Abstract
Neutrophils are mobilized to the vascular wall during vessel inflammation. Published data are conflicting on phagocytic nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase activation during the hypertensive state, and the capacity of angiotensin II (Ang II) to modulate the intracellular redox status has not been analyzed in neutrophils. We here describe that Ang II highly stimulates endogenous and extracellular O2- production in these cells, consistent with the translocation to the cell membrane of the cytosolic components of NADPH oxidase, p47phox, and p67phox. The Ang II–dependent O2- production was suppressed by specific inhibitors of AT1 receptors, of the p38MAPK and ERK1/2 pathways, and of flavin oxidases. Furthermore, Ang II induced a robust phosphorylation of p38MAPK, ERK1/2, and JNK1/2 (particularly JNK2), which was hindered by inhibitors of NADPH oxidase, tyrosine kinases, and ROS scavengers. Ang II increased cytosolic Ca2+ levels—released mainly from calcium stores—enhanced the synthesis de novo and activity of calcineurin, and stimulated the DNA-binding activity of the transcription factor NF-κB in cultured human neutrophils. Present data demonstrate for the first time a stimulatory role of Ang II in the activation of phagocytic cells, underscore the relevant role of ROS as mediators in this process, and uncover a variety of signaling pathways by which Ang II operates in human neutrophils.
Introduction
Angiotensin II (Ang II), the main peptide hormone of the renin-angiotensin system, induces leukocyte recruitment to the vessel wall, which constitutes a hallmark of the early stages of atherosclerosis and several hypertensive diseases.1 In addition, it plays a regulatory role on blood pressure and circulation volume and on the proliferation of vascular smooth muscle cells.2 Ang II acts through high-affinity cell surface receptors (AT1), which are linked to pathways classically associated with G-protein–coupled and tyrosine-kinase–mediated responses.3 Although most studies on Ang II have been carried out in smooth muscle and endothelial cells, experimental evidence has been obtained on its effects on circulating cells. AT1 receptors for Ang II have been found recently in circulating neutrophils4 and human peripheral monocytes,5 and Ang II–induced cell activation in the latter has been reported.6 In this context, the migration of leukocytes from blood to sites of inflammation and their adhesion to endothelial cells are primary events taking place during the acute inflammatory response and the pathogenesis of vascular diseases.7
Because chronic inflammation of vessel walls is a hallmark of atherosclerosis,8 and reactive oxygen species (ROS) such as superoxide anion (O2-) and H2O2 constitute the main intermediary molecules responsible for inflammation,9 a link between atherosclerosis and ROS production has been postulated.10 Nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase of phagocytes catalyzes the reduction of oxygen to O2-. In resting cells this enzyme is inactive, and its components are distributed between the cytosol and the membrane of secretory vesicles. When phagocytic cells are activated, the enzyme's cytosolic components associate to membrane-bound components and assemble into catalytically active NADPH oxidase.11 It has been reported that Ang II–induced hypertrophy of vascular smooth muscle cells is mediated by both O2- and H2O2.12 In addition, ROS has gained acceptance as a modulator of receptor-mediated signal transduction in a variety of cell types.9,13 Relevant ROS targets are proteins of the mitogen-activated protein kinase (MAPK) family, which are responsible for the transduction of signals from the cell membrane to the nucleus in response to classical growth factors and G-protein–coupled receptor agonists and under cellular stress conditions.14,15
Conflicting results have been reported about the activation of NADPH oxidase from neutrophils during the hypertensive state, and the activity of respiratory-burst oxidase has been found to become enhanced,16 unaffected,17 or decreased18 compared with activity in normotensive subjects. Most studies have paid attention to the role of ROS release by vascular oxidase activity and the regulation of superoxide-induced leukocyte-endothelial cell interactions.19,20 Present experimental approaches take into account that neutrophils possess a robust O2--forming NADPH oxidase, an enzyme that could constitute a potential target for Ang II. Preliminary data from the present work illustrated that neutrophils are highly responsive to Ang II in the context of O2- production, which prompted us to use these cells as a model to assess the physiological significance of Ang II–dependent O2- production. Given that exogenous oxidants are known to promote phosphorylation and, hence, activation of p38MAPK and extracellular signal-regulated kinases 1 and 2 (ERK1/2, also termed p42/44MAPK) in human neutrophils,20-23 we set about to analyze whether endogenous O2- production is linked to MAPK activation in these cells. The results obtained indicate that Ang II, at concentrations effective on vascular and endothelial cells, promotes the mobilization to the plasma membrane of cytosolic subunits of NADPH oxidase, together with the activation of p38MAPK, ERK1/2, and JNK1/2 in human neutrophils. The parallel cancellation of the activity of NADPH oxidase and of these MAPKs by specific pharmacologic inhibitors suggests the existence of crosstalk between Ang II–dependent O2- production and MAPK activation pathways. Furthermore, a functional significance of Ang II effects on ROS release and MAPK activation can be inferred from the increase in intracellular Ca2+, in calcineurin (CaN) protein and activity levels, and in NF-κB DNA-binding activity elicited by this hormone. Present data indicate a positive role for Ang II in the activation of human neutrophils, which could be related to the mobilization of these cells to the vascular wall occurring during inflammatory responses.19
Materials and methods
Chemicals and reagents
Angiotensin II, cytochrome c, bis-N-methylacridinium nitrate (lucigenin), phenol-red, diphenylene iodonium (DPI), 4-hydroxy-3-methoxyacetophenone (HMAP), 4-(2-aminoethyl)-benzene sulfonyl fluoride (AEBSF), 5-amino-2,3-dihydrophthalazine-1,4-dione (luminol), N-acetyl-L-cysteine (NAC), pyrrolidine dithiocarbamate (PDTC), aminotriazole (AMT), genistein, TEMPO, lipopolysaccharide (LPS) from Escherichia coli, phorbol 12-myristate-13-acetate (PMA), Fura-2 acetoxymethyl ester, monoclonal mouse antihuman β-actin, and horseradish peroxidase (HRP)–conjugated rabbit antigoat, goat antirabbit, and goat antimouse immunoglobulin G (IgG) antibodies were from Sigma Aldrich (Madrid, Spain). The double-stranded consensus oligonucleotide probe 5′-AGTTGAGGGGACTTTCCCAGGC-3′ (κB-binding site underlined) was from Boehringer Mannheim GmbH (Mannheim, Germany). Polyvinylidene fluoride (PVDF) membranes were from Bio-Rad (Hercules, CA). Recombinant human interferon-γ (IFN-γ) was from Endogen (Woburn, MA). Wortmannin, pertussis toxin (Ptx), herbimycin A, PD089059, and SB203580 were purchased from Calbiochem (San Diego, CA). The rabbit polyclonal antiphosphospecific p38MAPK (Thr180/Tyr182) and mouse monoclonal antiphosphospecific ERK1/2 (Thr202/Tyr204) antibodies used for the detection of the phosphorylated forms of these enzymes, and antibodies against their unphosphorylated counterparts were obtained from New England Biolabs (Beverly, MA). Rabbit antihuman IκB-α and NF-κB subunits p50 and p65 (sc-371, sc-114, and sc-109, respectively) and mouse antiphosphospecific JNK1/2 (Thr183/Tyr185) (sc-6254) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Goat antisera raised against p47phox and p67phox were kindly donated by Dr T. Leto (National Institutes of Health [NIH], Bethesda, MD).24 The rabbit antiserum against bovine calcineurin was kindly provided by Dr C. B. Klee (NIH).
Human neutrophil extraction and processing
Human peripheral neutrophils were isolated and further processed as previously described.25 In all experimental conditions, neutrophils were preincubated for 7 hours in RPMI without serum at 37°C and washed twice in HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)–buffered Krebs-Ringer solution (KR-HEPES) (composed of 118 mM NaCl, 4.75 mM KCl, 1.18 mM H2PO4, 1.18 mM Mg SO4, 1.25 mM CaCl2,10 mM glucose, and 25 mM HEPES, pH 7.4) at 37°C before treatment in vitro with Ang II or other additions.
O2-, H2O2, and total ROS production
The production of ROS was analyzed by 4 separate methods, each with a different specificity toward the location and type(s) of ROS produced. The (1) lucigenin-based luminescence method was specific for O2-, whereas (2) luminol-based luminescence correlated well with total ROS produced by the cells.26 Lucigenin (15 μM) or 15 μM luminol was added, and the assays were carried out as described previously,25 except that HRP (8 mU/mL) was included when luminol was used. Given that luminol and lucigenin can permeate freely through the cell membrane, their luminescences were indications of the sum of intracellular plus extracellular ROS. On the other hand, (3) the assay based on cytochrome c reduction27 was highly specific for O2- released to the extracellular medium because the cytochrome was unable to access the cell interior. Cytochrome c was added at 80 μM, and the absorbance at 550 nm was recorded. Finally, (4) the phenol-red method for the measurement of H2O2 production by human neutrophils was applied as described.28
Mobilization of p47phox and p67phox
The neutrophil plasma membrane fraction was subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and was electro-blotted onto PVDF membranes as previously described.29 Antibody probing was carried out overnight without the need for prior blocking30 with goat anti-p67phox or anti-p47phox IgG, and it was diluted 1:2000 in PBS supplemented with 0.02% Tween 20 and 1% bovine serum albumin (BSA). Antibody binding was detected by incubation with HRP-conjugated antigoat IgG (1:20 000 dilution) in PBS with 0.5% BSA followed by enhanced chemiluminescence, as previously indicated.31
p38MAPK, ERK1/2, and JNK1/2 phosphorylation
Neutrophils were preincubated for 7 hours in RPMI at 37°C to reduce the p38MAPK basal phosphorylation levels usually found in our preparations of human neutrophils. They were then subjected to the drug treatment indicated in each case, washed twice with ice-cold KR-HEPES, and immediately lysed by incubation for 30 minutes in a buffer composed of 20 mM Tris-HCl (pH 7.4), 50 mM β-mercaptoethanol, 2 mM EDTA (ethylenediaminetetraacetic acid), 25 mM NaF, 1% Nonidet P-40, 1% Triton X-100, 2 mM diisopropyl fluorophosphate, and the protease inhibitors. Protein extracts (50 μg) were resolved on 10% SDS-PAGE gels and electrotransferred to PVDF membranes, which were probed for 2 hours with antiphospho-p38MAPK, antiphospho-ERK1/2, or antiphospho-JNK1/2 antibodies (1:2000 dilution). Antibody binding was detected by using HRP-conjugated antirabbit or antimouse IgG antibodies (1:20 000 dilution) followed by enhanced chemiluminescence.31
Immunoprecipitation and p38MAPK activity assay
Human neutrophils were preincubated for 7 hours and were washed as indicated, before treatment in vitro with Ang II or other additions, and p38MAPK activity was assessed as described previously.14 Endogenous p38MAPK was immunoprecipitated from the supernatants using rabbit polyclonal antibodies against unphosphorylated p38 MAPK (1:50 dilution) for 12 to 18 hours at 4°C and were incubated with 30 μL protein A-Sepharose beads for 3 hours at 4°C with gentle rocking. The beads were washed 4 times with 500 μL lysis buffer and twice with 500 μL kinase buffer (20 mM HEPES, pH 7.6, 200 mM Cl2 Mg, 2 mM dithiothreitol [DTT], 25 mM β-glycerophosphate, 1 mM Na3VO4 and the protease inhibitors mentioned). Kinase activity was assayed for 30 minutes at 30°C in the presence of 6 μg myelin basic protein (MBP) as the substrate, 10 μM adenosine triphosphate (ATP), and 10 μCi (0.37 MBq) γ-32P]ATP in 25 μL kinase buffer. The proteins were resolved by SDS-PAGE and subjected to autoradiography.
Calcineurin protein and phosphatase activity levels
Calcineurin (CaN) phosphatase activity was measured as previously described.31 For immunoblotting analysis of CaN subunits A and B, neutrophil lysates were subjected to SDS-PAGE. This was followed by electroblotting onto PVDF membranes, which were then probed for 2 hours with rabbit antibovine CaN IgG (1:2000 dilution) and thereafter with HRP-conjugated antirabbit IgG.
Intracellular Ca2+ levels
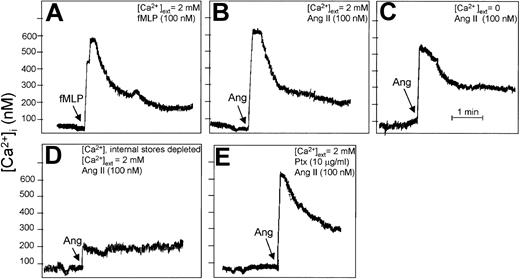
Cytosolic [Ca2+] was measured in cell populations using the fluorescent probe Fura-2.32 Concentrations of external CaCl2 and additions were as indicated in the Figure 7 legend. Intracellular Ca2+ stores were depleted by treatment with 1 μM thapsigargin and 0.1 mM EGTA (ethyleneglycotetraacetic acid) for 5 minutes, followed by cell washing. Batches (0.6 mL) containing 6 × 106 cells were incubated in the presence of 100 nM Ang II or 100 nM N-formyl-Met-Leu-Phe (fMLP) in a thermostatically maintained cuvette at 37°C.
Angiotensin II increases cytosolic Ca2+ levels in human neutrophils. Fura-2–loaded neutrophils (107 cells/mL) were incubated with 100 nM fMLP (A) or with 100 nM Ang II (B-E) under the indicated conditions for 4 minutes, and changes in intracellular Ca2+ levels were fluorometrically recorded. The arrow in each panel indicates the time of fMLP, Ang II, or Ptx addition. (A-B) Cell suspension was incubated in the presence of external 2 mM CaCl2, and either fMLP (A) or Ang II (B) was added. (C) Cells were treated with 0.1 mM EGTA for 5 minutes in the absence of external Ca2+ before the addition of Ang II. (D) Intracellular Ca2+ stores were first depleted with 1 μM thapsigargin and 0.1 mM EGTA for 5 minutes, followed by washing. Then the cells were treated simultaneously with Ang II and 2 mM CaCl2. (E) Cells were pretreated with Ptx before the addition of Ang II. Each graph is representative of a set of 3 experiments that yielded similar results.
Angiotensin II increases cytosolic Ca2+ levels in human neutrophils. Fura-2–loaded neutrophils (107 cells/mL) were incubated with 100 nM fMLP (A) or with 100 nM Ang II (B-E) under the indicated conditions for 4 minutes, and changes in intracellular Ca2+ levels were fluorometrically recorded. The arrow in each panel indicates the time of fMLP, Ang II, or Ptx addition. (A-B) Cell suspension was incubated in the presence of external 2 mM CaCl2, and either fMLP (A) or Ang II (B) was added. (C) Cells were treated with 0.1 mM EGTA for 5 minutes in the absence of external Ca2+ before the addition of Ang II. (D) Intracellular Ca2+ stores were first depleted with 1 μM thapsigargin and 0.1 mM EGTA for 5 minutes, followed by washing. Then the cells were treated simultaneously with Ang II and 2 mM CaCl2. (E) Cells were pretreated with Ptx before the addition of Ang II. Each graph is representative of a set of 3 experiments that yielded similar results.
NF-κB activation
Electrophoretic mobility shift assays (EMSAs) for NF-κB DNA binding were performed as described,31 using as a probe the γ-32P]dATP-radiolabeled (specific activity, 3000 Ci/mmol [11 100 × 1010 Bq/mmol]) double-stranded 22-bp NF-κB consensus oligonucleotide indicated in “Chemicals and reagents.” Reactions were performed in 20 μL binding buffer (10 mM HEPES, pH 7.9, 10% [vol/vol] glycerol, 0.1 mM EDTA, 8 mM MgCl2, 0.2 μg/mL poly[dI-dC], 1 mM DTT) containing 5 μg nuclear extract and were allowed to proceed for 20 minutes at room temperature. The protein-DNA complexes were separated on 6% polyacrylamide gels.
For immunoblotting analysis of IκB-α levels, the neutrophil cytoplasmic fraction was subjected to SDS-PAGE, and electroblotting. PVDF membranes were probed with rabbit antihuman IκB-α (1:2000 dilution) and thereafter with HRP-conjugated antirabbit IgG.
Results
Effect of Ang II on O2-/ROS production and p67phox/p47phox membrane translocation
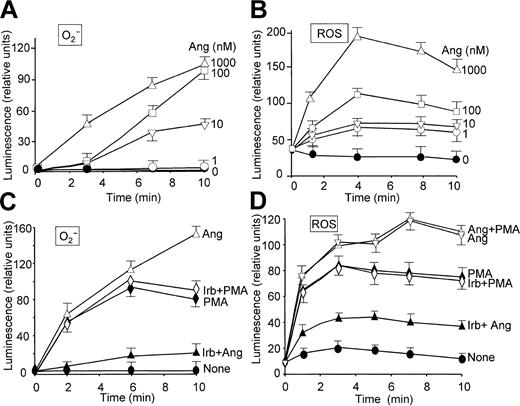
The effect of Ang II on O2- production by human neutrophils was examined by chemiluminescence measurements based on the use of lucigenin and luminol, which give an indication of the levels of O2- and total ROS, respectively.26 Figure 1 illustrates that Ang II induced O2- (Figure 1A, C) and general ROS (Figure 1B, D) production in a dose-dependent manner and that a maximal release of these species was attained after approximately 10 and 5 minutes of treatment, respectively, at 1 μM Ang II. These effects of Ang II were specific because irbesartan, an inhibitor of AT1 receptors,33 substantially hindered O2- and ROS release induced by Ang II (Figure 1C-D). Irbesartan alone did not have any effect on O2- or ROS release (data not shown), and neither affected the response induced by PMA (Figure 1C-D). Ang II–dependent extracellular O2- production was also examined on the basis of cytochrome c reduction. Table 1 shows that neutrophil stimulation with Ang II elicited the release of O2- to the medium, until a 15-fold increase was reached at the maximal dose of Ang II tested (1 μM) compared with control, unstimulated cells. Similarly, when neutrophils in a resting state or primed with LPS/IFN-γ were incubated with Ang II for 2 hours, this hormone was found to enhance H2O2 release, though a significant synergistic effect between Ang II and the LPS/IFN-γ mixture was not observed (Table 2). The values found with these stimuli agree closely with those previously reported.34 PMA was, however, inapplicable to the study of Ang II–dependent H2O2 production because it elicited by itself a robust production of H2O2 (data not shown). On the other hand, the priming role of LPS/IFN-γ on neutrophils is well documented.34-36
Angiotensin II enhances O2- and ROS production in a time- and dose-dependent manner in human neutrophils. Inhibitory effect of irbesartan. Neutrophils were suspended in KR-HEPES buffer at 106 cells/mL in the presence of 15 μM lucigenin (A,C) or 15 μM luminol (B,D). Reactions were started by the addition of angiotensin II (Ang) at the indicated concentrations. The effect of irbesartan (Irb, 10 μg/mL) on PMA (100 nM)– and Ang II (1 μM)–dependent O2- and ROS production was analyzed after incubating the cells with Irb for 5 minutes at 37°C (C,D). Chemiluminescence intensity was recorded in a Wallac Victor2 (Perkin Elmer) apparatus for 10 minutes. Data are expressed as the mean ± SEM of 3 experiments.
Angiotensin II enhances O2- and ROS production in a time- and dose-dependent manner in human neutrophils. Inhibitory effect of irbesartan. Neutrophils were suspended in KR-HEPES buffer at 106 cells/mL in the presence of 15 μM lucigenin (A,C) or 15 μM luminol (B,D). Reactions were started by the addition of angiotensin II (Ang) at the indicated concentrations. The effect of irbesartan (Irb, 10 μg/mL) on PMA (100 nM)– and Ang II (1 μM)–dependent O2- and ROS production was analyzed after incubating the cells with Irb for 5 minutes at 37°C (C,D). Chemiluminescence intensity was recorded in a Wallac Victor2 (Perkin Elmer) apparatus for 10 minutes. Data are expressed as the mean ± SEM of 3 experiments.
Angiotensin II enhances the extracellular release of O2- by human neutrophils
Additions to cells . | O2-, nmol/106 cells × min . |
|---|---|
| None | 0.056 |
| Ang (10) | 0.142 |
| Ang (100) | 0.417 |
| Ang (1000) | 0.781 |
| Ang (100) + PD098059 | 0.056 |
| Ang (1000) + PD098059 | 0.170 |
Additions to cells . | O2-, nmol/106 cells × min . |
|---|---|
| None | 0.056 |
| Ang (10) | 0.142 |
| Ang (100) | 0.417 |
| Ang (1000) | 0.781 |
| Ang (100) + PD098059 | 0.056 |
| Ang (1000) + PD098059 | 0.170 |
Neutrophils (1 × 106 cells/mL) were preincubated alone or with PD098059 (50 μM) for 30 minutes. Reactions were started by the addition of the indicated concentrations (nM) of Ang II (Ang) and were allowed to proceed for 10 minutes. OD550 from cytochrome c was recorded
Angiotensin II enhances the extracellular release of H2O2 by human neutrophils
. | H2O2, nmol/106 cells × min . | . | |
|---|---|---|---|
| Additions to cells . | Resting . | IFN-γ/LPS treated . | |
| None | 0.37 ± 0.02 | 0.53 ± 0.01 | |
| Ang (100) | 0.59 ± 0.01 | 0.82 ± 0.01 | |
| Ang (1000) | 1.00 ± 0.02 | 1.30 ± 0.02 | |
. | H2O2, nmol/106 cells × min . | . | |
|---|---|---|---|
| Additions to cells . | Resting . | IFN-γ/LPS treated . | |
| None | 0.37 ± 0.02 | 0.53 ± 0.01 | |
| Ang (100) | 0.59 ± 0.01 | 0.82 ± 0.01 | |
| Ang (1000) | 1.00 ± 0.02 | 1.30 ± 0.02 | |
Neutrophils (9 × 106 cells/mL) were preincubated alone (resting) or with IFN-γ/LPS (100 U/mL and 1 μg/mL, respectively) for 60 minutes and were further treated with Ang II for 2 hours at 37°C. The amount of H2O2 released was measured by the phenol-red method. Data are expressed as the means ± SEMs of 3 separate experiments
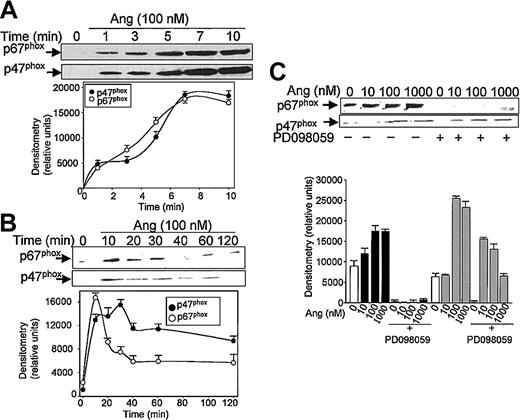
Subsequent experiments were addressed to analyze the effect of Ang II on the mobilization of the NADPH oxidase subunits p47phox and p67phox, from the cytosol to the plasma membrane. Brief kinetic experiments (Figure 2A) illustrate that an effective Ang II–dependent translocation of p47phox and p67phox was observed after 1 minute of treatment with the hormone; maximal membrane levels were reached at 7 minutes for each protein. Thereafter, a slow return to basal levels was observed, attained after 40 to 60 minutes of exposure to Ang II for p67phox and after 120 minutes for p47phox (Figure 2B). NADPH oxidase subunit mobilization to the plasma membrane was prominent at concentrations of Ang II as low as 10 nM (p67phox) or 100 nM (p47phox). These data clearly suggested that Ang II is capable of eliciting the activation of phagocytic NADPH oxidase.
Angiotensin II promotes p47phox and p67phox mobilization to the cell membrane in human neutrophils. Neutrophils were suspended in KR-HEPES buffer at 107 cells/mL and were incubated with 100 nM angiotensin II (Ang) for short (until 10 minutes) (A) or longer (until 120 minutes) durations (B), or the cells were previously incubated in the absence or presence of PD098059 (50 μM) for 30 minutes and Ang II was then added at the indicated concentrations for 15 minutes (C). Cells were lysed, and the plasma-membrane–enriched fraction was separated from the cytosolic fraction by ultracentrifugation at 100 000g. Membrane proteins (50 μg/lane) were subjected to SDS-PAGE, transferred to PVDF membranes, and probed with antisera against p47phox and p67phox. Each blot is representative of a set of 3 experiments that yielded similar results.
Angiotensin II promotes p47phox and p67phox mobilization to the cell membrane in human neutrophils. Neutrophils were suspended in KR-HEPES buffer at 107 cells/mL and were incubated with 100 nM angiotensin II (Ang) for short (until 10 minutes) (A) or longer (until 120 minutes) durations (B), or the cells were previously incubated in the absence or presence of PD098059 (50 μM) for 30 minutes and Ang II was then added at the indicated concentrations for 15 minutes (C). Cells were lysed, and the plasma-membrane–enriched fraction was separated from the cytosolic fraction by ultracentrifugation at 100 000g. Membrane proteins (50 μg/lane) were subjected to SDS-PAGE, transferred to PVDF membranes, and probed with antisera against p47phox and p67phox. Each blot is representative of a set of 3 experiments that yielded similar results.
Ang II activates p38MAPK and ERK1/2
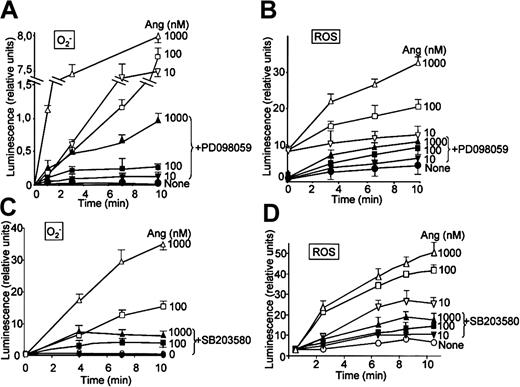
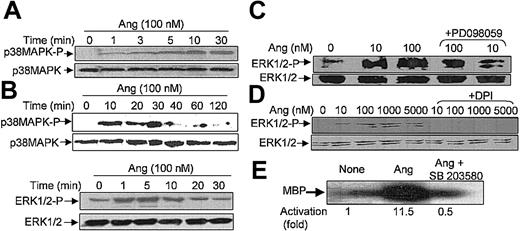
It has been recently reported that neutrophils use the p38MAPK cascade to link LPS, granulocyte-macrophage colony-stimulating factor, and fMLP-promoted activation to an array of functional responses.20,21,23,37-40 In this context, we investigated the relationship between Ang II–dependent ROS production and the activation of p38 and ERK1/2 proteins of the MAPK family, and we explored their potential participation in the transduction of the Ang II signal in human neutrophils. Toward this end, we made use of 2 specific compounds, PD098059, which inhibits MEK1/2, the upstream activator of ERK1/2,41 and SB203580, which directly inhibits p38MAPK.42 We found that PD098059 virtually canceled the extracellular O2- production triggered by Ang II (Table 1). This inhibitor also acted negatively on Ang II–dependent mobilization of the p47phox and p67phox NADPH oxidase subunits to the cell membrane, especially hindering p67phox mobilization (Figure 2C). Studies conducted to analyze whether PD098059 also inhibited intracellular O2- and ROS production elicited by Ang II in human neutrophils demonstrated that it did (Figure 3A-B) and that only at the highest Ang II concentrations tested (0.1-1 μM) was certain, albeit low, O2- production detected in the presence of the mitogen extracellular kinase (MEK) inhibitor. Similar results were obtained using the p38MAPK inhibitor SB203580 regarding O2- and total ROS release (Figure 3C-D). This molecule was also found to block the translocation of p47phox and p67phox to the plasma membrane (data not shown). These results thus pointed out the existence of cross-signaling (defined as the regulation of one signaling pathway by another43 ) between the Ang II–dependent activation of MAPKs and the products of NADPH oxidase. Next, we examined the effect of Ang II on p38MAPK activation in human neutrophils. As shown in Figure 4A, a certain phosphorylation level of p38MAPK was already detectable after 1 minute of treatment with 100 nM Ang II. It remained high for 30 minutes and then began to decrease. Other studies have demonstrated that an important pathway in determining the activation of neutrophils is that constituted by Raf-MEK-ERK1/2.40 We show in this context that Ang II was also able to promote a rapid phosphorylation and, hence, activation of ERK1/2. This remained high for 5 to 10 minutes and then began to decrease until it returned to basal levels at 30 minutes of treatment (Figure 4B). We have detected in most of our preparations a basal, nonspecific activation of ERK1/2 in untreated neutrophils. An inhibitory effect of PD098059 on Ang II–dependent ERK1/2 activation was also systematically observed (Figure 4C). Catalytic activation of p38MAPK by Ang II treatment was confirmed by direct measurement of its kinase activity in immune complex assays using MBP as the substrate (Figure 4E). As expected, Ang II–dependent p38MAPK activity was strongly suppressed on the treatment of cells with 5 μM SB203580 before their stimulation with Ang II.
PD098059 and SB203580 inhibit angiotensin II–dependent O2- and ROS production in human neutrophils. Neutrophils were suspended in KR-HEPES buffer at 106 cells/mL and were incubated with PD098059 (50 μM) or SB203580 (50 μM) for 30 minutes. Lucigenin (15 μM) (A,C) or luminol (15 μM) (B,D) was added, and the reactions were started by the addition of angiotensin II (Ang) at the indicated concentrations. Chemiluminescence intensity was recorded in BioOrbit 1252 luminometer for the indicated times. Data are expressed as the mean ± SEM of 3 experiments.
PD098059 and SB203580 inhibit angiotensin II–dependent O2- and ROS production in human neutrophils. Neutrophils were suspended in KR-HEPES buffer at 106 cells/mL and were incubated with PD098059 (50 μM) or SB203580 (50 μM) for 30 minutes. Lucigenin (15 μM) (A,C) or luminol (15 μM) (B,D) was added, and the reactions were started by the addition of angiotensin II (Ang) at the indicated concentrations. Chemiluminescence intensity was recorded in BioOrbit 1252 luminometer for the indicated times. Data are expressed as the mean ± SEM of 3 experiments.
Angiotensin II stimulates p38MAPK and ERK1/2 phosphorylation and p38 kinase activity in human neutrophils. Neutrophils (9 × 106 cells/mL) were preincubated at 37°C for 7 hours in KR-HEPES without additions. Next, they were treated with Ang II (100 nM) alone for the indicated times (A, B), or they were incubated with PD098059 (50 μM) for 30 minutes (C) or with DPI (10 μM) (D) for 30 minutes and then with Ang II for another 30 minutes. Cells were lysed, and protein extracts (50 μg/lane) were subjected to SDS-PAGE, transferred to PVDF, and probed with antiphospho-p38MAPK (A) or antiphospho-ERK1/2 (B-D), and the corresponding phosphorylated bands were detected by means of luminol-enhanced chemiluminescence. To verify even protein loading, the blots were subsequently stripped and reprobed with antibodies against unphosphorylated p38MAPK (A) or ERK1/2 (B-D). (E) Neutrophils were previously incubated with 5 μM SB203580 for 30 minutes and then with 100 nM Ang II. After cell lysis, p38MAPK activity was assessed by immune complex kinase assay, and intensities of the 28-kDa MBP bands in the autoradiogram were measured by densitometry scanning. Numbers represent the average activity increase from 3 independent experiments. Each blot is representative of a set of 3 experiments that yielded similar results.
Angiotensin II stimulates p38MAPK and ERK1/2 phosphorylation and p38 kinase activity in human neutrophils. Neutrophils (9 × 106 cells/mL) were preincubated at 37°C for 7 hours in KR-HEPES without additions. Next, they were treated with Ang II (100 nM) alone for the indicated times (A, B), or they were incubated with PD098059 (50 μM) for 30 minutes (C) or with DPI (10 μM) (D) for 30 minutes and then with Ang II for another 30 minutes. Cells were lysed, and protein extracts (50 μg/lane) were subjected to SDS-PAGE, transferred to PVDF, and probed with antiphospho-p38MAPK (A) or antiphospho-ERK1/2 (B-D), and the corresponding phosphorylated bands were detected by means of luminol-enhanced chemiluminescence. To verify even protein loading, the blots were subsequently stripped and reprobed with antibodies against unphosphorylated p38MAPK (A) or ERK1/2 (B-D). (E) Neutrophils were previously incubated with 5 μM SB203580 for 30 minutes and then with 100 nM Ang II. After cell lysis, p38MAPK activity was assessed by immune complex kinase assay, and intensities of the 28-kDa MBP bands in the autoradiogram were measured by densitometry scanning. Numbers represent the average activity increase from 3 independent experiments. Each blot is representative of a set of 3 experiments that yielded similar results.
Effect of NADPH oxidase inhibitors and ROS scavengers on Ang II–promoted p38MAPK and ERK1/2 activation
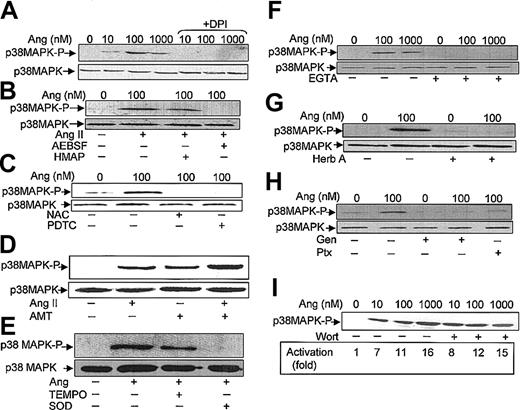
It has been described that in neutrophils stimulated with a variety of agents, the activation of p38MAPK is dependent on ROS production.20-23 However, the effect on p38MAPK of drugs that alter NADPH oxidase activity has not been analyzed. We used 3 types of such pharmacologic inhibitors aimed at testing the involvement of NADPH oxidase in Ang II–dependent p38MAPK activation. The first inhibitor tested, DPI, a reagent that blocks flavin adenine dinucleotide (FAD) binding to a flavin site of the oxidase,44 was found to effectively cancel Ang II–dependent ERK1/2 (Figure 4D) and p38MAPK (Figure 5A) phosphorylation. The second inhibitor tested, HMAP, which competes with NADPH for a binding site on oxidase but does not effectively compete for the 2-electron transfer to FAD,45 decreased Ang II–promoted p38MAPK phosphorylation only slightly, whereas the third inhibitor, AEBSF, which blocks the assembly of the NADPH oxidase subunits at the plasma membrane,46 completely abolished the Ang II–dependent activation of p38MAPK (Figure 5B). Additionally, we assessed the effects of 2 structurally distinct reducing reagents known to block the oxidative effects of O2-/ROS in many biologic systems, namely NAC and PDTC. Figure 5C illustrates that both reagents drastically reduced Ang II–dependent p38MAPK activation. Conversely, when oxidative stress was exacerbated in the presence of AMT—an inhibitor of the activity of the intracellular H2O2-removing enzyme, catalase— and of myeloperoxidase, p38MAPK phosphorylation was increased (Figure 5D). Moreover, AMT potentiated the effect of Ang II. Furthermore, the effects of TEMPO, a well-known scavenger of hydroxyl radicals and other oxygen free radicals, and of superoxide dismutase (SOD), an O2--removing enzyme, were also tested. As shown in Figure 5E, TEMPO partially reduced Ang-dependent p38MAPK phosphorylation, whereas SOD drastically blocked this process. Taken together, the present data unequivocally indicate that O2-/ROS is involved in the Ang II–promoting activation of this protein kinase in human neutrophils.
Angiotensin II–induced p38MAPK activation is dependent on ROS production, Ca2+ levels, tyrosine kinase, and Ptx-sensitive G proteins. Neutrophils (9 × 106 cells/mL) were incubated previously for 7 hours in KR-HEPES at 37°C without additions. Further, they were incubated in the absence or presence of DPI (10 μM) (A); HMAP (500 μM) or AESBF (500 μM) for 30 minutes (B); NAC (50 mM) or PDTC (100 μM) for 60 minutes (C); aminotriazole (D); TEMPO (100 μM) or SOD (500 U/mL) for 30 minutes (E); EGTA (2.5 mM) for 30 minutes (F); herbimycin A (Herb A) (3 μM) for 30 minutes (G); genistein (Gen) (100 μM) for 10 minutes or pertussis toxin (Ptx) (10 ng/mL) for 2 hours (H); and wortmannin (Wort) (100 nM) for 30 minutes (I). Cells were then treated with angiotensin II (Ang) at the indicated concentrations, or at 100 nM (D-E), for 30 minutes and subsequently lysed. Protein extracts (50 μg/lane) were subjected to SDS-PAGE, transferred to PVDF membrane, and probed with antiphospho-p38MAPK. To verify even protein loading, the blots were subsequently stripped and reprobed with anti-p38 antibody (A-C, E-H). Results shown in all panels are representative of 3 independent experiments.
Angiotensin II–induced p38MAPK activation is dependent on ROS production, Ca2+ levels, tyrosine kinase, and Ptx-sensitive G proteins. Neutrophils (9 × 106 cells/mL) were incubated previously for 7 hours in KR-HEPES at 37°C without additions. Further, they were incubated in the absence or presence of DPI (10 μM) (A); HMAP (500 μM) or AESBF (500 μM) for 30 minutes (B); NAC (50 mM) or PDTC (100 μM) for 60 minutes (C); aminotriazole (D); TEMPO (100 μM) or SOD (500 U/mL) for 30 minutes (E); EGTA (2.5 mM) for 30 minutes (F); herbimycin A (Herb A) (3 μM) for 30 minutes (G); genistein (Gen) (100 μM) for 10 minutes or pertussis toxin (Ptx) (10 ng/mL) for 2 hours (H); and wortmannin (Wort) (100 nM) for 30 minutes (I). Cells were then treated with angiotensin II (Ang) at the indicated concentrations, or at 100 nM (D-E), for 30 minutes and subsequently lysed. Protein extracts (50 μg/lane) were subjected to SDS-PAGE, transferred to PVDF membrane, and probed with antiphospho-p38MAPK. To verify even protein loading, the blots were subsequently stripped and reprobed with anti-p38 antibody (A-C, E-H). Results shown in all panels are representative of 3 independent experiments.
Effect of Ca2+ chelators and inhibitors of protein tyrosine kinases, G proteins, and phosphatidylinositol 3-kinase (PI-3K) on Ang II–promoted p38MAPK activation
It has been reported that Ang II–dependent MAPK activation becomes partially hampered in the absence of Ca2+ in adrenal glomerulosa cells, suggesting a permissive role for calcium in this response.47 Consistent with this finding, we observed, using EGTA as a Ca2+ chelator, that the absence of this cation completely prevented Ang II–induced p38MAPK activation in human neutrophils (Figure 5F). It may be assumed, therefore, that transduction of the Ang II signal is a Ca2+-dependent process in phagocytic cells.
Subsequent experiments were conducted to examine whether the activity of cellular tyrosine kinase(s) was required for Ang II–induced p38MAPK activation. In this context, herbimycin A, a protein tyrosine kinase inhibitor, completely blocked the stimulatory effect of Ang II on p38MAPK phosphorylation (Figure 5G). Similarly, genistein, another tyrosine kinase inhibitor, inhibited Ang II–promoted p38MAPK activation, as shown in Figure 5H, further underscoring the dependence on protein tyrosine phosphorylation of this process. This figure also illustrates that Ptx, a potent inhibitor of certain guanine nucleotide-binding proteins (G proteins), blocked the activation of p38MAPK by Ang II. These results suggested that Ptx-sensitive G proteins are involved in Ang II–receptor–mediated signal transduction in human neutrophils. Finally, the effect of wortmannin, a potent inhibitor of PI-3K, was also tested. It was found that a 100 nM concentration of this compound failed to inhibit Ang II–dependent p38MAPK activation (Figure 5I). In separate experiments it was found that 100 nM wortmannin inhibited by approximately 75% the total ROS production induced by Ang II (data not shown). In summary, the results obtained with this set of inhibitors strongly indicate that Ang II elicits the activation of p38MAPK through a Ca2+- and a tyrosine kinase–dependent pathway.
Ang II activates JNK1/2
In mammals, a third major group of MAPKs comprises c-Jun NH2–terminal kinases (JNK), which exhibit the dual phosphorylation site Thr-Pro-Tyr and the ability to bind to the NH2-terminal activation domain of c-Jun.48 These kinases become activated under conditions of cellular and environmental stress elicited by ultraviolet irradiation, heat shock, protein synthesis inhibitors, hyperosmolarity, and inflammatory cytokines. JNK has been found in human neutrophils, and it has been shown that inflammatory cytokines such as tumor necrosis factor (TNF)–α induce the activation of the JNK pathway in these cells.49
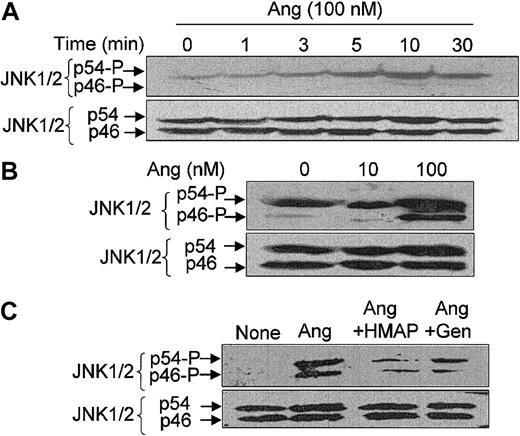
We also analyzed whether JNK1/2 (also termed p46/p54JNK) activity is modulated by Ang II in neutrophils and found that it is. Figure 6A illustrates that phosphorylation levels of JNK2 (p54) were significantly increased at 3 minutes after the addition of Ang II, peaked at 10 minutes, and decreased slightly at 30 minutes. In this particular cellular preparation, only a faint signal of JNK1 (p46) phosphorylation was found at 10 to 30 minutes of Ang II treatment. However, in other neutrophil extracts, a higher JNK2 (p54) phosphorylation signal was obtained under basal conditions, which further increased with the addition of Ang II (Figure 6B). In these cases, the presence of Ang II could be detected to strongly enhance the phosphorylation levels of JNK1 and JNK2. As described for p38MAPK, the NADPH oxidase inhibitor HMAP and the tyrosine kinase inhibitor genistein also attenuated the Ang II–dependent activation of JNK1/2 (Figure 6C).
Angiotensin II–induced JNK1/2 activation in human neutrophils and its dependence on ROS and tyrosine kinase activity. Neutrophils (9 × 106 cells/mL) were suspended in KR-HEPES buffer and preincubated for 7 hours at 37°C without additions. Further, they were incubated in the presence of Ang II (100 nM) for the indicated times (A) or of the indicated Ang II doses for 15 minutes (B), or in the absence or presence of HMAP (500 μM) for 30 minutes or genistein (Gen) (100 μM) for 10 minutes and then with Ang II (100 nM) for 15 minutes (C). After cell lysis, protein extracts (50 μg/lane) were subjected to SDS-PAGE, transferred to PVDF membranes, and probed with antiphospho-JNK1/2. Arrows indicate phosphorylated JNK1 (p46) and JNK2 (p54). To verify even protein loading, the blots were subsequently stripped and reprobed with anti-JNK1/2 antibody. Data are representative of 3 independent experiments that yielded similar results.
Angiotensin II–induced JNK1/2 activation in human neutrophils and its dependence on ROS and tyrosine kinase activity. Neutrophils (9 × 106 cells/mL) were suspended in KR-HEPES buffer and preincubated for 7 hours at 37°C without additions. Further, they were incubated in the presence of Ang II (100 nM) for the indicated times (A) or of the indicated Ang II doses for 15 minutes (B), or in the absence or presence of HMAP (500 μM) for 30 minutes or genistein (Gen) (100 μM) for 10 minutes and then with Ang II (100 nM) for 15 minutes (C). After cell lysis, protein extracts (50 μg/lane) were subjected to SDS-PAGE, transferred to PVDF membranes, and probed with antiphospho-JNK1/2. Arrows indicate phosphorylated JNK1 (p46) and JNK2 (p54). To verify even protein loading, the blots were subsequently stripped and reprobed with anti-JNK1/2 antibody. Data are representative of 3 independent experiments that yielded similar results.
Ang II increases intracellular Ca2+ levels and stimulates calcineurin synthesis
It has been reported that Ang II promotes an increase of intracellular Ca2+ in vascular smooth muscle cells.50 Because human neutrophils constitute target cells for Ang II, as shown in the present work, we analyzed whether intracellular Ca2+ underwent mobilization in response to Ang II. As shown, Ang II promoted an immediate cytosolic Ca2+ accumulation of a magnitude similar to that obtained on cell treatment with fMLP (Figure 7A-B; compare panels). Calcium mobilization induced by Ang II still occurred when extracellular Ca2+ was chelated with 0.1 mM EGTA (Figure 7C), though the intensity of the response was slightly lower (approximately 75%-80%) than that elicited by Ang II in the absence of this cation in the medium, indicating that most intracellular Ca2+ was mobilized from intracellular stores. This fact was confirmed when intracellularly stored calcium was depleted by preincubation with thapsigargin (a specific inhibitor of Ca2+-dependent ATPase) and EGTA. Under these conditions, when cells were stimulated with Ang II in the simultaneous presence of 2 mM external Ca2+, the hormone evoked only a slight increase in intracellular Ca2+ (Figure 7D). Therefore, present data indicate that the Ang II–dependent rise of intracellular Ca2+ resulted mainly from the mobilization of Ca2+ from intracellular stores and that only a smaller fraction resulted from the influx of extracellular Ca2+ through the plasma membrane. The major Ptx substrate in neutrophils is the G-protein α subunit, αi2. To determine whether activation of Gαi2 was involved in Ang II–induced Ca2+ mobilization, neutrophils were exposed to Ptx before stimulation. As seen in Figure 7E, pretreatment with Ptx basically altered the intracellular Ca2+ response, suggesting the involvement in Ang II–induced Ca2+ mobilization of a mechanism insensitive to Ptx and independent of Gαi2 protein. It must be noted, however, that Ang II–induced p38MAPK phosphorylation was strongly sensitive to Ptx (Figure 5H), indicating complex cross-signaling between Ang II–dependent Ca2+ mobilization and p38MAPK phosphorylation.
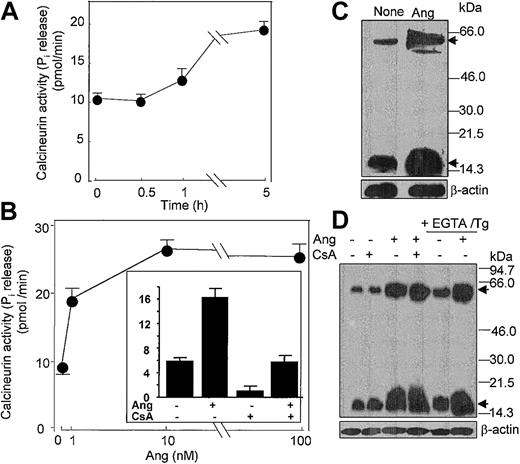
In addition, it has been shown that Ang II activates the Ca2+/calmodulin-dependent protein phosphatase CaN in cardiocytes.51 Given the pivotal role that CaN plays in signal transduction processes in neutrophils and cardiocytes,30,51 among many other cell types, we hypothesized that Ang II could act to modulate CaN phosphatase activity. To assess this possibility, we assayed CaN activity in neutrophils incubated with different doses of Ang II. It was found that treatment with 10 nM Ang II led to a slow increase in CaN activity, which became apparent only after 5 hours of incubation with the hormone (Figure 8A), and that a maximal 3.5-fold (n = 4) stimulation of CaN activity with respect to control values was obtained at 10 nM Ang II (Figure 8B). Pretreatment of neutrophils with the specific CaN inhibitor cyclosporin A (CsA) resulted in a complete hindrance of Ang II–induced CaN activation (Figure 8B, inset), which reflected the implication of CaN in the Ang II–mediated activation of neutrophils. Through the use of immunologic methods, we observed that Ang II induced de novo synthesis of CaN protein because the 19-kDa and 59-kDa CaN subunit levels clearly increased after the 5-hour–long addition of Ang II to neutrophils (Figure 8C). These results provided evidence that the Ang II signal is transduced, at least partially, through Ca2+-dependent signaling pathway(s). Thus, it seemed possible that Ang II–promoted induction of CaN synthesis could result from the activation of CaN gene expression in response to increased intracellular calcium levels. Figure 8D illustrates that when neutrophils were pretreated with thapsigargin and EGTA to deplete cytosolic Ca2+ and to chelate all external calcium, the further addition of Ang II enhanced the de novo synthesis of CaN protein. In addition, the pretreatment of cells with CsA did not alter the response elicited by Ang II. Recently, it has been shown that CaN protein content is increased in the heart in response to pressure-overload hypertrophy52 and that in vivo CsA treatment for 14 days cancels this effect. The discrepancy between these and our results about the CsA effect probably reflects the duration of cell treatment with the immunosuppressive reagent; in our case it was only 1 hour in vitro. Nonetheless, present data show for the first time that the expression of CaN may be modulated at the translational level by Ang II in human neutrophils.
Angiotensin II increases the activity and synthesis of calcineurin in human neutrophils. (A) Neutrophils (6 × 106 cells/mL) were incubated with 10 nM Ang II for the indicated times, (B) with the indicated Ang II concentrations for 5 hours, or (inset) previously with 1 μg/mL CsA for 60 minutes and then with 10 nM Ang II for 5 hours. After cell lysis, calcineurin activity was assayed. Phosphatase activity is expressed as picomoles of phosphate released per minute per milligram protein. Mean ± SE values from 4 separate experiments performed in triplicate are presented. (C-D) Neutrophils (107 cells/mL) were previously incubated in the absence or presence of CsA for 1 hour or with 0.1 mM EGTA and 1 μM thapsigargin for 20 minutes, as indicated, and then were treated with 10 nM Ang II for another 5 hours. Proteins from lysed cells were resolved by SDS-PAGE, followed by immunoblotting with anticalcineurin. Both A (59-kDa) and B (19-kDa) subunits of calcineurin were detected (arrows). Thereafter, antibody stripping and reprobing of the membrane with antihuman β-actin was carried out.
Angiotensin II increases the activity and synthesis of calcineurin in human neutrophils. (A) Neutrophils (6 × 106 cells/mL) were incubated with 10 nM Ang II for the indicated times, (B) with the indicated Ang II concentrations for 5 hours, or (inset) previously with 1 μg/mL CsA for 60 minutes and then with 10 nM Ang II for 5 hours. After cell lysis, calcineurin activity was assayed. Phosphatase activity is expressed as picomoles of phosphate released per minute per milligram protein. Mean ± SE values from 4 separate experiments performed in triplicate are presented. (C-D) Neutrophils (107 cells/mL) were previously incubated in the absence or presence of CsA for 1 hour or with 0.1 mM EGTA and 1 μM thapsigargin for 20 minutes, as indicated, and then were treated with 10 nM Ang II for another 5 hours. Proteins from lysed cells were resolved by SDS-PAGE, followed by immunoblotting with anticalcineurin. Both A (59-kDa) and B (19-kDa) subunits of calcineurin were detected (arrows). Thereafter, antibody stripping and reprobing of the membrane with antihuman β-actin was carried out.
Ang II promotes NF-κB activation
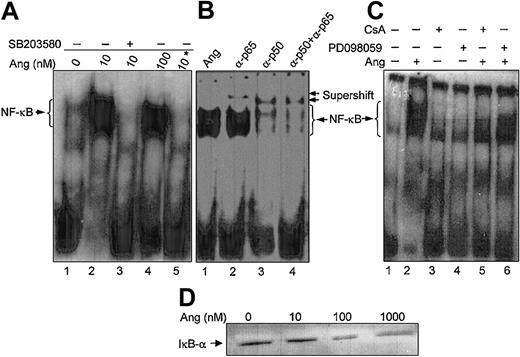
We have previously demonstrated that CaN activity from human neutrophils is modulated by the intracellular redox status, which results in the simultaneous alteration of DNA-binding activity of the transcription factor NF-κB.31 Therefore, subsequent experiments were addressed to analyze whether the described effects of Ang II in neutrophils also extended to NF-κB activity. DNA-bound NF-κB was selectively detectable as a retarded band in EMSA experiments using nuclear extracts from human neutrophils stimulated with Ang II (Figure 9A). On the basis of the supershifts obtained in the presence of anti-p50 and anti-p65 antibodies in the binding assays (Figure 9B), it could be concluded that this retarded band contained both types of NF-κB subunits. Ang II–triggered NF-κB nuclear activity was completely suppressed on cell treatment with SB203580 (Figure 9A) or PD098059 (Figure 9C), indicating that nuclear localization of this transcription factor was dependent on p38MAPK and ERK1/2 activation in human neutrophils. Additionally, the dependence of this process on CaN activity was evidenced by the abolition of NF-κB DNA-binding activity in cells treated with CsA (Figure 9C). The specificity of this elicited Ang II response was further underscored by the increased degradation of the NF-κB inhibitor, IκB-α, occurring in the cytosol on cell treatment with increasing doses of Ang II (Figure 9D). Present data, therefore, provide strong evidence of the existence of an Ang II–dependent NF-κB activity regulated by MAPKs in human neutrophils, in agreement with previous reports on cardiac fibroblasts.53
Angiotensin II enhances NF-κB DNA-binding activity and IκB degradation in human neutrophils. (A) Neutrophils (9 × 106 cells/mL) were previously incubated in the absence (lanes 1, 2, 4) or presence (lane 3) of SB203580 (50 μM) for 30 minutes and then were treated with the indicated concentrations of angiotensin II (Ang) for another 30 minutes. Nuclear extracts were obtained, and EMSA was carried out as indicated in “Materials and methods.” Where indicated by an asterisk (lane 5), the binding reaction was performed in the presence of a 100-fold molar excess of unlabeled oligonucleotide under the same conditions as in lane 4. (B) Nuclear extracts from 100 nM Ang II–treated cells were preincubated for 30 minutes with, or without (lane 1), 1 μL antihuman p50 or p65 antibodies, as indicated, before the addition of the radiolabeled probe (lanes 2-4). (C) Cells were previously incubated in the absence or presence of PD098059 (50 μM) for 30 minutes (lanes 4, 6) or of CsA (1 μg/mL) for 60 minutes (lanes 3, 5) and then were incubated with Ang II (100 nM) for 30 minutes where indicated (lanes 2, 5, 6). Untreated controls are also shown (lane 1). Neutrophils were lysed, and nuclear extracts were used for EMSA analysis. (D) The cytosolic fractions (45 μg/lane) from cells incubated for 30 minutes with Ang II at the indicated concentrations were subjected to SDS-PAGE and then to immunoblotting analysis using an antihuman IκB-α antiserum. Data are representative of similar results of 3 independent experiments.
Angiotensin II enhances NF-κB DNA-binding activity and IκB degradation in human neutrophils. (A) Neutrophils (9 × 106 cells/mL) were previously incubated in the absence (lanes 1, 2, 4) or presence (lane 3) of SB203580 (50 μM) for 30 minutes and then were treated with the indicated concentrations of angiotensin II (Ang) for another 30 minutes. Nuclear extracts were obtained, and EMSA was carried out as indicated in “Materials and methods.” Where indicated by an asterisk (lane 5), the binding reaction was performed in the presence of a 100-fold molar excess of unlabeled oligonucleotide under the same conditions as in lane 4. (B) Nuclear extracts from 100 nM Ang II–treated cells were preincubated for 30 minutes with, or without (lane 1), 1 μL antihuman p50 or p65 antibodies, as indicated, before the addition of the radiolabeled probe (lanes 2-4). (C) Cells were previously incubated in the absence or presence of PD098059 (50 μM) for 30 minutes (lanes 4, 6) or of CsA (1 μg/mL) for 60 minutes (lanes 3, 5) and then were incubated with Ang II (100 nM) for 30 minutes where indicated (lanes 2, 5, 6). Untreated controls are also shown (lane 1). Neutrophils were lysed, and nuclear extracts were used for EMSA analysis. (D) The cytosolic fractions (45 μg/lane) from cells incubated for 30 minutes with Ang II at the indicated concentrations were subjected to SDS-PAGE and then to immunoblotting analysis using an antihuman IκB-α antiserum. Data are representative of similar results of 3 independent experiments.
Discussion
This study demonstrates for the first time that Ang II elicits in human neutrophils a series of events involving (1) an increase of intracellular and extracellular O2-/ROS production associated with the translocation of the cytosolic subunit of NADPH oxidase to the cell membrane; (2) a rapid phosphorylation and hence activation of p38MAPK, ERK1/2, and JNK1/2 kinases; (3) an enhanced mobilization of Ca2+ from intracellular stores; (4) an increase of CaN protein content and activity; and (5) the activation of DNA-binding capability of NF-κB. Our data also demonstrate that ROS production and MAPK activation are mutually regulated processes given that specific pharmacologic inhibitors acting on either of the 2 pathways reciprocally abrogated activation of the other. These Ang II–promoted effects will be discussed separately.
The phenomenon of activation of phagocytic NADPH oxidase by Ang II raises further questions about the contribution of neutrophils to inflammatory processes associated with diseases of the vascular system and the heart. The results here reported are consistent with the previous finding of abnormally high levels of NADPH oxidase in the course of activation of neutrophils from patients with hypertension,16 though other authors have found no alteration in the activation pattern of this enzyme in this condition.17,18 It is noteworthy that Ang II triggers more potent and sustained O2- and ROS production in vitro in human neutrophils than that elicited by PMA, a classical NADPH oxidase stimulator, which evinces an especially high sensitivity level of neutrophils toward Ang II. The activation of phagocytic membrane NADPH oxidase promoted by this hormone is demonstrated by the observation that Ang II–dependent O2- production in neutrophils occurs in parallel to a dose-dependent translocation of p47phox and p67phox subunits of the enzyme to the cell membrane. Specificity of the Ang II response was demonstrated by the complete inhibition of O2- production provoked by irbesartan, an inhibitor of AT1-type Ang II receptors. In addition, the present data demonstrate that Ang II could constitute a pivotal factor involved in the activation of neutrophils in the hypertensive state, in agreement with previous reports.16,19,54
Another goal of this study focused on the presumptive role of ROS in the activation of kinases of the MAPK family in human neutrophils. Several lines of evidence have indicated the participation of MAPK activation in the transduction of Ang II–dependent signals in different cell types, such as vascular smooth muscle cells,15 cardiac cells,55 and adrenal glomerulosa cells.47 In neutrophils, however, no data on the Ang II effect on MAPK activation were hitherto available, though MAPK activation promoted by oxidants,21,22 PMA,56 LPS,39 or fMLP38,40 has been previously documented. Recent results from our laboratory support a role for MAPK activation as an intermediate step between the intracellular events triggered by Ang II and the release of inflammatory cytokines by human neutrophils (R.E.B. and F.S., unpublished observations, September 2001). Among the 3 main classes of MAPK described in other cell systems (p38MAPK, ERK1/2, and JNK1/2), we found the p38MAPK and JNK1/2 proteins gave the highest phosphorylation signal in immunoblotting assays, and an increase in its phosphorylation status in the presence of Ang II was consistently obtained. In contrast, the phosphorylation of ERK1/2 was difficult to detect in some of our neutrophil preparations. Given that the experimental protocol included a routine 7-hour preincubation period to cancel the basal p38MAPK phosphorylation levels usually found in untreated neutrophils, it can be hypothesized that such protein might undergo partial degradation during preincubation. In addition, the phosphorylation of p38MAPK induced by Ang II in human neutrophils was shown to bear characteristics similar to those found on their activation with TNF-α, fMLP, or serum-opsonized zymosan, such as a dependence on cytosolic Ca2+, protein kinase C, and protein tyrosine kinases.57,58
Data presented here indicate that protein tyrosine kinase activity is indispensable for Ang II–induced p38MAPK activation, and they are in agreement with previous reports showing this same dependence in vascular smooth muscle cells and human neutrophils stimulated with Ang II and TNF-α, respectively.58,59 Additionally, our finding that the activation of p38MAPK is independent of PI-3K activity is in line with previous observations on fMLP-stimulated neutrophils60 or on Ang II–stimulated vascular smooth muscle cells.61 Another study reveals that whereas the oxidative burst was inhibited by low concentrations of wortmannin (ID50, 5 nM), MAPK became only slightly attenuated at high doses (ID50, 150 nM) of this inhibitor,62 raising doubts about PI-3K involvement in the transmission of signals to MAPK. In addition, recent data63 reveal the presence of 2 separate pools of NADPH oxidase in neutrophils, located at the plasma membrane and in granules, that differ regarding the involvement of PI-3K in the modulation of their activities and the presence in neutrophils of the p100γ PI-3K isoform, which becomes inhibited by much higher concentrations of wortmannin than does the classical p110α isoform. All this adds new levels of complexity to the issue of cross-signaling between ROS and MAPK activation in neutrophils. Other reports, however, claim the partial involvement of PI-3K in the switch-on of the p38MAPK pathway in neutrophils.62,64
To study the relationship between ROS and MAPK activation, studies performed in neutrophils have addressed the effect of exogenous oxidants.21,22 We have taken into account the fact that human neutrophils are highly sensitive to Ang II and are active in the production of endogenous and extracellular O2-/ROS. Recently, it has been demonstrated that p38MAPK15 and ERK1/258 in vascular smooth muscle cells are critical components of the oxidative stress-sensitive pathway(s) activated by Ang II. Our data are in agreement with these observations and illustrate that intracellular pro-oxidative conditions promoted by Ang II are coupled to the activation of MAPKs in a reciprocal manner, though the exact hierarchical order of activation of NADPH oxidase and MAPKs must still be clarified. Our data are consistent with findings in studies on human neutrophils that provide evidence that the inhibition of p38MAPK results in decreased O2- production.20 However, other reports have shown functional uncoupling between MAPK activation and the oxidative burst in human neutrophils and in the promyelocytic HL-60 cell line in response to cyclin adenosine monophosphate (cAMP).56 The complex response elicited by this cyclic nucleotide suggests that the presumptive cross-talk between O2- release and MAPK activation mechanisms is probably dependent on the participation of other putative signaling pathways.
Regarding JNK1/2 activation, an increasing body of evidence points to its Ang II–promoted phosphorylation in a wide variety of cell lines. In this context, Ang II has been shown to activate JNK1/2 in vascular smooth muscle cells,65 in neonatal rat myocytes,55 and in cardiac fibroblasts.66 Additionally, some authors have observed a positive relationship between Ang II–dependent JNK1/2 activation and increased intracellular ROS levels.53 Although the presence of JNK1/2 has been shown in neutrophils,49 the present data illustrate for the first time the occurrence of activation of JNK1/2 by Ang II in neutrophils and its dependence on intracellular oxidative stress because the simultaneous presence of the NADPH oxidase inhibitor, HMAP, partially canceled the Ang II–promoted JNK1/2 activation.
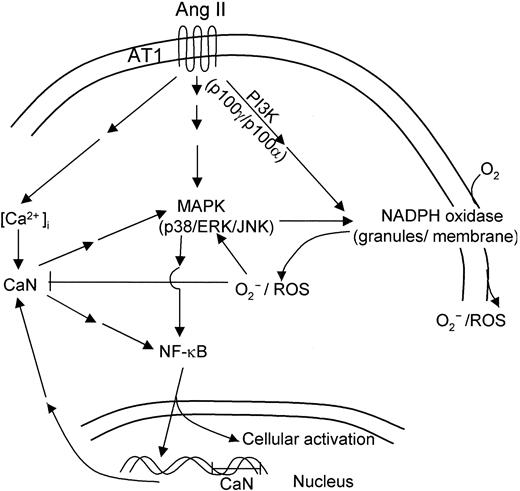
These results emphasize the potential role that neutrophils may play as sensitive cell targets for Ang II in vivo. This notion is further supported by our observations that Ang II enhances CaN expression and NF-κB DNA-binding activity in human neutrophils, which constitute 2 important intracellular events. Our results are consistent with recently published data showing that Ang II induces CaN activation, and they suggest the existence of a pathway linking CaN and MAPK in cardiac myocytes.51 It has also been shown that Ang II modulates cellular immune response through a CaN-dependent pathway.67 Interestingly, CaN synergizes with protein kinase C (PKC) to activate Rac in T cells68 and to engage in cross-talk with PKC and MAPK activation in transducing the Ang II stimulus in cardiomyocytes.69 Both proteins (Rac and PKC) are constituents of the machinery responsible for the activation of NADPH oxidase,70,71 and they may participate in Ang II–dependent signal transduction pathways. In this context, experimental evidence for an involvement of CaN in the enhancement of agonist-stimulated cAMP formation by Ang II in adrenal glomerulosa cells has been reported.72 Furthermore, the observed Ang II–promoted stimulation of NF-κB DNA-binding activity described here is in agreement with the recent observation of Ang II–dependent activation of NF-κB in human monocytes.73 Our data are consistent with previous reports describing the induction of TNF-α secretion by Ang II in monocytes, an effect also dependent on NF-κB activation.74 The scheme presented in Figure 10 summarizes the putative Ang II–induced signaling pathways in human neutrophils, as derived from the results reported in the present work.
Hypothetical scheme of intracellular signaling pathways involved in human neutrophil activation in response to angiotensin II. Binding of Ang II to AT1 receptors in human neutrophils would initiate a complex cascade of signaling pathways leading to the activation of NADPH oxidase, with the concomitant production of ROS and of kinases p38, ERK1/2, and JNK1/2 of the MAPK family. The increase in cytosolic Ca2+ resulting from AT1 receptor activation would in turn stimulate CaN activity, promoting nuclear translocation of NF-κB. This transcription factor would activate the expression of a number of genes involved in neutrophil cell activation, including CaN subunits encoding genes.
Hypothetical scheme of intracellular signaling pathways involved in human neutrophil activation in response to angiotensin II. Binding of Ang II to AT1 receptors in human neutrophils would initiate a complex cascade of signaling pathways leading to the activation of NADPH oxidase, with the concomitant production of ROS and of kinases p38, ERK1/2, and JNK1/2 of the MAPK family. The increase in cytosolic Ca2+ resulting from AT1 receptor activation would in turn stimulate CaN activity, promoting nuclear translocation of NF-κB. This transcription factor would activate the expression of a number of genes involved in neutrophil cell activation, including CaN subunits encoding genes.
Regarding the possible physiologic relevance of the present data, inflammation may be considered a crucial process in the pathogenesis of diseases such as atherosclerosis and hypertension. In this context, the activating effects of Ang II on polymorphonuclear cells may contribute to inflammation at the vessel wall level, a notable feature of such diseases, by favoring their cooperation with endothelial and vascular cells during the initiation of the inflammatory process. Neutrophils provide mobile pathways by which cell-bound or released enzymes can activate and amplify the renin-angiotensin pathway.75 Experimental evidence indicates the inhibition of angiotensin-converting enzyme, resulting in impeded adherence of polymorphonuclear cells to the endothelium and in diminished intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) expression.76 This work presents a new picture of neutrophils as circulating cells that are highly sensitive to Ang II, and it provides novel evidence to support the view that essential hypertension is a generalized type of membranopathy in which vascular contractile cells are not the only cell type involved.77
Prepublished online as Blood First Edition Paper, March 27, 2003; DOI 10.1182/blood-2002-09-2785.
Supported by Ministerio de Ciencia y Tecnología grants SAF/2000-117 (F.S.) and SAF/2000-161 (F.J.B.) and by grants from the Fundación SEIAC, Bial-Arístegui, and Hycor Biomedical Inc (J.M.). A.V. was supported by a predoctoral fellowship from the Ministerio de Ciencia y Tecnología.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs T. Leto and C. B. Klee for kindly providing the anti-p47phox and anti-p67phox and the anti-CaN antisera, respectively.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal