Abstract
Animal models with impaired thymic negative selection do not always cause autoimmune diseases despite the development of an autoreactive T-cell repertoire. We investigated the requirements for the de velopment of systemic autoimmune disease by using bone marrow chimeras that lacked expression of major histocompatibility complex (MHC) class II on thymic antigen-presenting cells (APCs), leading to impaired negative selection. We found that impaired negative selection mediated by absence of MHC class II, but not MHC class I, permitted the development of systemic autoimmune disease that is indistinguishable from acute graft-versus-host disease (GVHD). Thymectomy prevented disease, confirming the causal association of the thymus with its development. Adoptive transfer of CD4+ T cells caused GVHD in secondary hosts only when they were irradiated, and cotransfer of peripheral CD4+ and CD8+ T cells from naive mice prevented the disease. These results demonstrate that impaired thymic negative selection can cause lethal autoimmune disease indistinguishable from acute GVHD in the context of a proinflammatory milieu when peripheral regulatory mechanisms are absent.
Introduction
T cells are both positively and negatively selected in the thymus during their development through the interaction of T-cell receptors with self-peptides bound to self-major histocompatibility complex (MHC) molecules, resulting in a self-MHC-restricted, self-tolerant T-cell repertoire.1-5 T cells with strong affinity for thymic MHC-peptide complexes are deleted in the thymic medulla via negative selection, which is mediated primarily by dendritic cells (DCs) and less efficiently by thymic medullary epithelial cells (MECs),6-12 whereas positive selection is mediated by the thymic cortical epithelium.1,13-17
Several animal models of impaired thymic negative selection retain normal positive selection, including K14 mice that express MHC only on thymic cortical epithelium,17,18 RelB-deficient mice lacking DCs and functional MECs,19,20 H2-M-deficient mice,21-23 transgenic mice expressing MHC linked to a single peptide,24-26 and bone marrow (BM) chimeras lacking MHC expression on hematopoietic cells.27,28 A common characteristic of all these models is the generation of a peripheral T-cell repertoire containing a large number of autoreactive T cells because of the impaired thymic negative selection. However, the pathogenic role of these autoreactive T cells remains unclear and the only significant in vivo activity caused by these cells is the suppression of engraftment of normal BM cells by K14 T cells.29 Otherwise, autoreactive T cells are largely incapable of inducing autoimmune diseases in vivo,20,28,29 a phenomenon called “split tolerance.”30
On the other hand, a recent study demonstrated the development of colitis in BM chimeras lacking MHC class II on hematopoietic cells but the authors suggested an impaired peripheral regulatory mechanism as a possible cause of the colitis rather than impairment of thymic negative selection.31
To test the hypothesis that impaired thymic negative selection could induce autoimmune disease, we created BM chimeras in which MHC molecules were expressed on the radioresistant thymic epithelium that supports positive selection, but not on the radiosensitive hematopoietic elements responsible for negative selection. We demonstrate that impaired thymic negative selection can cause autoimmune disease that is indistinguishable from acute graft-versus-host disease (GVHD) in the presence of proinflammatory cytokines and absence of peripheral regulatory mechanisms. Thymectomy prevented the development of GVHD, providing direct evidence for a causal association between the thymus and development of the disease.
Materials and methods
Mice
Female C57BL/6 (B6, CD45.2+: wild type [wt]), B6.Ly-5a (CD45.1+), and B6-background mice deficient in MHC class I expression (B6.129P2-β2-microglobulin [β2mtm1Unc]: β2m–/–) because of targeted disruption of the β2m gene32 were purchased from the Jackson Laboratories (Bar Harbor, ME). B6-backgound class II-deficient mice (the deficiency caused by an introduced null mutation in the I-Aβb gene [B6.129-Abbtm1 N5: II–/–])33 were from Taconic (Germantown, NY). The age range of mice used for chimera generation was between 10 and 16 weeks.
Generation of bone marrow chimeras
Wild-type (wt) B6 mice were exposed to 13 Gy total body irradiation (TBI, 137Cs source), split into 2 doses separated by 3 hours to minimize gastrointestinal toxicity, and then injected intravenously with 5 × 106 T-cell-depleted (TCD) BM cells from wt, β2m–/–, or II–/– mice on day 0. T-cell depletion was performed using CD90 microbeads and the AutoMACS system (Miltenyi Biotec, Bergisch Gladbach, Germany). Wt recipients of β2m–/– TCD BM received 200 μg anti-NK1.1 monoclonal antibodies (mAbs; clone PK136) on days –2 and –134 in addition to TBI. Mice were housed in sterilized microisolator cages and received autoclaved hyperchlorinated drinking water for the first 3 weeks after BM transplantation (BMT), and filtered water thereafter. In some experiments, wt mice were thymectomized prior to BMT and used as recipients. Thymectomy was performed under anesthesia. The sternum was divided in its superior portion, the prepericardial soft tissue including the thymus was removed by gentle suction, and the thorax and skin were closed with stainless steel wound clips.
Systemic and histopathologic analysis of GVHD
Survival after BMT was monitored daily and the degree of clinical GVHD was assessed weekly by a scoring system that sums changes in 5 clinical parameters: weight loss, posture, activity, fur texture, and skin integrity (maximum index = 10) as previously described.35 We have found this index to be a more sensitive measure of the severity of allogeneic GVHD than weight loss alone, a reliable indicator of systemic GVHD in multiple murine models.35 Acute GVHD was also assessed by detailed histopathologic analysis of liver, small intestine, large intestine, and skin, and of primary GVHD target organs as described.36,37 Slides were stained with hematoxylin and eosin, coded without reference to mouse type or prior treatment status, and examined systematically by a single pathologist (C.L.) using a semiquantitative scoring system that incorporates both the severity and extent of histopathology.35,38,39 For skin GVHD, the histologic grading system of Lerner et al was used: grade I, epidermal basal cell vacuolization; grade II, epidermal basal cell apoptosis with lymphoid infiltrates; grade III, bulla formation; and grade IV, ulceration of skin.40
Adoptive transfer experiments
Splenocytes were isolated from spleens of chimeric animals 6 to 11 weeks after BMT. CD4+ T cells were negatively selected from nylon wool-purified T cells by depleting CD8+, DX5+, and B220+ cells using the AutoMACS system. From naive wt mice, T cells were negatively isolated from splenocytes by depleting MHC class II+ and CD11b+ cells; non-T cells were isolated by depleting CD4+ and CD8+ cells; and CD4+ and CD8+ T cells were negatively isolated from splenic T cells by depleting CD8+ and CD4+ cells, respectively. Purity of each T-cell subset was higher than 80%, and contamination of either CD4+ or CD8+ cells was less than 3%. For adoptive transfer experiments, 1 × 107 CD4+ T cells from [II–/– → wt] chimeras were injected intravenously into wt mice following nonmyeloablative 650 cGy TBI. In some experiments, 1 × 107 CD4+ T cells along with 5 × 106 TCD BM cells were injected into wt mice following 850 cGy TBI. A cohort of mice was also coinjected with either T cells, non-T cells, CD4+ T cells, or CD8+ T cells from wt mice.
Blockade of inflammatory cytokines
Mice were injected intraperitoneally with a combination of dimeric human soluble tumor necrosis factor α (TNF-α) receptor p80-immunoglobulin G1 (IgG1) Fc fusion protein (TNFR/Fc, 100 μg/d) and hamster antimouse type 1 interleukin-1 receptor (αIL-1R, 200 μg/d) (Immunex, Seattle, WA) on day 0 and then on alternate days up to day 21 as described.37 Mice in control groups received injections of control human or hamster immunoglobulin.
Flow cytometric analysis
The mAbs used were fluorescein isothiocyanate-, phycoerythrin-, or allophycocyanin-conjugated antimouse CD45.1, CD3ϵ, CD4, CD8α, CD11c (HL3), CD45RB, CD62L, H-2Kb, and I-Ab (BD Pharmingen, San Diego, CA). Cells were preincubated with 2.4G2 mAbs to block FcγR, and then incubated with the relevant mAbs for 20 minutes at 4°C. Finally, cells were washed twice with 0.2% bovine serum albumin in phosphate-buffered saline (PBS), fixed with 1% paraformaldehyde in PBS and analyzed by EPICS Elite ESP cell sorter (Beckman-Coulter, Miami, FL). Irrelevant IgG2a/b mAbs were used as a negative control. Acquired for analysis were 10 000 live events.
Isolation of thymic DCs
Thymic DCs were isolated as described.41 Briefly, after digestion with collagenase D (1 mg/mL, Boehringer Mannheim, Indianapolis, IN), cells were resuspended in 1.035 g/mL Percoll (Pharmacia Biotech, Uppsala, Sweden) and underlain with an equivalent volume of 1.075 g/mL Percoll. After centrifugation, the resulting band was harvested, washed twice, and DCs were isolated using CD11c (N418) microbeads and the AutoMACS.
Cell culture and enzyme-linked immunosorbent assay (ELISA)
Splenic CD4+ T cells were negatively selected from BM and were used as responders at 2 × 105/well against irradiated (20 Gy) peritoneal cells (1 × 105/well). Cultures were maintained in 10% heat-inactivated fetal calf serum in complete Dulbecco modified Eagle medium at 37°C in 7.5% CO2. After 3 days of culture, supernatants were harvested from the culture for cytokine measurements and cells were then pulsed with [3H]thymidine (1 μCi [0.037 MBq] per well) for an additional 16 hours. Proliferation was determined on a TopCount NTX (Packard Instrument, Meriden, CT). ELISAs for interferon γ (IFN-γ), IL-2, and IL-4 (BD Pharmingen) were performed as described.42 Samples and standards were run in duplicate.
Statistical analysis
Survival curves were plotted using Kaplan-Meier estimates. The Mann-Whitney U test was used for the statistical analysis of in vitro data and clinical scores, while the Mantel-Cox log-rank test was used to analyze survival data. A P value less than .05 was considered statistically significant.
Results
Mice lacking negative selection dependent on MHC class II expression, but not on MHC class I expression, develop autoimmune GVHD
To test the hypothesis that impaired thymic negative selection could induce autoimmune disease, we created BM chimeras in which MHC molecules were expressed on the radioresistant thymic epithelium that supports positive selection, but not on the radiosensitive hematopoietic elements responsible for negative selection. It has been shown that negative selection of CD4+ T cells is impaired in [II–/– → wt] chimeras, while [wt → II–/–] chimeras lack positive selection of CD4+ T cells.12,27 Similarly, negative selection of CD8+ T cells is impaired in [β2m–/– → wt] chimeras.27 Flow cytometric analysis of thymic DCs 4 weeks after syngeneic BMT either from II–/– or β2m–/– donors confirmed the replacement of host thymic DCs by donor-derived DCs: thymic DCs from [II–/– → wt] chimeras did not express MHC class II, whereas more than 98% DCs isolated from [β2m–/– → wt] chimeras did not express MHC class I, as previously shown.27 Analysis of the thymus 10 weeks after BMT showed the emergence of CD4 single-positive (SP) thymocytes and CD8 SP thymocytes in [wt → wt] chimeras comparable with naive wt mice (Table 1), demonstrating preservation of efficient positive selection by the thymus after 13 Gy TBI, as previously shown for 10 Gy TBI.27 At 4 weeks after BMT, [II–/– → wt] chimeras developed weight loss, scaling of skin, hunched posture, and decreased activity. This constellation of signs was quite similar to acute GVHD seen after allogeneic BMT.35 Assessment of these mice with a clinical GVHD scoring system35 showed a rapid and sustained rise in [II–/– → wt] chimeras beginning 4 weeks after BMT (Figure 1A). Scores were slightly elevated in all mice 1 week after BMT due to radiation toxicity, but returned to baseline at 2 weeks after BMT and remained within normal range thereafter in both [wt → wt] and [wt → II–/–] chimeras. This systemic illness proved lethal in the majority of animals so that only 40% survived at day 130 after BMT (Figure 1B, P < .005).
Development of multiple organ injury in [II–/– → wt] chimeras
. | Naive wt . | [wt → wt] . | [II-/- → wt] . | [β2m-/- → wt] . |
|---|---|---|---|---|
| Pathology scores | n = 6 | n = 15 | n = 14 | n = 3 |
| Liver | ||||
| Portal tracts | 0.5 ± 0.3 | 0.3 ± 0.2 | 4.9 ± 0.6* | 0.0 ± 0.0 |
| Bile ducts | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.6 ± 0.5* | 0.0 ± 0.0 |
| Vessels | 0.2 ± 0.2 | 0.1 ± 0.1 | 1.4 ± 0.3† | 0.0 ± 0.0 |
| Hepatocytes | 1.2 ± 0.6 | 1.0 ± 0.2 | 3.4 ± 0.4* | 0.0 ± 0.0 |
| Total | 1.8 ± 0.6 | 1.3 ± 0.2 | 12.6 ± 1.5* | 0.0 ± 0.0 |
| Small bowel | ||||
| Architecture | 1.1 ± 0.5 | 1.1 ± 0.3 | 2.6 ± 0.2* | 0.0 ± 0.0 |
| Epithelium | 1.3 ± 0.5 | 1.5 ± 0.4 | 3.1 ± 0.2† | 0.0 ± 0.0 |
| Total | 2.5 ± 1.0 | 2.5 ± 0.6 | 5.8 ± 0.3* | 0.0 ± 0.0 |
| Large bowel | ||||
| Architecture | 1.0 ± 0.0 | 0.5 ± 0.2 | 1.7 ± 0.3† | 0.0 ± 0.0 |
| Epithelium | 1.5 ± 0.5 | 0.7 ± 0.2 | 2.9 ± 0.2* | 0.0 ± 0.0 |
| Total | 2.3 ± 0.8 | 1.2 ± 0.4 | 4.6 ± 0.4* | 0.0 ± 0.0 |
| Skin | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.3 ± 0.2* | 0.0 ± 0.0 |
| Immune reconstitution | n = 3 | n = 4 | n = 4 | ND |
| Thymus, cells × 106 | ||||
| Total thymocytes | 82 ± 5 | 62 ± 13 | 1 ± 2 | ND |
| CD4 SP cells | 11 ± 1 | 8 ± 2 | 0 ± 0 | ND |
| CD8 SP cells | 2 ± 1 | 2 ± 1 | 0 ± 0 | ND |
| Spleen, cells × 106 | ||||
| CD4+ T cells | 21 ± 2 | 18 ± 2 | 8 ± 4† | ND |
| CD8+ T cells | 9 ± 1 | 6 ± 1 | 3 ± 1† | ND |
| B220+ cells | 43 ± 3 | 55 ± 5.8 | 22 ± 8.6† | ND |
. | Naive wt . | [wt → wt] . | [II-/- → wt] . | [β2m-/- → wt] . |
|---|---|---|---|---|
| Pathology scores | n = 6 | n = 15 | n = 14 | n = 3 |
| Liver | ||||
| Portal tracts | 0.5 ± 0.3 | 0.3 ± 0.2 | 4.9 ± 0.6* | 0.0 ± 0.0 |
| Bile ducts | 0.0 ± 0.0 | 0.0 ± 0.0 | 2.6 ± 0.5* | 0.0 ± 0.0 |
| Vessels | 0.2 ± 0.2 | 0.1 ± 0.1 | 1.4 ± 0.3† | 0.0 ± 0.0 |
| Hepatocytes | 1.2 ± 0.6 | 1.0 ± 0.2 | 3.4 ± 0.4* | 0.0 ± 0.0 |
| Total | 1.8 ± 0.6 | 1.3 ± 0.2 | 12.6 ± 1.5* | 0.0 ± 0.0 |
| Small bowel | ||||
| Architecture | 1.1 ± 0.5 | 1.1 ± 0.3 | 2.6 ± 0.2* | 0.0 ± 0.0 |
| Epithelium | 1.3 ± 0.5 | 1.5 ± 0.4 | 3.1 ± 0.2† | 0.0 ± 0.0 |
| Total | 2.5 ± 1.0 | 2.5 ± 0.6 | 5.8 ± 0.3* | 0.0 ± 0.0 |
| Large bowel | ||||
| Architecture | 1.0 ± 0.0 | 0.5 ± 0.2 | 1.7 ± 0.3† | 0.0 ± 0.0 |
| Epithelium | 1.5 ± 0.5 | 0.7 ± 0.2 | 2.9 ± 0.2* | 0.0 ± 0.0 |
| Total | 2.3 ± 0.8 | 1.2 ± 0.4 | 4.6 ± 0.4* | 0.0 ± 0.0 |
| Skin | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.3 ± 0.2* | 0.0 ± 0.0 |
| Immune reconstitution | n = 3 | n = 4 | n = 4 | ND |
| Thymus, cells × 106 | ||||
| Total thymocytes | 82 ± 5 | 62 ± 13 | 1 ± 2 | ND |
| CD4 SP cells | 11 ± 1 | 8 ± 2 | 0 ± 0 | ND |
| CD8 SP cells | 2 ± 1 | 2 ± 1 | 0 ± 0 | ND |
| Spleen, cells × 106 | ||||
| CD4+ T cells | 21 ± 2 | 18 ± 2 | 8 ± 4† | ND |
| CD8+ T cells | 9 ± 1 | 6 ± 1 | 3 ± 1† | ND |
| B220+ cells | 43 ± 3 | 55 ± 5.8 | 22 ± 8.6† | ND |
The extent of GVHD was assessed 10 weeks after BMT. Liver, small intestine, large intestine, and skin were analyzed using the histopathologic scoring system described in “Materials and methods.” Thymus and spleens were phenotyped by a flow cytometric analysis. II-/- mice have normal counts of DP cells in the thymus and of CD8+ and B220+ cells in spleen.43 Data are expressed as mean ± SE. SP indicates single positive; ND, not done; and DP, double positive.
P < .001.
P < .01.
[II–/– → wt] chimeric mice develop systemic and lethal autoimmune disease. Wt or II–/– mice were lethally irradiated and received transplants from either wt or II–/– B6 donors. (A) Clinical GVHD scores35 and (B) survivals after BMT were monitored. [wt → wt], n = 38; [wt → II–/–], n = 29; and [II–/– → wt], n = 35. Results from 3 similar experiments are combined and mean ± SE of clinical scores is shown. *P < .001, ** P < .005.
[II–/– → wt] chimeric mice develop systemic and lethal autoimmune disease. Wt or II–/– mice were lethally irradiated and received transplants from either wt or II–/– B6 donors. (A) Clinical GVHD scores35 and (B) survivals after BMT were monitored. [wt → wt], n = 38; [wt → II–/–], n = 29; and [II–/– → wt], n = 35. Results from 3 similar experiments are combined and mean ± SE of clinical scores is shown. *P < .001, ** P < .005.
Histologic examination of the liver, skin, and small and large intestine 10 weeks after BMT showed standard pathologic features of acute GVHD in [II–/– → wt] chimeras (Table 1). GVHD was severe in the liver, showing characteristic damage of acute GVHD in portal tracts, bile ducts, vasculature, and parenchyma.44 The portal tract was expanded with dense mononuclear cell infiltrates and necrotic hepatocytes (Figure 2A), producing a hepatitis-like picture characterized by acidophilic bodies. Mononuclear cells infiltrated the bile duct epithelium, causing nuclear pleomorphism, multilayering, pyknosis, and endothelialitis (Figure 2B). GVHD was also in the skin with mononuclear cell infiltration of the dermis (Figure 2C), and in the intestine with villous atrophy, crypt cell apoptosis, and lymphocytic infiltrates (Figure 2D). Immunodefi-ciency, another cardinal feature of GVHD after allogeneic BMT,45 was present with profound reductions in the number of double-positive (DP) thymocytes, splenic CD4+ T, CD8+ T, and B cells (Table 1).
Histologic analysis of [II–/– → wt] chimeric mice reveals systemic GVHD. Histologic analysis of [II–/– → wt] chimeras 10 weeks after BMT showed periportal mononuclear infiltrates (A) and endothelialitis (B, arrow) in liver, dermal mononuclear infiltrates in the skin (C), and crypt cell apoptosis in the small intestine (D, arrow). Original magnification × 200 (A, C, D) and × 400 (B).
Histologic analysis of [II–/– → wt] chimeric mice reveals systemic GVHD. Histologic analysis of [II–/– → wt] chimeras 10 weeks after BMT showed periportal mononuclear infiltrates (A) and endothelialitis (B, arrow) in liver, dermal mononuclear infiltrates in the skin (C), and crypt cell apoptosis in the small intestine (D, arrow). Original magnification × 200 (A, C, D) and × 400 (B).
We then investigated whether the similar absence of MHC class I expression on thymic antigen-presenting cells (APCs) could also cause GVHD. In contrast to [II–/– → wt] chimeras, [β2m–/– → wt] chimeras did not show any clinical signs of GVHD and 95% of these mice survived at day 140 after BMT (Figure 3). Analysis of the liver, intestine, and skin 10 weeks after BMT showed no histologic evidence of GVHD (Table 1).
[β2m–/– → wt] chimeric mice do not develop autoimmune diseases. Wt mice were lethally irradiated and received transplants from wt or β2m–/– B6 donors. Clinical GVHD scores (A) and survival (B) after BMT were monitored in [wt → wt], n = 32; and [β2m–/– → wt], n = 56. Results from 3 similar experiments are combined and mean ± SE of clinical scores is shown.
[β2m–/– → wt] chimeric mice do not develop autoimmune diseases. Wt mice were lethally irradiated and received transplants from wt or β2m–/– B6 donors. Clinical GVHD scores (A) and survival (B) after BMT were monitored in [wt → wt], n = 32; and [β2m–/– → wt], n = 56. Results from 3 similar experiments are combined and mean ± SE of clinical scores is shown.
Intact thymus is required for the development of autoimmune GVHD
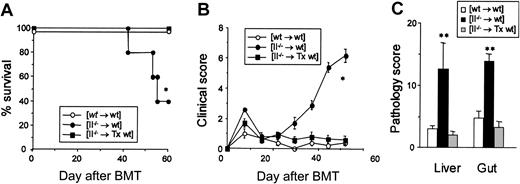
To confirm the causative role of thymus in the development of GVHD, wt mice were thymectomized prior to BMT and underwent transplantation as above. Control [II–/– → wt] chimeras developed severe, lethal GVHD with only 40% survival at day 60 after BMT, whereas all thymectomized [II–/– → wt] chimeras survived this period (Figure 4A) without any evidence of GVHD as judged by clinical GVHD scores (Figure 4B) and histology scores of the liver and intestine (Figure 4C). Thus, we confirmed the causal association of the thymus with the development of autoimmune GVHD.
Thymectomy of BM transplant recipients prevents the development of GVHD. (A-C) Wt mice were thymectomized (Tx) and received transplants of TCD BM from II–/– donors following 13 Gy TBI. Survival (A) and clinical GVHD scores (B) are shown after BMT (n = 5-9/group). (C) Pathology scores of the liver and intestine at 7 weeks after BMT (n = 3/group). Mean ± SE is shown. *P < .01, **P < .05.
Thymectomy of BM transplant recipients prevents the development of GVHD. (A-C) Wt mice were thymectomized (Tx) and received transplants of TCD BM from II–/– donors following 13 Gy TBI. Survival (A) and clinical GVHD scores (B) are shown after BMT (n = 5-9/group). (C) Pathology scores of the liver and intestine at 7 weeks after BMT (n = 3/group). Mean ± SE is shown. *P < .01, **P < .05.
Autoreactive CD4+ T cells confer GVHD to the secondary hosts when peripheral regulatory mechanisms are eliminated
We next investigated the pathogenic role of CD4+ T cells in this disease process. CD4+ T cells that were isolated 8 weeks after BMT from spleens of [II–/– → wt] chimeras proliferated and produced IL-2 and IFN-γ in response to B6 (self) stimulators in vitro, while CD4+ T cells isolated from [wt → wt] chimeras did not (Table 2). [II–/– → wt] CD4+ T cells showed an activation/memory phenotype with the reduction of CD62L and CD45RB expression compared with [wt → wt] CD4+ T cells (CD62L+, 11% vs 66%; CD45RB+, 60% vs 90%), respectively, confirming the emergence of activated, autoreactive CD4+ Th1 cells in the absence of thymic negative selection.
Development of autoreactive CD4+ T cells in the [II–/– → wt] chimeras
Mice . | Stimulation with B6-PC . | Proliferation, cpm . | IFN-γ, pg/mL . | IL-2, pg/mL . |
|---|---|---|---|---|
| wt B6 | ||||
| - | 538 ± 34 | UD | UD | |
| + | 860 ± 164 | UD | UD | |
| [wt → wt] | ||||
| - | 916 ± 24 | UD | UD | |
| + | 780 ± 157 | UD | UD | |
| [wt → II-/-] | ||||
| - | 733 ± 237 | UD | UD | |
| + | 535 ± 181 | UD | UD | |
| [II-/- → wt] | ||||
| - | 949 ± 54 | UD | UD | |
| + | 5640 ± 459 | 698 ± 22 | 64.1 ± 9.5 |
Mice . | Stimulation with B6-PC . | Proliferation, cpm . | IFN-γ, pg/mL . | IL-2, pg/mL . |
|---|---|---|---|---|
| wt B6 | ||||
| - | 538 ± 34 | UD | UD | |
| + | 860 ± 164 | UD | UD | |
| [wt → wt] | ||||
| - | 916 ± 24 | UD | UD | |
| + | 780 ± 157 | UD | UD | |
| [wt → II-/-] | ||||
| - | 733 ± 237 | UD | UD | |
| + | 535 ± 181 | UD | UD | |
| [II-/- → wt] | ||||
| - | 949 ± 54 | UD | UD | |
| + | 5640 ± 459 | 698 ± 22 | 64.1 ± 9.5 |
CD4+ T cells were isolated from spleens (3 spleens/group) 8 weeks after BMT and were stimulated at 2 × 105 cells/well with irradiated (20 Gy) peritoneal cells (1 × 105/well) from B6 mice (B6-PC). After 3 days of culture, supernatants were harvested for cytokine measurements and cell proliferation was determined after pulsing with [3H]thymidine for an additional 16 hours. Data represent mean ± SD. UD indicates undetectable.
To determine whether [II–/– → wt] CD4+ T cells transmit the disease to secondary hosts, CD4+ T cells isolated from spleens of [II–/– → wt] chimeras were adoptively transferred to secondary syngeneic wt recipients. Transfer of 1 × 107 [II–/– → wt] CD4+ T cells into unirradiated wt B6 mice produced no clinical disease (Figure 5A). We therefore hypothesized that unirradiated recipients did not develop disease after transfer of autoreactive CD4+ T cells because (a) the CD4+ T cells were suppressed by peripheral regulatory cells; and/or (b) the absence of proinflammatory milieu in unirradiated secondary hosts did not sustain activation of the CD4+ T cells. To test this hypothesis, wt B6 mice were sublethally irradiated (6.5 Gy) and then injected with 1 × 107 CD4+ T cells isolated either from [wt → wt] or [II–/– → wt] chimeras. Recipients of [II–/– → wt] CD4+ T cells developed lethal GVHD with only 17% surviving 5 weeks after transfer (Figure 5A). These mice exihibited significantly elevated clinical GVHD scores (4.5 ± 1.0, P < .01) and showed hepatic and intestinal pathology similar to the original [II–/– → wt] chimeras (Figure 5B). Flow cytometric analysis of the spleen 6 weeks after adoptive transfer of [II–/– → wt] CD4+ T cells (CD45.2+) into irradiated congeneic B6.Ly-5a (CD45.1+) mice confirmed the expansion of donor-derived CD45.2+CD4+ T cells (79 ± 3%).
Autoreactive CD4+ T cells that escape thymic negative selection cause GVHD only when peripheral regulatory mechanisms by T cells are eliminated. At 10 weeks after BMT, splenic CD4+ T cells (1 × 107) isolated from [wt → wt] and [II–/– → wt] chimeras were injected into either irradiated (6.5 Gy) or unirradiated wt mice (n = 5 or 6 per group). Survival after transfer (A) and pathology scores of the liver and small and large intestine at 6 weeks after transfer (B) (n = 3 per group) are shown. Results from 2 similar experiments are combined and represent mean ± SE. Survival (C) and clinical score (D) after adoptive transfer of CD4+ T cells from [II–/– → wt] chimeras into lethally irradiated wt mice with or without cotransfer of splenic CD4–CD8– cells (non-T cells, 2 × 107), CD4+ T cells (1 × 107), 1 × 107 CD8+ T cells (1 × 107), or splenic CD4+ plus CD8+ T cells (2 × 107) isolated from naive wt mice. (E) DP thymocytes were enumerated 5 weeks after transfer. UD indicates undetectable. *P < .05.
Autoreactive CD4+ T cells that escape thymic negative selection cause GVHD only when peripheral regulatory mechanisms by T cells are eliminated. At 10 weeks after BMT, splenic CD4+ T cells (1 × 107) isolated from [wt → wt] and [II–/– → wt] chimeras were injected into either irradiated (6.5 Gy) or unirradiated wt mice (n = 5 or 6 per group). Survival after transfer (A) and pathology scores of the liver and small and large intestine at 6 weeks after transfer (B) (n = 3 per group) are shown. Results from 2 similar experiments are combined and represent mean ± SE. Survival (C) and clinical score (D) after adoptive transfer of CD4+ T cells from [II–/– → wt] chimeras into lethally irradiated wt mice with or without cotransfer of splenic CD4–CD8– cells (non-T cells, 2 × 107), CD4+ T cells (1 × 107), 1 × 107 CD8+ T cells (1 × 107), or splenic CD4+ plus CD8+ T cells (2 × 107) isolated from naive wt mice. (E) DP thymocytes were enumerated 5 weeks after transfer. UD indicates undetectable. *P < .05.
We then tested whether normal, resting T cells could suppress the pathology of autoreactive CD4+ T cells. Transfer of [II–/– → wt] CD4+ T cells (1 × 107) with TCD BM (5 × 106) from wt mice into lethally irradiated wt mice conferred GVHD. However, cotransfer of both CD4+ and CD8+ T cells from wt mice completely prevented GVHD development, while cotransfer of non-T cells or either CD4+ or CD8+ T cells alone did not prevent it (Figure 5C-D). Cotransfer of CD4+ and CD8+ T cells completely prevented GVHD-associated thymic damage measured by the number of DP thymocytes (Figure 5E). These results demonstrate that autoreactive CD4+ T cells that escape negative selection in the thymus become pathogenic when peripheral T cells are eliminated by irradiation.
CD4+ effector T cells lyse target cells primarily by Fas ligand and cytokines.46 Serum levels of TNF-α (46 ± 11 pg/mL) and IL-1 (20 ± 3 pg/mL) were elevated in [II–/– → wt] chimeras but not in controls. Therefore, in order to determine the functional relevance of these proinflammatory cytokines, we neutralized both cytokines by intraperitoneal injections of TNFR/Fc and αIL-1R, as described in “Materials and methods.” This neutralization significantly delayed GVHD compared with control-treated animals (median survival time 22 days versus 12 days, P < .05). These results demonstrate an important role for inflammatory cytokines in autoimmune GVHD caused by autoreactive CD4+ T cells that escaped negative thymic selection, as has been shown in other autoimmune diseases.47
Discussion
The original formulation of the requirements for the development of GVHD included the following: first, the graft must contain immunologically competent cells; second, the recipient must be incapable of mounting an effective response to destroy the transplanted cells; and third, the recipient must express tissue antigens that are not present in the transplant donor.48 According to these criteria, GVHD normally develops in various clinical settings when allogeneic tissues that contain immunocompetent cells (BM, blood products, and solid organs) are transferred to immunocompromised hosts, a frequent scenario in allogeneic BMT. However, GVHD can also develop after autologous or syngeneic BMT, even though the recipients do not express tissue antigens not present in the donor.49,50 Administration of cyclosporine (CsA) following syngeneic or autologous BMT results in the induction of a T-cell-mediated autoimmune syndrome with pathology similar to GVHD after allogeneic BMT.50 T cells from animals with this type of GVHD recognize a peptide from the MHC class II invariant chain present in the context of MHC class II.51 In the current study, we demonstrate the development of acute GVHD after syngeneic BMT when MHC class II-dependent thymic negative selection is impaired, although we have not yet identified the antigen being recognized. CsA-induced GVHD is relatively mild and primarily limited to skin, and CsA treatment markedly reduces expression of MHC class II in the thymic medulla.50 Our results suggest that such reduction is critical to the development of CsA-induced GVHD, and severe GVHD observed in our model might be explained by the complete, rather than the partial, loss of MHC class II expression in thymic DCs. Thymic negative selection also requires interaction of the costimulatory molecules B7-1 and B7-2, and CSA might affect expression of these molecules as well.52
Our results extend an earlier report demonstrating the development of autoimmune colitis in [II–/– → wt] chimeras,31 but they contrast with another study that failed to demonstrate development of autoimmune diseases in [β2m–/–II–/– → wt] chimeras despite the emergence of autoreactive T cells.28 Interestingly, Marguerat et al31 demonstrated that autoimmune colitis was lethal in [II–/– → wt] chimeras but not in [β2m–/– II–/– → wt] chimeras. Thus, the absence of MHC class I on hematopoietic cells may reduce morbidity and mortality of an autoimmune disease when both MHC class I and MHC class II molecules are absent.28
Marguerat et al31 suggested that development of autoimmune colitis in [II–/– → wt] chimeras is due to a failure to activate regulatory CD4+ T cells by peripheral APCs that lack MHC class II expression. However, our study clearly demonstrated a causal association between the lack of thymic negative selection and the development of autoimmune GVHD because (1) thymectomy completely prevented GVHD, and (2) GVHD did not develop in [wt → II–/–] chimeras (Figure 1) where regulatory CD4+ T cells could not develop.53 Normal naive T cells require costimulation to be activated, and the activated phenotype of CD4+ T cells in the absence of MHC class II on APCs in the periphery in [II–/– → wt] chimeras is thus surprising. One explanation is that autoreactive T cells developed in the absence of negative selection may be already “antigen-experienced cells” whose activation depends less on costimulation.54 A second possibility is the survival of a very small number of wt APCs after irradiation, as previously shown55 ; these few APCs could then activate autoreactive T cells.37,56,57 The precise nature of the antigen recognized by the CD4+ T cells is currently under investigation.
Consistent with previous reports,18,31 we demonstrate that absence of functional MHC class I on thymic APCs failed to cause autoimmune GVHD in [β2m–/– → wt] chimeras. Taken together, these results underscore the critical role for MHC class II-dependent negative selection, rather than MHC class I-dependent negative selection, in the induction of self-tolerance. Adoptive transfer experiments have shown that CD4+ T cells alone are sufficient to transfer disease in irradiated secondary recipients. Similar results have been reported in murine models of autoimmune diseases, such as experimental allergic encephalomyelitis, systemic lupus erythematosus, and diabetes.58 Furthermore, these results support the strong linkage of the genetic susceptibility of several autoimmune diseases and MHC class II, but not class I, alleles in animals and humans.59,60
Our study demonstrates that impaired thymic negative selection causes significant autoimmune diseases when peripheral regulatory mechanisms are impaired. CD4+ T cells isolated from [II–/– → wt] chimeras conferred GVHD after transfer to irradiated, but not unirradiated, hosts. A similar phenomenon was observed in adoptive transfer experiments of autoreactive K14 T cells.29 In that study, the authors suggested that irradiation renders target tissues vulnerable to effector cell attack by inducing tissue damage and an inflammatory milieu.29 Although this remains a possibility, our study suggests that irradiation not only induced an inflammatory milieu but also eliminated peripheral regulatory mechanisms because cotransfer of peripheral T cells suppressed the development of the disease. Although the detailed phenotype of regulatory cells remains to be determined, both CD4+ and CD8+ T cells were required to completely prevent GVHD after adoptive transfer, similar to the peripheral regulatory mechanisms that inhibit CsA-induced syngeneic GVHD.49 These results are also consistent with the studies of cardiac allografts in the rat where CD4+ suppressor cells require CD8+ T cells to mediate their suppressive effects on allograft rejection.61 Similarly, transgenic mice H3-TKO develop CD4-dependent autoimmune neuritis due to impaired MHC class II-dependent thymic negative selection only in the absence of peripheral CD8+ T cells.26 Autoreactive T cells developed after impairing thymic negative selection by perinatal blockade of B7-1 and B7-2; these autoreactive T cells caused systemic autoimmune disease only after transfer into lethally irradiated or T-cell-deficient syngeneic hosts.52 The interaction between autoreactive T cells and regulatory T cells in the generation of acute (syngeneic) GVHD thus may help to provide insight into the development of other autoimmune diseases.
Prepublished online as Blood First Edition Paper, March 27, 2003; DOI 10.1182/blood-2003-01-0266.
Supported by National Institutes of Health (NIH) grant CA39542 (J.L.M.F.). K.R.C. is a National Marrow Donor Program Amy Strelzer-Manasevit Scholar and a Fellow of the Robert Wood Johnson Minority Medical Faculty Development Program. J.L.M.F. is the recipient of a Doris Duke Distinguished Clinical Scientist Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.
We thank Keith Bishop for helpful discussions and Janet Hoff for expert technical assistance.

![Figure 1. [II–/– → wt] chimeric mice develop systemic and lethal autoimmune disease. Wt or II–/– mice were lethally irradiated and received transplants from either wt or II–/– B6 donors. (A) Clinical GVHD scores35 and (B) survivals after BMT were monitored. [wt → wt], n = 38; [wt → II–/–], n = 29; and [II–/– → wt], n = 35. Results from 3 similar experiments are combined and mean ± SE of clinical scores is shown. *P < .001, ** P < .005.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/2/10.1182_blood-2003-01-0266/6/m_h81434618001.jpeg?Expires=1767713868&Signature=mwHImjDDgJkRu96Cb9C9DfPFKKY04euszdfjQUEFnqVnDvQRj8zUDurQXK8uCLptwAdZd9~A27ptkSlbyCjFo3RQC-Guo6E-TEkxzxp16vZaMf9QnqL1Pcm6qF49EtNQonApCM~m5gvl23DTuyTyg~D4ffnUiGGnBmxe-spLQEPN6JHSRbVPRNkDSeeJq9wHmBS28nywLTyfZjSnrWPmDECevU687WDE1qUzE4Yb2sw0Fct~Y~FsraW2thK9NzNLOUyRmQBMm7e~A7eFLU~jBwxnwEMQYMzdXNFI~9nnDFAlECZb7Dq6YmOGjR3ojl3FzKpF6ggasIOcnxQlJoY6OA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Histologic analysis of [II–/– → wt] chimeric mice reveals systemic GVHD. Histologic analysis of [II–/– → wt] chimeras 10 weeks after BMT showed periportal mononuclear infiltrates (A) and endothelialitis (B, arrow) in liver, dermal mononuclear infiltrates in the skin (C), and crypt cell apoptosis in the small intestine (D, arrow). Original magnification × 200 (A, C, D) and × 400 (B).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/2/10.1182_blood-2003-01-0266/6/m_h81434618002.jpeg?Expires=1767713868&Signature=KkJ7uKeBExZPp9Aw-RRUP~WmnxXrsS9PLcF1B-sdqYarU3x5x9gClt3AzF84V3YQ9UspxtzvNrdUHPKhJELkLXT8EFRqzUaVcod~5G7AbWmnVFJl9q4uxt11uC2nV7FmKvQniRjMLj-qO-UylFOiF1eJjclDJhb2vXYeh8a8OfUouDwKC9II-6l~3MTZ9W1l512QGVhOc-OUni9WP~K4Tyy~mvca4YcoRBSL4tN0WxX1lglKQV6I5kXfSu0llOZTaBM3nEOD~YtofaFZnvMZplnOv~1f1kPx9rcxN3c7s-yrRmTL0H44AE9WpaQwsrbReMb7ZedOoAhwiqdrGhhlDg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. [β2m–/– → wt] chimeric mice do not develop autoimmune diseases. Wt mice were lethally irradiated and received transplants from wt or β2m–/– B6 donors. Clinical GVHD scores (A) and survival (B) after BMT were monitored in [wt → wt], n = 32; and [β2m–/– → wt], n = 56. Results from 3 similar experiments are combined and mean ± SE of clinical scores is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/2/10.1182_blood-2003-01-0266/6/m_h81434618003.jpeg?Expires=1767713868&Signature=n51kRdZasLtLRgyifockAmP3wO0WjFN2If2s2F5qkXvS1QrWf-4oBTlpYX8~Z9dmBr7SOUIQxS6NRMLJ3ladi6MmkxVc~CqLWkT~SG7fJE0lkJ4LWEkecVf368qW9ilXx3b-SqMmTb4Rnrpbr5usFmQUJknQeSx2LEbz60L3htsKoP~ZqjSutd-12WJJNdeos2-NrgpJuanaLiapro5jWCegwFX4mjPvyFXmjjGF0e9XWeCWaNMr18Ay~J7e1JbFdxv-OWb7eKdNFBIadIs3GwcijfEBVR3AUylLyKDqjbHcLO9t~nBzCQuRLKagccz1un8Ikwbr-QF0ZIuVKT-s-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Autoreactive CD4+ T cells that escape thymic negative selection cause GVHD only when peripheral regulatory mechanisms by T cells are eliminated. At 10 weeks after BMT, splenic CD4+ T cells (1 × 107) isolated from [wt → wt] and [II–/– → wt] chimeras were injected into either irradiated (6.5 Gy) or unirradiated wt mice (n = 5 or 6 per group). Survival after transfer (A) and pathology scores of the liver and small and large intestine at 6 weeks after transfer (B) (n = 3 per group) are shown. Results from 2 similar experiments are combined and represent mean ± SE. Survival (C) and clinical score (D) after adoptive transfer of CD4+ T cells from [II–/– → wt] chimeras into lethally irradiated wt mice with or without cotransfer of splenic CD4–CD8– cells (non-T cells, 2 × 107), CD4+ T cells (1 × 107), 1 × 107 CD8+ T cells (1 × 107), or splenic CD4+ plus CD8+ T cells (2 × 107) isolated from naive wt mice. (E) DP thymocytes were enumerated 5 weeks after transfer. UD indicates undetectable. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/2/10.1182_blood-2003-01-0266/6/m_h81434618005.jpeg?Expires=1767713868&Signature=o-85NvVqjpJQiWplGlPGE9bG-LwWXxw~PvDVT8cJ4p7pu~rYXGAABYxAE7ZsxrRCSfQNwnDpS-hC37teG~qsm9k0dPjJs9bmoCB6v3gyVnM7Ya1gcSS0xHullrvrbiQlMeWQ~I8s4b97r0dC6d9BMb~3vbbAys23ByCcziNycZJvnaTjiFthHpW6YORcjPiLJxovuVTbLLJay33DuNdbAh0CPq8M5oJXi-nfnpOG0NvkoCKOm9Dyke-7tb9fMp~MEKPPYeG7rvbSVFHhRWz-lLk0vMbml~gjzVsg1xyFVUl57Pf7tOVjw7deHNnQznHm7mpwvbpHHOVvPn45cywnng__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal