Abstract
When cells are injured they release their contents, resulting in a local accumulation of free heme proteins and heme. Here, we investigated the involvement of heme and its degrading enzyme heme oxygenase (HO) in the inflammatory process during wound healing. We observed that heme directly accumulates at the edges of the wound after inflicting a wound in the palate of Wistar rats. This coincided with an increased adhesion molecule expression and the recruitment of leukocytes. To prove that heme is responsible for the recruitment of leukocytes, heme was administered intradermally 24 hours prior to injury. A clear heme-induced influx of both macrophages and granulocytes was observed. When examining the HO isoforms, HO-1 and HO-2, we found that HO-2 was present in the entire submucosa. Surprisingly, we observed also that HO-1 is significantly expressed in the epithelium of both the mucosa and the skin of animals without wounds. On inflammation, HO-1 expression increased, particularly in infiltrating cells during the resolution phase of inflammation. Interestingly, we observed that heme-induced influx of leukocytes was highly elevated after pharmacologic inhibition of HO activity. These observations suggest that the heme-HO system is closely involved in the control of wound healing. Our results demonstrate that the local release of heme may be a physiologic trigger to start inflammatory processes, whereas HO-1 antagonizes inflammation by attenuating adhesive interactions and cellular infiltration. Moreover, the basal level of HO expression in the skin may serve as a first protective environment against acute oxidative and inflammatory insults.
Introduction
The skin, consisting of an epidermal and a dermal layer divided by the stratum basale, forms a versatile organ with highly adaptive properties. In addition to the various homeostatic mechanisms, the skin has to cope continuously with changes in the external environment, such as oxidative insults, heat, cold, UV radiation, and injury.1,2 The skin microenvironment changes dramatically after injury.3-6 One of the earliest changes in the skin after wounding is the release of modified, denatured tissue proteins. This may result from the direct effect of the trauma or by proteolysis following lysosomal rupture. Wounds are associated with severe hemorrhage, hemolysis, and cell injury and may result in a local accumulation of free heme proteins and heme.
Heme and heme proteins play a crucial role in diverse biologic processes and are present in every human cell. We and others have recently shown that free heme also possesses several pro-oxidative and proinflammatory properties.7-11 It induces the expression of adhesion molecules and causes vascular permeability and leukocyte infiltration.9
Wound healing consists of a complex series of processes to stop and prevent blood loss, to kill possible intruding pathogens, and to restore the site of injury.5,12 In chronic wounds, resolution of inflammation is interrupted, resulting in continuous influx of inflammatory cells and subsequently exacerbation of cellular injury.
It is well accepted that bacterial products, such as lipopolysaccharide (LPS) or CpG-containing DNA, initiate inflammatory processes. However, nonopen wounds, such as contusions, do not involve invasion of pathogens, but nevertheless result in inflammatory processes and wound healing. This may be explained by the local release of heme and heme proteins at the site of injury. Elevated levels of free heme may act as a “danger signal” and initiate a wide range of proinflammatory signals to recruit and alert the immune system.
In the resolution phase of inflammation, heme and other proinflammatory signals need to be neutralized. Interestingly, heme oxygenase 1 (HO-1), the heme-degrading enzyme, is one of the major acute-phase proteins that are induced on stress.13,14 HO expression represents an important mechanism of protective response to a wide variety of cellular stresses.13 HO is the enzyme that converts heme into biliverdin/bilirubin, iron, and carbon monoxide (CO).13,15
There are currently 3 isoforms of HO described. HO-2 and, to a lesser extent, the recently discovered HO-3 isoenzyme are predominantly constitutively expressed and function mainly in normal heme capturing and metabolism.16,17
The inducible HO-1 isoform is known to function in a wide range of processes that could be important in the resolution phase of wound healing, such as amelioration of inflammation, proliferation, and protection against apoptosis.18-20 These actions are likely mediated via the downstream effector molecules of HO. Biliverdin and bilirubin function as a potent antioxidant system, whereas CO mediates vasodilation via cyclic guanosine monophosphate (cGMP) induction.21 Furthermore, the iron, released from heme by HO-1 activity, enhances ferritin synthesis and thereby renders it inactive by sequestering.22 In this regard, overexpression of HO-1 in endothelial cells has been shown to protect from heme- and hemoglobin-mediated toxicity and is associated with increased tolerance of cells to ischemia and trauma in a variety of tissues.23-25 Interestingly, HO-1 overexpression has been demonstrated to down-modulate inflammatory adhesion molecules and to diminish leukocyte adhesion to the vascular endothelium on proinflammatory stimuli both in vitro and in vivo.8,9,26-28
Here, we hypothesize that HO alleviates the inflammatory process present after wounding and provides protection against further injurious insults. To investigate this hypothesis, we examined the role of the heme-HO system on adhesion molecule expression and leukocyte recruitment during wound healing in rat oral mucosa.
Materials and methods
Porphyrin solutions
The following porphyrins were used: hemin (Sigma Chemical, St Louis, MO) and tin (stannic) mesoporphyrin (SnMP; Porphyrin Products, Logan, UT). At low concentrations, SnMP acts as a potent and selective competitive inhibitor of HO activity in vitro and in vivo.29 All porphyrin solutions were freshly prepared as previously described.30 In short, hemin was dissolved together with Trizma base in a 0.1 M NaOH solution and diluted in saline (Braun Melsungen, Melsungen, Germany). Next, the pH was adjusted to pH 8 with HCl. The solution was then filter-sterilized, protected from light, and directly used. The heme solution was negative for the presence of endotoxin contamination as determined by the limulus amebocyte lysate (LAL) test (BioWhittaker, Walkersville, MD).
Antibodies
The following monoclonal antirat antibodies were used: anti-intercellular adhesion molecule 1 (ICAM-1; Endogen, Woburn, MA), anti-β1 (kind gift from K. Löster, Berlin, Germany), antimacrophage marker, antigranulocyte marker, and anti–T-cell marker (Serotec, Oxford, United Kingdom). In addition, the polyclonal rabbit antibodies SPA895 (antirat-HO-1; Stressgen/Sanbio, Uden, The Netherlands) and OSA200 (antirat-HO-2; Stressgen/Sanbio) were used. As secondary antibodies, biotin-labeled goat-antimouse IgG or biotin-labeled antirabbit IgG antibodies were used.
Animal and human material
Male Wistar rats, 5 to 7 weeks of age, were obtained from Harlan (Horst, The Netherlands). The rats were kept under specified pathogen-free conditions in the Central Animal Laboratory (University Medical Center Nijmegen, The Netherlands). The rats had free access to water and were fed standard laboratory chow (Hope Farms, Woerden, The Netherlands). All experiments were performed in accordance with the guidelines of the Nijmegen Animal Experiments Committee.
Human skin material was obtained according to the guidelines of the medical and ethics committee of the University Medical Center Nijmegen and after informed consent was given.
Experimental wounding protocol
All rats were anesthetized with an intraperitoneal injection of fentanyl and fluanisone, 0.4 mL/kg body weight (Janssen-Cilag, Beerse, Belgium). Full-thickness excisional circular wounds were made in the mucoperiosteum of the hard palate between the second molars using a 3-mm biopsy punch.31,32 The day on which this biopsy was taken is further referred to as day 0. The initial wounds included the submucosa attached to the midpalatal suture.31 Wounded rats were medicated postoperatively with a subcutaneous injection of buprenorphine, 0.1 mg/kg body weight (Schering-Plough, Maarssen, The Netherlands) as an analgesic. Animals were killed with a mixture of CO2/carbogene at 1, 2, 3, 5, 8, 15, 30, 60, or 90 days after wounding. To ensure the inclusion of the wound margins, 4-mm circular biopsy punches of rats (n = 3-6) were then obtained per time point. The tissues were immediately immersed in Tissue-Tek (Sakura Finetek Europe, Zoeterwoude, The Netherlands), frozen in liquid nitrogen, and stored at –80°C.
In subsequent experiments, 30 μL SnMP (20 μM; day –2) and/or 30 μL heme (750 μM; day –1) or 30 μL saline (day –1) was given intradermally with a fine needle (29 gauge) prior to the described wounding (n = 6).
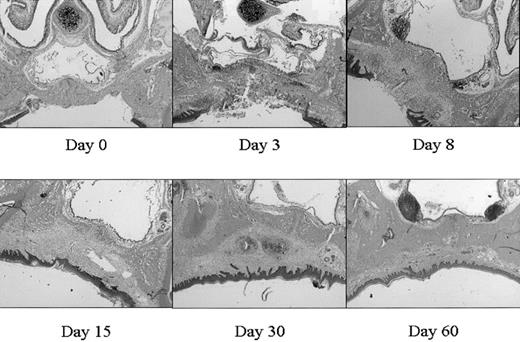
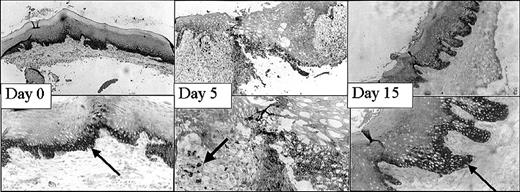
This mucosal wound-healing model was characterized in time using hematoxylin-eosin staining (Figure 1). Tissue from day 0 showed the normal mucosa, in which no inflammatory leukocyte infiltrates were present. Acute cellular injury and inflammatory reactions and cellular influx are evident at days 1 to 5 as exemplified by day 3. From day 5 on, resolution of inflammation started resulting in a decreased cell influx (data not shown). From day 8 until day 15, wound contraction by myofibroblasts resulted in the closure of the wound. Around day 15 to 30 remodeling started, and at day 90 to 120 the wound site was restored, resulting in scar tissue.
Wound healing in time. Various stages (days 0, 3, 8, 15, 30, 60) in wound healing are shown. The tissues are stained with hematoxylin and eosin. A clear influx of inflammatory cells can be seen at day 3 to 8, whereas from day 15 remodeling occurs, which was completed at day 60 (original magnification, × 25).
Wound healing in time. Various stages (days 0, 3, 8, 15, 30, 60) in wound healing are shown. The tissues are stained with hematoxylin and eosin. A clear influx of inflammatory cells can be seen at day 3 to 8, whereas from day 15 remodeling occurs, which was completed at day 60 (original magnification, × 25).
Primary fibroblast cultures
For fibroblast isolation, rats were killed with a mixture of CO2/carbogene at 3, 5, 8, 15, 30, 60, and 90 days after initial wounding (n = 5). Biopsy specimens from the wound area were transported into sterile phosphate-buffered saline (PBS), washed extensively, cut into small pieces, and placed into a single well of a 24-well culture plate, containing Dulbecco modified Eagle medium (DMEM) with 25 mM HEPES (N-2-hydroxyethylpiperazine-N′2-ethanesulfonic acid), sodium pyruvate, 1000 mg/L glucose, and pyridoxine (Gibco Life Technologies, Paisley, Scotland) supplemented with 10% fetal bovine serum (FBS; Gibco Life Technologies) and 100 U/mL penicillin (Gibco Life Technologies) and 100 μg/mL streptomycin (Gibco Life Technologies). The tissue explants were left undisturbed for 1 week in an incubator (37°C, 5% CO2) to allow the outgrowth of fibroblasts. After 7 days, the cultures were trypsinized and transferred to a 25-cm2 culture flask (Nunc, Naperville, IL). Cultures were successively passaged to one and three 75-cm2 culture flasks. Finally, cell cultures were frozen in 7.5% dimethyl sulfoxide (DMSO; ICN Biomedicals, Aurora, OH) in culture medium and stored in liquid nitrogen until use.32,33
FACS analysis of HO-1 expression
The fibroblasts isolated at the different time points were analyzed for the expression of HO-1 using fluorescence-activated cell sorting (FACS) analysis. Cells were fixated for 20 minutes in 1% paraformaldehyde on ice. Next, they were permeabilized by incubation with 0.1% saponin/PBA (PBAS; PBS containing 0.5% wt/vol bovine serum albumin [Roche Molecular Biochemicals, Mannheim, Germany] and 0.01% sodium azide [Merck, Hohenbrunn, Germany]) for 10 minutes on ice. Cells were incubated (30 minutes, 4°C) with primary antibody against HO-1. After extensive washing (PBAS) cells were incubated with fluorescein isothiocyanate (FITC)–conjugated goat-antirabbit IgG for 30 minutes at 4°C. Cells were washed and the relative fluorescence intensity was measured by FACScan analysis (Becton Dickinson, Oxnard, CA).
Heme staining
Tissue slices, 4 μm thick, were stained with hematoxylin and eosin using standard protocols. The presence of heme in the sections was analyzed using a benzidine staining technique as described before.9 Tissue was counterstained with hematoxylin.
Immunohistochemistry
Cryostat sections (4 μm thick) were collected on Super frost slides (Menzel-Gläser, Braunschweig, Germany) and analyzed for the expression of ICAM-1, β1, HO-1 and HO-2, and leukocyte subsets using immunohistochemistry.
Immunohistochemical analysis was performed using protocols of the provider (Vector Laboratories, Burlingame, CA). AEC (3-amino-9-ethylcarbazole) or diaminobenzidine (DAB) were used as chromogens. Slides were microscopically analyzed after counterstaining with hematoxylin.
Results
Heme accumulation, adhesion molecule expression, and cellular infiltrates during wound healing
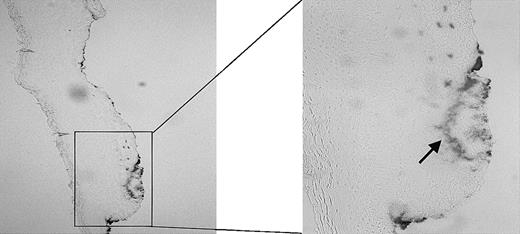
Using the wound-healing model as described in “Materials and methods,” we first examined the effect of injury on heme release using benzidine staining. As demonstrated in Figure 2, large amounts of free heme/hemoproteins are visible at the site of injury, whereas in the surrounding noninjured tissue no heme can be detected.
Presence of heme. The presence of heme in a fresh wound of a Wistar rat was analyzed using benzidine staining. The heme (arrow) has clearly accumulated at the sites of injury. Original magnification, × 100 (left panel) and × 265 (right panel).
Presence of heme. The presence of heme in a fresh wound of a Wistar rat was analyzed using benzidine staining. The heme (arrow) has clearly accumulated at the sites of injury. Original magnification, × 100 (left panel) and × 265 (right panel).
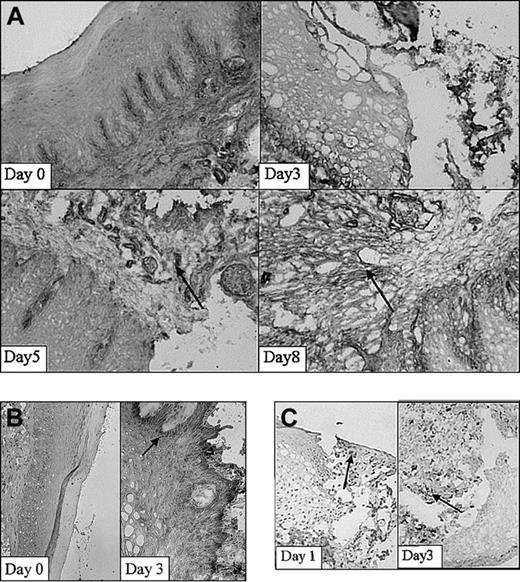
Next, expression of adhesion molecules was analyzed in the wound area in time using immunohistochemical techniques. Using immunohistochemical analysis, a clear increase in expression of β1-integrin was demonstrated on injury, which was localized mainly on fibroblasts and infiltrating cells (Figure 3A). The β1 integrins are engaged in interactions with extracellular matrix components, such as collagen and fibronectin, and cell surface ligands. In parallel studies, it was recently found that about 70% of the fibroblasts isolated at day 0 expressed the β1 subunit on their cell surface.32 Highest amounts of positive cells (90%) were observed at 30 days after wounding. Peaks in the fluorescence intensity were observed at 5 and 60 days after wounding.32 In addition, nontreated rats (day 0) hardly showed ICAM-1 expression (Figure 3B). After injury (day 1-5) ICAM-1 expression was clearly induced. Interestingly, activated infiltrating leukocytes also showed intense ICAM-1 staining.
Adhesion molecule expression and the infiltration of leukocytes. Adhesion molecule expression and the infiltration of leukocytes subset in the wound shown at 2 different stages in wound healing as determined using immunohistochemistry. (A) β1-Integrins are highly up-regulated 5 and 8 days after wounding (arrows; original magnification, × 100). (B) ICAM-1 expression is clearly up-regulated within the wounded tissue at day 3 compared with day 0 (arrow; original magnifications, × 100 and × 250, respectively). (C) Massive infiltrates of macrophages are present 1 and 3 days after wounding. Arrows indicate examples of positive staining (original magnification, × 250).
Adhesion molecule expression and the infiltration of leukocytes. Adhesion molecule expression and the infiltration of leukocytes subset in the wound shown at 2 different stages in wound healing as determined using immunohistochemistry. (A) β1-Integrins are highly up-regulated 5 and 8 days after wounding (arrows; original magnification, × 100). (B) ICAM-1 expression is clearly up-regulated within the wounded tissue at day 3 compared with day 0 (arrow; original magnifications, × 100 and × 250, respectively). (C) Massive infiltrates of macrophages are present 1 and 3 days after wounding. Arrows indicate examples of positive staining (original magnification, × 250).
Infiltration of leukocytes into the wound area was investigated using subset-specific antibodies. The first infiltrating leukocytes appeared already 1 day after inflicting the wound (Figure 3C). They consisted mainly of granulocytes (data not shown). After 3 days massive infiltrates of leukocytes, consisting of large amounts of macrophages (Figure 3C) and granulocytes, were present. T lymphocytes were observed both in normal mucosa and in the wound and did not significantly increase. After 15 days most wounds were closed and filled up with new granulation tissue. The infiltrated granulocytes and macrophages remained only at the initial wound site. Thus, the release of heme is followed by adhesion molecule expression and leukocyte recruitment.
Effects of heme administration on adhesion molecule expression and leukocyte infiltration
Because we observed that after injury large quantities of heme were locally released in conjunction with elevated inflammatory adhesion molecules and leukocyte infiltrates, we hypothesized that heme is playing an active role in this inflammatory process. To investigate the inflammatory effects of heme the rat mucosa was exposed to heme, or saline control, and analyzed for the expression of adhesion molecules and leukocytes.
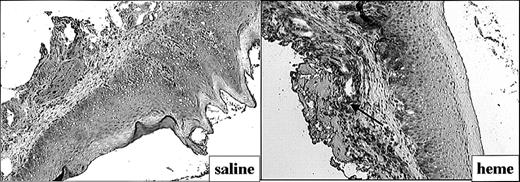
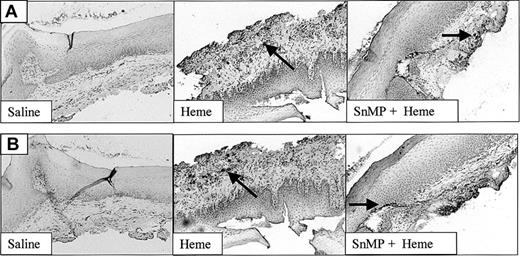
The effect of heme on ICAM-1 expression (Figure 4) and leukocyte infiltration (Figure 5) in the mucosa was measured by immunohistochemistry. ICAM-1 expression was highly upregulated on endothelial cells and keratinocytes in animals exposed for 24 hours to heme when compared with saline controls (Figure 4). Moreover, 24 hours after administration of heme the mucosa showed a clear increase of granulocyte and macrophage infiltration when compared with saline controls (Figure 5A-B). Thus, the sheer presence of heme mimics several of the inflammatory effects observed on wounding.
Adhesion molecule expression after administration of saline or heme. Administration of heme prior to injury caused a clear increase in ICAM-1 expression in endothelial cells, infiltrating leukocytes, and keratinocytes (arrow; original magnification, × 100).
Adhesion molecule expression after administration of saline or heme. Administration of heme prior to injury caused a clear increase in ICAM-1 expression in endothelial cells, infiltrating leukocytes, and keratinocytes (arrow; original magnification, × 100).
The recruitment of leukocytes after administration of heme, saline, or SnMP. Both infiltrated granulocytes (A) and macrophages (B) are highly up-regulated after administration of heme (arrows). Inhibition of HO activity using SnMP exacerbated heme-induced leukocyte infiltration (original magnification, × 100). In some cases HO inhibition using SnMP alone already caused leukocyte infiltration (data not shown).
The recruitment of leukocytes after administration of heme, saline, or SnMP. Both infiltrated granulocytes (A) and macrophages (B) are highly up-regulated after administration of heme (arrows). Inhibition of HO activity using SnMP exacerbated heme-induced leukocyte infiltration (original magnification, × 100). In some cases HO inhibition using SnMP alone already caused leukocyte infiltration (data not shown).
Expression of HO-1 and HO-2 isoforms in the mucosa and skin
Because HO-1, the heme-degrading enzyme, is up-regulated by a multitude of stress-causing conditions, we hypothesized that HO may also play an important function in the response to injury. The expression levels of the HO-1 and HO-2 isoforms during wound healing were therefore examined using immunohistochemistry.
Surprisingly, HO-1 protein expression was not only evident in the mucosal epithelium throughout the healing process, but also in normal noninjured mucosa (Figure 6). High levels of HO-1 were observed in both keratinocytes and infiltrating leukocytes 1 or 2 days after wounding (data not shown). In addition, high HO-1 expression was observed in infiltrated immune cells at day 3 (data not shown) and day 5 (Figure 6).
Expression of HO-1 in the mucosa in time. HO-1 is clearly expressed in the epithelium of the mucosa (arrows). Furthermore, at day 5 HO-1 is highly evident in infiltrated leukocytes. At day 15 the expression returned to initial levels (in the top and lower panels, original magnifications are × 100 and × 250, respectively).
Expression of HO-1 in the mucosa in time. HO-1 is clearly expressed in the epithelium of the mucosa (arrows). Furthermore, at day 5 HO-1 is highly evident in infiltrated leukocytes. At day 15 the expression returned to initial levels (in the top and lower panels, original magnifications are × 100 and × 250, respectively).
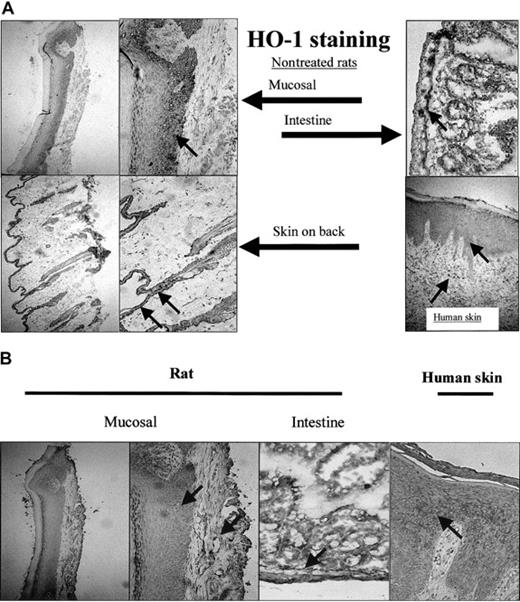
To analyze whether this basal level of HO-1 expression was confined to the epithelium of the mucosa, the expression of HO-1 (Figure 7A) and HO-2 (Figure 7B) was also investigated in different sections of the skin. Although the epidermis in skin sections of the back of the rat is narrow compared with the mucosal epithelium, both HO-1 and HO-2 were highly expressed (Figure 7). Furthermore, skin sections of patients with chronic inflammation of the skin showed expression of HO-1 in the epidermis and in infiltrated leukocytes (Figure 7).
Expression of HO-1 and HO-2 isoforms in mucosa and skin. (A) HO-1 is not exclusively expressed in the oral mucosal epithelium, but also present in the epidermis of skin from the back of a rat and in the intestines (original magnifications, × 100 and × 250, respectively). Furthermore, HO-1 expression is evident in human epidermis and infiltrated leukocytes of a patient suffering from chronic inflammation (arrows; original magnification, × 250). (B) HO-2 is expressed both in the epithelial as well as the dermal layer of mucosa and skin (mucosal, back of the rat, and intestines; arrows; original magnifications, × 100 and × 250, respectively). HO-2 is also expressed in a patient suffering from chronic inflammation.
Expression of HO-1 and HO-2 isoforms in mucosa and skin. (A) HO-1 is not exclusively expressed in the oral mucosal epithelium, but also present in the epidermis of skin from the back of a rat and in the intestines (original magnifications, × 100 and × 250, respectively). Furthermore, HO-1 expression is evident in human epidermis and infiltrated leukocytes of a patient suffering from chronic inflammation (arrows; original magnification, × 250). (B) HO-2 is expressed both in the epithelial as well as the dermal layer of mucosa and skin (mucosal, back of the rat, and intestines; arrows; original magnifications, × 100 and × 250, respectively). HO-2 is also expressed in a patient suffering from chronic inflammation.
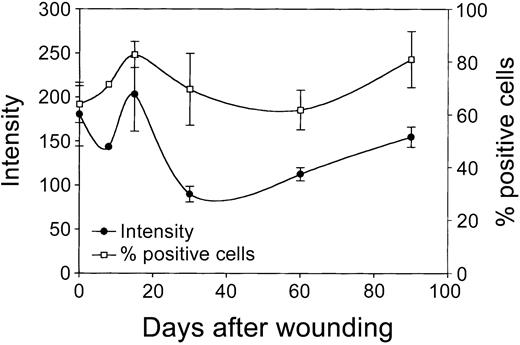
Next, cultured rat skin fibroblasts, isolated from the mucosa at several time intervals after wounding (day 0 to day 90; see “Materials and methods”) were evaluated for the expression of HO-1 using flow cytometric analysis. It has previously been demonstrated that the phenotype of fibroblasts from biopsies remains remarkably stable on prolonged in vitro culturing.33-35 These cultured fibroblasts showed no significant changes in expression of a panel of markers (eg, integrins and cytoskeletal markers) and morphology compared to the in vivo situation.32,33,35 Figure 8 shows that shortly after wounding (day 1) fibroblasts initially show a small decrease in HO-1 expression, which is directly followed by a rise in HO-1 expression, which peaks at day 15. Next, the HO-1 expression levels decrease and return at day 90 slowly to day 0 levels. Furthermore, a clear increase in HO-1+ fibroblast populations was observed on wounding, which peaks at day 15 (Figure 8). Next, the percentage of HO-1+ cells returns to day 0 levels at day 60 and then slightly increases again.
Expression of HO-1 in wound fibroblasts in time. Fibroblast populations isolated from the wound area (see “Materials and methods”) were analyzed for HO-1 expression levels (left y-axis; mean fluorescence intensity) and percentage HO-1+ cells (right y-axis) using flow cytometry. After initial decrease, HO-1 expression is elevated and peaks after 15 days, after which it decreased and returned to control levels (left y-axis). After wounding, the population of infiltrated HO-1+ cells swiftly increased, peaked after 15 days, and returned to control levels at day 60, after which it slowly increased again (right y-axis).
Expression of HO-1 in wound fibroblasts in time. Fibroblast populations isolated from the wound area (see “Materials and methods”) were analyzed for HO-1 expression levels (left y-axis; mean fluorescence intensity) and percentage HO-1+ cells (right y-axis) using flow cytometry. After initial decrease, HO-1 expression is elevated and peaks after 15 days, after which it decreased and returned to control levels (left y-axis). After wounding, the population of infiltrated HO-1+ cells swiftly increased, peaked after 15 days, and returned to control levels at day 60, after which it slowly increased again (right y-axis).
The effects of HO activity on leukocyte infiltration
The layer of HO-1+ and HO-2+ cells in the noninjured mucosa suggests that HO activity may play a prominent modulating role in the observed heme-induced leukocyte infiltration. To further investigate the effects of HO on wound healing, the mucosa was pretreated with SnMP, an inhibitor of HO activity, followed by exposure to heme or saline. Twenty-four hours of incubation with SnMP, followed by 24 hours of exposure to heme, resulted in an exacerbated influx of both granulocytes as well as macrophages into the mucosa, compared with heme treatment alone (Figure 5). Surprisingly, in some saline control animals (2 of 6) as well as SnMP-treated animals (4 of 6) increased numbers of infiltrating leukocytes were observed after injection (data not shown), whereas rats receiving no injection never showed inflammatory infiltrates (data not shown). Thus, inhibition of HO activity seems to sensitize the tissue for leukocyte infiltration.
Discussion
In this study, we investigated the effects of heme and the heme-degrading enzyme, HO, on the inflammatory response in a mucosal wound-healing model in rats. Cellular injury inflicted upon the skin is associated with repair processes involving complex interactions among coagulation, platelet aggregation, and the immune response. However, the exact mechanism of wound healing remains largely obscure. Wounding is associated with severe hemorrhage and hemolysis, and the injured cells get ruptured and release their content resulting in a local accumulation of free heme proteins and heme. We postulate that in a wounded area a pro-oxidant microenvironment is created providing signals to generate an inflammatory process.
We found that large amounts of heme are released at the wound site shortly after wounding, which is rapidly followed by the induction of adhesion molecules and the invasion of leukocyte subsets into the wounded area, which is indispensable for the elimination of pathogens and for the wound-healing process. Moreover, it was demonstrated that exposure of the mucosa to heme induces an increased expression of adhesion molecules and results in the recruitment of leukocytes. Interestingly, our observation that occasionally the injection of only saline also induces leukocyte recruitment, suggests that a minor injury evoked by the injection needle, probably releases sufficient amounts of heme to initiate local inflammation.
After injury, the life of organisms is at risk and wound repair is crucial, because of blood loss and possible influx of bacterial invaders. Previous data support a contribution of heme to this rescue operation because hemoglobin/heme can activate a wide array of processes, such as vasoconstriction, mononuclear cell-procoagulating effects, the activation of complement, the induction of inflammatory cytokines, vascular permeability, and platelet aggregation.9,36,37 Therefore, heme and heme proteins are likely implicated in initiation of inflammatory processes9,38 and homeostasis36 and may form an essential link between these important systems.
Previously, we and others demonstrated that excess of free heme and heme proteins possesses potent pro-oxidative and proinflammatory properties.9,39 Intriguingly, we demonstrate here that administration of solely heme is sufficient to induce inflammatory processes as exemplified by the influx of both granulocytes and macrophages.
In line with our findings of heme-induced inflammation in the rat model are earlier observations that administration of lysed blood, hemoglobin, or heme induced increased adhesion molecule expression,7,9 influx of leukocytes (F.A.D.T.G.W., A. Eggert, and colleagues, unpublished observations, November 2001), vascular permeability,9,40 vascular smooth muscle cell contraction,41 nuclear factor κB (NF-κB) activation, and chemokine production.38
It was previously demonstrated that heme activates NF-κB, AP-1, and AP-2 responsive elements.42,43 These regulatory sites are present on various proinflammatory genes (eg, ICAM-1, vascular cell adhesion molecule 1, E-selectin) as well as on anti-inflammatory/antioxidative genes (eg, HO-1).7 Recently, it was found that heme further controls gene expression via the transcriptional repressor Bach1.44 Interestingly, heme increases the nuclear translocation of Nrf2, which has recently been implicated in wound-healing processes,45 via increased Nrf2 stabilization.46 On binding of intracellular heme, Bach1 detaches from the MARE sequences, which allows binding of Nrf2 and other small maf proteins, resulting in transcriptional activation of target genes, such as HO-1, thioredoxin, and keratinocyte growth factor.44,45,47,48
The importance of the heme-HO system in wound healing is underscored by the finding that HO-1 is associated with keratinocyte proliferation49 and that HO-1 expression is highly elevated in infiltrated immune cells of patients with psoriasis,49 who suffer from hyperproliferation of keratinocytes.
Surprisingly, we found that both HO-1 and HO-2 were significantly expressed in normal mucosa and skin. HO-1 is present mainly near the basal membrane in proliferating keratinocytes.50 HO-2, the HO isoform that is able to bind heme, may therefore function as a first scavenger against heme-induced insults, whereas HO-1, present in the first layers of the epidermis and in infiltrating leukocytes, degrades heme to generate its potent cytoprotective breakdown products. Therefore, HO seems to form a general protective layer to withstand the continuous oxidative and mechanical insults of which the skin daily suffers. This hypothesis is supported by experiments of Tyrrell,51 who demonstrated that on UV radiation HO-1 and therefore ferritin are highly up-regulated in fibroblasts and provide potent cytoprotection. Interestingly, many reptiles and amphibians accumulate large amounts of the green antioxidant biliverdin, a heme breakdown product, in their skin, which may function as a protective wall as well.
The observed increased expression of HO-1 on wounding in fibroblasts seems to be mediated mainly by an increased cell population of HO-1+ fibroblasts, which probably represents newly recruited cells. The newly arrived fibroblasts and leukocytes in the wound area demonstrate clearly elevated HO-1 levels. Thus, by infiltrating, these cells import the HO system into the wounded area and cause protection via the immediate generation of the anti-inflammatory and antioxidative heme metabolites biliverdin/bilirubin and CO, and the up-regulation of ferritin.50
When HO activity is inhibited by pharmacologic agents such as SnMP, administration of heme aggravated influx of granulocytes and macrophages as compared with heme treatment alone. HO activity may, via the generation of antioxidants biliverdin/bilirubin and vasodilator CO, function as a feedback mechanism by down-regulating adhesion molecules and thereby alleviating recruitment of immune cells and the immune response. It is likely that the mechanism by which heme-induced leukocyte influx acts is also mediated by chemokine-triggered pathways. Heme may oxidize low-density lipoproteins (ox-LDL) that form potent chemoattractants (references 39 and 52 and F.A.D.T.G.W., J. Van Horssen and colleages, unpublished observations, 2002). Moreover, it was recently discovered that heme strongly induces monocyte chemoattractant protein 1 (MCP-1),38,42 and that exposure of granulocytes to heme induces the expression of the chemokine interleukin 8 (IL-8).53 These heme-mediated inflammatory processes may be counteracted by HO activity. In fact, it has been observed that heme breakdown products down-modulate ox-LDL–mediated leukocyte chemotaxis.52 Moreover, hypoxia-induced proinflammatory cytokines and chemokines are significantly suppressed in HO-1 transgenic mice.54
Matzinger recently described a novel view on immune regulation, in which the immune system does not primarily discriminate between “self and non-self,” but acts mainly on “danger signals.”55 The local release of heme and heme proteins may act as a “danger signal,” similar to inflammatory bacterial endotoxins, and alert the immune system to initiate inflammatory processes, resulting in the recruitment of leukocytes.
Similarly, heme may act as an inducer of inflammatory processes, by cellular activation, the formation of ox-LDL, and the induction of adhesion molecules.7,39 This results in signals for recruitment of leukocytes and for antigen processing by dendritic cells and macrophages.52,56 This may also explain the vivid response of the immune system toward nonopen wounds, such as contusions, despite the absence of “foreign” material.
Interestingly, Tolosano and coworkers recently described several anti-inflammatory effects of hemopexin and haptoglobin, scavengers for, respectively, heme and hemoglobin.57 They suggested that these scavengers modulate the inflammatory response by triggering their specific receptors.57 These receptors may also be able to sense and respond to the first signs of injury (eg, heme release). In fact, the haptoglobin receptor, CD163, and hemopexin receptors have been implicated in cell survival, stress response, and inflammatory processes.57-61
On the other hand, we and others previously observed that overexpression of HO-1 down-regulates adhesion molecule expression and subsequently reduces binding of leukocytes, whereas inhibition of HO activity exacerbates adhesion molecule expression and leukocyte infiltration mediated by proinflammatory mediators.8,9,26-28,62 This model of heme–HO-mediated modulation of inflammation is further strengthened by the findings of Willis and colleagues who demonstrated enhanced levels of HO-1 in monocytes during the resolution phase of inflammation.18,63 Thus, HO activity probably forms a feedback loop by attenuating adhesion and migration and by promoting resolution of inflammation.
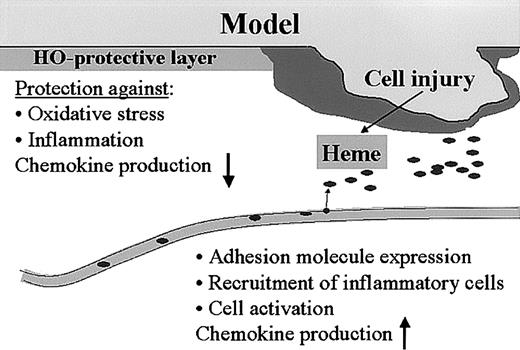
Thus, the heme-HO system seems tightly integrated in complex wound-healing processes such as hemostasis and inflammation (Figure 9). This novel view on wound-healing processes may provide new tools for therapeutic strategies.
Model for heme, HO, and wound healing. We hypothesize that the heme released after injury functions as a “danger signal” that can activate a whole range of inflammatory and immune regulatory cascades. Heme activates platelet aggregation and causes vasoconstriction, which are important parameters for “thrombus formation.” Furthermore, heme activates leukocytes, oxidizes LDL, increases adhesion molecule expression, and recruits leukocytes. The presence of HO in the skin protects against the heme-mediated pro-oxidative and proinflammatory microenvironment that is instantly created on injury, and might play a role in down-regulation of inflammatory cell recruitment and resolution of inflammation.
Model for heme, HO, and wound healing. We hypothesize that the heme released after injury functions as a “danger signal” that can activate a whole range of inflammatory and immune regulatory cascades. Heme activates platelet aggregation and causes vasoconstriction, which are important parameters for “thrombus formation.” Furthermore, heme activates leukocytes, oxidizes LDL, increases adhesion molecule expression, and recruits leukocytes. The presence of HO in the skin protects against the heme-mediated pro-oxidative and proinflammatory microenvironment that is instantly created on injury, and might play a role in down-regulation of inflammatory cell recruitment and resolution of inflammation.
Prepublished online as Blood First Edition Paper, March 20, 2003; DOI 10.1182/blood-2002-07-2248.
Supported in part by European Commission (EC) grant HO-1 QLK3-CT-2001-00422, the Vanderes Foundation, and Nederlandse organisatie voor Wetenschappelijk Onderzoek (NWO) grant “Gewrichtsaandoeningen en matrixpathologie 902-27.”
F.A.D.T.G.W. and H.E.v.B. contributed equally to this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ``advertisement'' in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal