium and analyzed for the presence of Lyn by Western blot. Cell lysate and exosomes from Daudi cells were also loaded on the gel as migration controls.
Discussion
In this report, we investigated the presence of lipid raft domains in exosomes. We show that typical raft components were associated in detergent-resistant membrane on the surface of secreted exosomes in the 3 cell types studied. These components belong to various families of molecules, such as glycolipids (ganglioside GM1), Src tyrosine kinases (Lyn), GPI-anchored proteins (AChE), and proteins containing prohibitin domains (stomatin and flotillin-1). The existence of such raft domains on the exosome surface was suspected from our previous data, especially on lipid and GPI-anchored protein sorting in reticulocyte exosomes.23,28 The fact that other cells, such as erythroleukemic K562 cells and lymphoblastoid Daudi cells, secrete exosomes containing similar organized lipid subdomains suggests that lipid domains may play a general role in exosome biogenesis and structure. Note that lipid rafts have already been described as key elements in the vesiculation process from the red cell membrane when there is a rise in cytosolic Ca2+. More recently, the shed vesicles were demonstrated to contain other raft components such as GM1, cholesterol, and stomatin.37 It was also suggested that lipid rafts are involved in virus budding from the cell surface, since GPI-anchored proteins are incorporated in virion membrane.44 These raft domains may therefore represent weak points on the membrane surface, prone to outward bending or budding.
Another possible role of lipid rafts during exosome formation could be related to the intrinsic low lateral diffusion of the proteins present in these subdomains.45 The presence of such lipid rafts in recycling endosomes has been suggested to slow down recycling of GPI-anchored proteins to the plasma membrane.46 Variable recycling capacities were also described for lipid molecules such as fluorescent-labeled lipids that, depending on the length and saturation of fatty acids, present different recycling aptitudes.47 Slowly recycling molecules would thus be more prone to be packaged in intralumenal vesicles, whereas fast recycling molecules would more easily escape MVB budding. Accordingly, we found that the fast recycling lipid (C6-NBD-SM [1-acyl-2[6-[N-(7-nitro-2,1,3-benzoxadiazol-4-yl) amino]caproyl] phosphatidylcholine]) was not incorporated in reticulocyte exosomes, unlike N-Rh-PE, which is known to be present on membrane in small clusters48 that are sorted in exosomes during red cell maturation.23 We show here that proteins such as flotillin-1 and stomatin, which have an intrinsic affinity for lipid rafts, are sorted in exosomes secreted by various cell types. It could be possible that other proteins interacting with raft components may be also sorted in exosomes. Note here that the glucose transporter Glut-1 found in reticulocyte exosomes was demonstrated to associate with stomatin in red cells.49 Stomatin, like caveolin and possibly flotillin-1, is an acylated protein found in oligomeric forms in lipid domains.34,50 This is highly reminiscent of another protein family, the tetraspan proteins (TSPs), which are also acylated and interact to form a protein web presenting some affinity for detergent-resistant domains,51 and which were found to be enriched in B-cell exosomes.52 Certain TSPs were described to interact with MHC class II molecules, while others can associate with integrins.53 While we were writing this manuscript, Wubbolts et al54 reported the colocalization of MHC class II molecules and TSP in a CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid)-resistant domain in B cell-derived exosomes. In addition, the integrin α4 chain was identified by mass spectrometry as a component of B-cell-secreted exosomes. Interestingly, we previously detected very late activation antigen-4 (VLA4) integrin (α4β1) in reticulocyte exosomes.55 In this scenario, interaction of MHC class II molecules and possibly integrins with TSP would reduce lateral diffusion of these molecules on endosomal membranes, favoring their incorporation in MVB buds. This hypothesis is strengthened by the observation that TfR recycles more slowly when interacting with oligomerized Tf,56 and is more efficiently sorted in exosomes when clustered by antibodies,23 which are thought to reduce their diffusion in the membrane.
Finally, lipid raft domains may serve as a ubiquitin-based sorting platform in exosomal protein sorting. Indeed, ubiquitination of cargo proteins was shown to be a sorting mechanism during MVB formation,18 involving various effectors that associate with lipid domains. For example, the ubiquitin ligases Cbl and Nedd4 become associated with lipid rafts upon activation of IgE signaling on mast cells.57 Similarly, upon insulin binding on adipocytes, Cbl, in association with the adapter c-Cbl-associated protein (CAP), translocates to lipid rafts through the interaction of the sorbin homology domain of CAP with flotillin-1.58 Very interestingly, Cbl was demonstrated to be present in exosomes produced by activated T cells.5 Tsg101 (tumor susceptibility gene 1), the mammalian homolog of vacuolar protein sorting-23 (Vps23), has also been found in dendritic cell-derived exosomes.59 Vps23 is a component of the endosomal sorting complex ESCRT-1, which recognizes ubiquinated cargoes and participates in their inclusion in MVB vesicles.11 Interestingly, tsg101 also interacts specifically with HIV-1 Gag protein and Ebola virus matrix viral protein 40 (Vp40)60 and recruits the Vp40 protein into lipid rafts.61 In agreement with this, we found that tsg101, which was present in the 3 types of exosomes tested, was also found in LDTI domains isolated from Daudi exosomes (data not shown).
A second major finding in this study is that the Src tyrosine kinase Lyn was selectively cleaved in exosomes secreted from the 3 cell types, leading to a 3-kDa truncated form that was no longer membrane bound. This is a major finding since such Lyn cleavage was already described as being induced by caspase-3 in apoptotic cells.43 Accordingly, we documented DEVDase activity in solubilized exosomes. The presence of caspase-3-like activity in reticulocytes is not really surprising since transient activation of several caspases was shown to be required during erythroid differentiation.62 Moreover, the remodeling occurring during red cell maturation is very favorable for caspase activation. Mitochondria destabilization by a 15-lipoxygenase63,64 expressed during reticulocyte maturation induces cytochrome C release,65 which can activate the caspase cascade. Accordingly, we found much higher DEVDase activity in reticulocyte exosomes relative to the 2 other cell types of vesicles. The presence of caspase activity in exosomes from nucleated cells is much more surprising because the cells were not undergoing apoptosis.
However, caspase-3 activation has already been reported to occur in cells that are not undergoing apoptosis, such as activated B or T cells66 and proliferative lymphoid cells.67 In fact, a growing body of evidence suggests that caspase-3 may be activated under physiologic states other than terminal apoptosis. Moreover, cells stained with annexin V, the early indicator of apoptosis, were shown to be able to re-enter the cell cycle and continue to proliferate.68 This suggests that mechanisms could rescue cells before commitment to apoptotic death. Packaging activated caspase within exosomes might be a mechanism developed by cells to ensure survival. At this point, it should be noted that members of the inhibitor-of-apoptosis protein family could regulate apoptosis by binding to and targeting caspase for ubiquitination.69,70 On the other hand, caspase-3 proenzyme and its activated counterpart have been shown to copurify with caveolin-enriched microdomains.71 The presence of other apoptosis-related proteins in exosomes has already been reported,59 in agreement with a possible role of exosomes in apoptosis regulation.
A major consequence of membrane sorting at the endosomal level is fine regulation of cellular processes. Lipid raft domains present on the endosomal membrane might be involved in setting up sorting platforms to concentrate cargo and effector proteins within MVB intralumenal vesicles that could be destined for lysosomal degradation or extracellular secretion.
Prepublished online as Blood First Edition Paper, July 24, 2003; DOI 10.1182/blood-2003-03-0871.
Supported by grants from the CNRS, the Institut Curie, the Ministère de la Recherche, and the Association pour la Recherche sur le Cancer (ARC) (no. 9580; M.V.); and by an ARC fellowship (C.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Rainer Prohaska for kindly providing MoAb antistomatin (GARP-50) and Dr Herisoa Rabesandratana (Hôpital St Eloi, Montpellier, France) for obtaining the blood samples.

![Figure 7. Evidence of caspase-3-like activity in exosomes secreted by reticulocytes, K562, and Daudi cells. (A) Left panel: Reticulocyte exosomes were solubilized, and various protein amounts were assayed for caspase-3-like activity (1-hour incubation) with the use of Z-DEVD-rhodamine 110. Data shown (means of duplicate experiments) represent total DEVDase activities (□) or activities measured in the presence (▨) of the inhibitor (Ac-DEVD-aldehyde [Ac-DEVD-CHO]). Right panel: Similar protein amounts (250 μg) of exosome from K562 cells and Daudi cells were assayed for caspase-3-like activity (1-hour incubation) as described. (B) Caspase-3 was immunoprecipitated by means of rabbit anti-caspase-3 from exosomes of the 3 cell types: Daudi (3 mg protein), K562 (3 mg protein), and reticulocytes (0.6 mg protein). Controls were carried out with the use of Jurkat cells incubated or not for 7 hours with 5 μM actinomycin D to activate caspase-3. The p20 subunit was revealed by Western blot with the use of the same anti-caspase-3 antibody. (C) Daudi cells were cultured in the presence of Z-DEVD-FMK (20 μM) or DMSO (0.2%), as described in “Materials and methods.” Exosomes were collected from the medium and analyzed for the presence of Lyn by Western blot. Cell lysate and exosomes from Daudi cells were also loaded on the gel as migration controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/13/10.1182_blood-2003-03-0871/6/m_h82435401007.jpeg?Expires=1769081522&Signature=d5~h5IW7Fq9Kuo~8AxmVrjIE7UegALI0VgodOA0VISh68xMC3cfhpfD0CmLbPSeDyAfWekxUP9sIfBf2LZqFCccQbRrC-Qj4GxkFEEIA~CzUUmh6SrtIKw~8xWpCqjyuxg~oshW5sLGZXyNx0z2kH7OrbXyVkJzIENZCnvXgAq2Q0xK~wnEdEUU1W5mftXF~5nfU2HGGaIDILm~pdEbEywnvTuUzPIkHhVlejVGiNePoghCB1C5YKqSf9GD45Tjd8jS5uxN2ObGBR4PjOR-DeHrC907aMIx-v7nWRDuxqVkaVd2Q5Mv0Qz4GTpzaDXGFDvEEf~8I9gXQDmyV91uMbQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal