Abstract
In the European Organization for Research and Treatment of Cancer Leukemia Group and Gruppo Italiano Malattie Ematologiche dell' Adulto (EORTC-LG/GIMEMA) acute myeloid leukemia (AML)–10 trial, patients in first complete remission (CR1) received a single intensive consolidation (IC) course. Subsequently, those patients younger than 46 years with an HLA-identical sibling donor were assigned to undergo allogeneic (allo) stem cell transplantation (SCT), and patients without such a donor were planned for autologous (auto) SCT. Between November 1993 and December 1999, of 1198 patients aged younger than 46 years, 822 achieved CR. The study group constituted 734 patients who received IC: 293 had a sibling donor and 441 did not. Allo-SCT and auto-SCT were performed in 68.9% and 55.8%, respectively. Cytogenetic determination was successfully performed in 446 patients. Risk groups were good (t(8;21), inv16), intermediate (NN or –Y only), and bad/very bad (all others). Median follow-up was 4 years; 289 patients relapsed, 66 died in CR1, and 293 died. Intention-to-treat analysis revealed that the 4-year disease-free survival (DFS) rate of patients with a donor versus those without a donor was 52.2% versus 42.2%, P = .044; hazard ratio = 0.80, 95% confidence interval (0.64, 0.995), the relapse incidence was 30.4% versus 52.5%, death in CR1 was 17.4% versus 5.3%, and the survival rate was 58.3% versus 50.8% (P = .18). The DFS rates in patients with and without a sibling donor were similar in patients with good/intermediate risk but were 43.4% and 18.4%, respectively, in patients with bad/very bad risk cytogenetics. In younger patients (15-35 years), the difference was more pronounced. The strategy to perform early allo-SCT led to better overall results than auto-SCT, especially for younger patients or those with bad/very bad risk cytogenetics.
Introduction
Current standard treatment strategy for patients with acute myelogenous leukemia (AML) younger than 60 years consists of 1 or 2 induction courses with cytosine arabinoside and an anthracycline followed in the case of complete remission (CR) by one or more intensive consolidation (IC) chemotherapy courses. There is no consensus about the subsequent treatment modality. Some prefer only chemotherapy,1 ie, continuation of consolidation courses and/or maintenance chemotherapy; others proceed with some form of allogeneic or autologous stem cell transplantation (allo- or auto-SCT). Several large randomized studies comparing IC courses with stem cell transplantation, in which patients with an HLA-identical donor were scheduled to receive an allograft, report contradictory results.2-11 However, the sizes of these studies were small, the follow-up was insufficient, or the intensity of chemotherapy preceding SCT was not optimal or different. In some studies comparing chemotherapy versus auto-SCT versus allo-SCT, the design was not appropriate to assess the difference between auto-SCT and allo-SCT in terms of disease-free survival (DFS).2,3,6,10 In other studies the analyses were performed according to treatment actually given, the data on cytogenetics were scarce, or the proportion of patients who received the intended randomized treatment modality or the intended allo-SCT was too low.12 The comparison of the effects of allo- and auto-SCT has also been performed on the basis of registration data of the International and European Blood and Bone Marrow Transplantation Registries.13-15 Because these registries contain only data of patients in whom a transplantation has been performed, this type of analysis is biased because of selection of patients with good performance, variability of the interval between the moment of remission and the transplantation, and exclusion of early relapses.16
In the previous AML-8A trial of the European Organization for Research and Treatment of Cancer (EORTC) Leukemia Group and the Italian Gruppo Italiano Malattie Ematologiche dell' Adulto (GIMEMA) group, it has been shown that auto-SCT leads to a significantly longer DFS than a second IC course, but significant differences between auto-SCT and allo-SCT could not be detected.6
The EORTC and GIMEMA researchers subsequently conducted a randomized study (AML-10 trial) for patients with AML younger than 61 years. All patients were randomly assigned at the time of diagnosis for 1 of 3 anthracyclines added to cytosine arabinoside and etoposide as remission induction treatment. After achievement of CR an IC course was given. This course was to be followed by an allo-SCT in the patients with a HLA-identical family donor or by auto-SCT in those who lacked such a donor. In the latter group of patients a second randomization was performed to investigate the long-term results of transplantation with peripheral blood stem cells compared with bone marrow–derived stem cells.17
The AML-10 trial offers the possibility in patients up to the age of 45 years to assess the value of early allo-SCT versus auto-SCT on the basis of intention to treat18 by comparing the outcome of the group of patients with an HLA-identical sibling donor (donor group) with the outcome of patients without such a donor (no donor group).
Our analysis indicates that the strategy to perform an early allo-SCT was indeed justified in this group of patients younger than 45 years, as it led to a longer DFS than the strategy to perform an early auto-SCT. Subgroup analysis shows that patients with cytogenetical bad/very bad risk characteristics have a better outcome when a sibling donor is available. Because of a high treatment-related mortality (TRM) observed in patients with a donor and with good cytogenetic features, one may question the wisdom of performing an allo-SCT in CR1 in this group of patients. Furthermore, because of a relatively high TRM in patients aged 36 to 45 years, the prognosis of these patients with or without a sibling donor is almost identical, whereas in younger patients the differences were prominent.
Patients and methods
Study design
The EORTC-LG/GIMEMAAML-10 protocol was approved by the EORTC Protocol Review Committee and by the Ethical Committee of each participating center. The study was conducted from November 1993 to December 1999 in 80 European centers. All patients, up to the age of 60 years, with previously untreated AML, except acute promyelocytic leukemia, with more than 30% blast cells in the bone marrow, were eligible. Patients with a blast crisis of chronic myeloid leukemia (CML), AML supervening after other myeloproliferative diseases, or AML following a myelodysplastic syndrome of more than 6 months' duration and patients with severe heart, lung, liver, renal, hepatic, or neurologic diseases were excluded from registration. Before randomization, all patients had to give their informed consent, which was in accordance with the Helsinki protocol.
Random assignment for daunorubicin, mitoxantrone, or idarubicin was performed at the time of registration. Remission induction treatment consisted of cytosine arabinoside 25 mg/m2 intravenous bolus followed immediately by 100 mg/m2 given as a continuous infusion daily for 10 days; etoposide 100 mg/m2 in 0.9% saline daily by intravenous infusion (1 hour) on days 1 to 5; and one of the following: daunorubicin 50 mg/m2 on days 1, 3, and 5 as a 5-minute infusion or mitoxantrone 12 mg/m2 as a 30-minute infusion on days 1, 3, and 5 or idarubicin 10 mg/m2 as a 5-minute infusion on days 1, 3, and 5. In case of partial remission a second remission induction course was given. In case of CR a single course of consolidation therapy was administered, which consisted of intermediate-dose cytosine arabinoside (500 mg/m2 every 12 hours in a 2-hour infusion on days 1-6) and the anthracycline, randomly assigned at registration, given on days 4 to 6. Patients with a sibling donor were assigned to undergo an allogeneic SCT. All patients without such a donor had to receive an autologous blood or bone marrow stem cell transplant without any purging. The recommended conditioning regimens were as follows: cyclophosphamide (60 mg/kg on 2 consecutive days) and total body irradiation, preferably fractionated (6 fractions of 2 Gy over 3 days; dose rate < 0.06 Gy/min), or busulphan 4 mg/kg per day on days –8, –7, –6, and –5 combined with cyclophosphamide 60 mg/kg on days –4 and –3. Graft-versus-host prophylaxis was performed according to the local policy. T-cell depletion of the allogeneic graft was performed in 49 cases by monoclonal antibodies (n = 24), elutriation (n = 17), and other techniques (n = 8).
Patients
Between November 1993 and December 1999, a total of 2157 patients were randomly assigned in the AML-10 study. Among 1198 patients aged between 15 and 46 years, 62 patients were considered as inevaluable for the treatment response, mainly because of the lack of clinical documentation. Of the remaining 1136 patients, 822 (72.4%) entered complete remission after 1 or 2 courses of induction therapy. Among them, 50 patients were off-protocol treatment because of toxicity (n = 27) or other reason (n = 8), or they were poorly documented thereafter (n = 15). Of 772 patients who received consolidation, 38 have not been HLA typed because of refusal (n = 9), early death/toxicity (n = 20), logistic reasons (n = 5), and other or unknown reasons (n = 4). Among the 734 patients, 55 had no sibling. Of the remaining 679 patients, 293 had an HLA-identical sibling donor (donor group) and 386 had no family donor. By adding to this latter group those 55 patients with no siblings, a group of 441 patients has been formed, which was designated as the no donor group. The median follow-up was 4 years; 289 patients relapsed, 66 died in CR1, and overall 293 patients died. With a total of 355 events, the a posteriori statistical power in detecting a difference in the 4-year DFS rates between 42.4% and 52.2% was approximately 0.70.
Criteria of evaluation
The Cancer and Leukemia Group B (CALGB) criteria for response to treatment and relapse were used.19 CR was defined as a morphologic normal marrow with less than 5% blasts. Normal findings for peripheral blood and differential counts (ie, platelets ≥ 100 × 109/L, polymorphonuclear leukocyte [PMN] ≥ 1.5 × 109/L, hemoglobin ≥ 12 g/dL) were required at the evaluation of the induction course or at the evaluation of consolidation. Among patients who reached CR, relapse was defined as the presence of more than 10% blasts in the bone marrow or blasts in extramedullary sites.
As cytogenetic classification the International System for Cytogenetic Nomenclature (ISCN) has been applied.20 A central review (by A.H.) of cytogenetics has been performed. Twenty analyzed metaphases were required to include a patient in the cytogenetic normal karyotype (NN group). Complex abnormalities were defined as a clone with at least 4 unrelated abnormalities. The patients with unknown, not done, or unsuccessful cytogenetics were grouped together as unknown. Regarding morphology, the French-American-British (FAB) cytologic classification has been used, and a central review has been done in 44% of cases.21,22
Statistical analysis
The disease-free survival (DFS) was calculated from the date of CR until the date of first relapse or of death in first CR. The time to relapse and time to death in CR were calculated as the DFS; patients who died in CR and those who relapsed were respectively censored at that moment for these 2 analyses. By definition all patients who died in CR were considered as death from treatment-related mortality (TRM). The duration of survival was calculated from the date of CR until the date of death; patients still alive were censored at their last follow-up.
Actuarial curves were calculated according to the Kaplan-Meier technique.23 The standard errors (SE) of the estimates were computed using the Greenwood formula.23 The estimates of the incidence of relapse and of death in CR, and their corresponding standard errors, were obtained using the cumulative incidence method, in which the risks of death in CR and of relapse were considered as competing risks.24 The differences between actuarial curves were tested for statistical significance using the 2-tailed log-rank test,23 whereas for the cumulative incidences the Gray test was used.24 The Cox proportional hazards model was used to obtain the estimate and the 95% confidence interval (CI) of the hazard ratio (HR) of the instantaneous event rate in one group versus the one in another group, as specified by a given variable, and the Wald test was used to determine the prognostic significance.23 The Cox model was also used to determine the independent prognostic factors among those that appeared important in univariate analyses (P < .1), or an a priori interaction with the donor availability group was suspected (eg, age). The prognostic interaction between 2 variables was tested by including the product of these 2 variables into the model. All analyses were based on the intent-to-treat principle.
The cutoff date was April 8, 2002. SAS 8.1 statistical software (SAS Institute, Cary, NC) was used.
Results
Relationship between initial patient characteristics and donor availability
The characteristics of patients with a sibling donor (donor group) and without a sibling donor (no donor group) are shown in Table 1. The distribution regarding age, white blood cell (WBC) count at diagnosis, FAB subtype, and the CR rate after the first induction course was similar in both groups. In each group approximately one third of patients had received 1 of the 3 anthracyclines, and the sex ratio (M/F) was 1.04 in both groups. Results of cytogenetic examination were unknown in 288 patients: 170 (38.5%) in the no donor group versus 118 (40.3%) in the donor group. In the remaining 446 patients, the following risk groups were considered: good (t(8;21) or inv16) in 123 patients, intermediate (NN or –Y only) in 165 patients, bad (all other abnormalities, without good and very bad cytogenetic features) in 78 patients, and very bad risk (–5, 5q–, –7, 7q–, complex abnormalities, abnormal (abn) 3q, t(9;22), t(6;9), or abn 11q23 and absence of good cytogenetic features) in 80 patients. These subgroups were quite well distributed between the 2 study groups (Table 1); the percentage of patients with very bad risk was slightly higher in the donor group (24.0%) than in the no donor group (14.0%).
Patient characteristics according to donor availability
. | No donor, N = 441 . | Donor, N = 293 . |
|---|---|---|
| Age, y, median (range) | 33 (15-45) | 35 (15-45) |
| Age group | ||
| Between 15 y and 25 y, n (%) | 114 (25.9) | 67 (22.9) |
| Between 26 y and 35 y, n (%) | 142 (32.2) | 88 (30.0) |
| Between 36 y and 45 y, n (%) | 185 (42.0) | 138 (47.1) |
| Median WBC count, × 109/L (range) | 19.2 (0.4-336) | 16.4 (0.6-420) |
| FAB type | ||
| Unknown, n (%) | 5 (1.1) | 2 (0.7) |
| M0, n (%) | 14 (3.2) | 9 (3.1) |
| M1, n (%) | 71 (16.1) | 46 (15.7) |
| M2, n (%) | 157 (35.6) | 106 (36.2) |
| M3, n (%)* | 2 (0.5) | 0 (0.0) |
| M4, n (%) | 75 (17.0) | 57 (19.5) |
| M4E, n (%) | 25 (5.6) | 22 (7.5) |
| M5, n (%) | 83 (18.8) | 40 (13.7) |
| M6-M7, n (%) | 9 (2.1) | 11 (3.7) |
| Courses needed to reach CR | ||
| 1, n (%) | 410 (93.0) | 267 (91.1) |
| 2, n (%) | 31 (7.0) | 26 (8.9) |
| Cytogenetics | ||
| Unknown, n (%) | 170 (38.5) | 118 (40.3) |
| Successful, n (%) | 271 (61.5) | 175 (59.7) |
| Good risk, n [%]† | 73 [26.9] | 50 [28.6] |
| Intermediate risk, n [%]‡ | 104 [38.4] | 61 [34.9] |
| Bad, n [%]§ | 56 [20.7] | 22 [12.6] |
| Very bad, n [%]∥ | 38 [14.0] | 42 [24.0] |
. | No donor, N = 441 . | Donor, N = 293 . |
|---|---|---|
| Age, y, median (range) | 33 (15-45) | 35 (15-45) |
| Age group | ||
| Between 15 y and 25 y, n (%) | 114 (25.9) | 67 (22.9) |
| Between 26 y and 35 y, n (%) | 142 (32.2) | 88 (30.0) |
| Between 36 y and 45 y, n (%) | 185 (42.0) | 138 (47.1) |
| Median WBC count, × 109/L (range) | 19.2 (0.4-336) | 16.4 (0.6-420) |
| FAB type | ||
| Unknown, n (%) | 5 (1.1) | 2 (0.7) |
| M0, n (%) | 14 (3.2) | 9 (3.1) |
| M1, n (%) | 71 (16.1) | 46 (15.7) |
| M2, n (%) | 157 (35.6) | 106 (36.2) |
| M3, n (%)* | 2 (0.5) | 0 (0.0) |
| M4, n (%) | 75 (17.0) | 57 (19.5) |
| M4E, n (%) | 25 (5.6) | 22 (7.5) |
| M5, n (%) | 83 (18.8) | 40 (13.7) |
| M6-M7, n (%) | 9 (2.1) | 11 (3.7) |
| Courses needed to reach CR | ||
| 1, n (%) | 410 (93.0) | 267 (91.1) |
| 2, n (%) | 31 (7.0) | 26 (8.9) |
| Cytogenetics | ||
| Unknown, n (%) | 170 (38.5) | 118 (40.3) |
| Successful, n (%) | 271 (61.5) | 175 (59.7) |
| Good risk, n [%]† | 73 [26.9] | 50 [28.6] |
| Intermediate risk, n [%]‡ | 104 [38.4] | 61 [34.9] |
| Bad, n [%]§ | 56 [20.7] | 22 [12.6] |
| Very bad, n [%]∥ | 38 [14.0] | 42 [24.0] |
Percentages between brackets were calculated for those with a successful examination.
M3 according to the cytology review, but M5 and M2 according to the local cytologist.
Presence of t(8;21) or inv(16).
NN or -Y only.
Presence of other abnormalities without good or very bad cytogenetic features.
Presence of -5, 5q-, -7, 7q-, complex abnormalities, abn 3q, t(9;22), t(6;9), or abn 11q23, and absence of good cytogenetic features.
Relationship between donor availability and stem cell transplantation
Allo-SCT in first CR was performed in 202 (68.9%) of 293 patients with a sibling donor, and auto-SCT was performed in 246 (55.8%) of 441 patients without such a donor. The conditioning regimen consisting of either busulphan + cyclophosphamide or cyclophosphamide + total body irradiation (TBI) was administered in 56% and 44%, respectively, of patients receiving allografts and in 75% and 25%, respectively, in patients receiving autotransplants. Eleven (2.5%) patients in the no donor group received transplants in first CR with stem cells of a matched unrelated or phenotypically identical related donor. Seven (2.5%) patients with a sibling donor received an autologous SC transplant. The percentages of actually performed SCTs in the cytogenetic subgroups are shown in Table 2.
Performed stem cell transplantation according to the donor availability group in each cytogenetic subgroup and each age group
. | No donor, N = 441 . | . | Donor, N = 293 . | . | ||
|---|---|---|---|---|---|---|
. | Autologous (%) . | Allogeneic (%) . | Autologous (%) . | Allogeneic (%) . | ||
| Cytogenetics | ||||||
| Unknown | 92 (54.1) | 1 (0.6) | 2 (1.7) | 73 (61.9) | ||
| Successful | 154 (56.8) | 10 (3.7) | 5 (2.9) | 129 (73.7) | ||
| Good risk | 46 (63.0) | 1 (1.4) | 4 (8.0) | 36 (72.0) | ||
| Intermediate risk | 65 (62.5) | 2 (1.9) | 1 (1.6) | 46 (75.4) | ||
| Bad risk | 29 (51.8) | 2 (3.6) | 0 (0.0) | 17 (77.3) | ||
| Very bad risk | 14 (36.8) | 5 (13.2) | 0 (0.0) | 30 (71.4) | ||
| Age, y | ||||||
| Between 15 and 25 | 62 (54.4) | 4 (3.5) | 0 (0.0) | 45 (67.2) | ||
| Between 26 and 35 | 85 (59.9) | 2 (1.4) | 1 (1.1) | 67 (76.1) | ||
| Between 36 and 45 | 99 (53.5) | 5 (2.7) | 6 (4.3) | 90 (65.2) | ||
| Total | 246 (55.8) | 11 (2.5) | 7 (2.4) | 202 (68.9) | ||
. | No donor, N = 441 . | . | Donor, N = 293 . | . | ||
|---|---|---|---|---|---|---|
. | Autologous (%) . | Allogeneic (%) . | Autologous (%) . | Allogeneic (%) . | ||
| Cytogenetics | ||||||
| Unknown | 92 (54.1) | 1 (0.6) | 2 (1.7) | 73 (61.9) | ||
| Successful | 154 (56.8) | 10 (3.7) | 5 (2.9) | 129 (73.7) | ||
| Good risk | 46 (63.0) | 1 (1.4) | 4 (8.0) | 36 (72.0) | ||
| Intermediate risk | 65 (62.5) | 2 (1.9) | 1 (1.6) | 46 (75.4) | ||
| Bad risk | 29 (51.8) | 2 (3.6) | 0 (0.0) | 17 (77.3) | ||
| Very bad risk | 14 (36.8) | 5 (13.2) | 0 (0.0) | 30 (71.4) | ||
| Age, y | ||||||
| Between 15 and 25 | 62 (54.4) | 4 (3.5) | 0 (0.0) | 45 (67.2) | ||
| Between 26 and 35 | 85 (59.9) | 2 (1.4) | 1 (1.1) | 67 (76.1) | ||
| Between 36 and 45 | 99 (53.5) | 5 (2.7) | 6 (4.3) | 90 (65.2) | ||
| Total | 246 (55.8) | 11 (2.5) | 7 (2.4) | 202 (68.9) | ||
The exact number of patients in each cytogenetic or age group is given in Table 1.
Although the percentages of actually performed SCTs in the donor group were only slightly different among the cytogenetic subgroups, in the no donor group a lower percentage (45.7%) of patients with bad/very bad cytogenetics received an autologous SC transplant, and 7.4% of this subgroup received transplants with stem cells of a matched unrelated donor. The low autologous transplantation rate was due mainly to a high early relapse rate before SCT could be performed in the bad/very bad risk group; of 44 patients (Table 3) who have not received transplants, 37 relapsed early. The reasons for no transplantation according to the donor availability, in each cytogenetic subgroup, are indicated in Table 3.
Number of patients for each reason for not performing stem cell transplantation (SCT) according to the donor availability group in each cytogenetic subgroup
. | No donor, N = 184 . | . | . | . | . | Donor, N = 84 . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Total . | Relapse . | Toxicity . | Refusal of protocol violation . | Other/not documented . | Total . | Relapse . | Toxicity . | Refusal of protocol or violation . | Other/not documented . | ||||||||
| Unknown | 77 | 12 | 20 | 23 | 22 | 43 | 14 | 7 | 5 | 17 | ||||||||
| Good risk | 26 | 1 | 8 | 14 | 3 | 10 | 1 | 2 | 4 | 3 | ||||||||
| Intermediate risk | 37 | 9 | 12 | 13 | 3 | 14 | 7 | 1 | 3 | 3 | ||||||||
| Bad risk | 25 | 12 | 4 | 6 | 3 | 5 | 1 | 1 | 1 | 2 | ||||||||
| Very bad risk | 19 | 9 | 7 | 2 | 1 | 12 | 8 | 1 | 3 | 0 | ||||||||
. | No donor, N = 184 . | . | . | . | . | Donor, N = 84 . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Total . | Relapse . | Toxicity . | Refusal of protocol violation . | Other/not documented . | Total . | Relapse . | Toxicity . | Refusal of protocol or violation . | Other/not documented . | ||||||||
| Unknown | 77 | 12 | 20 | 23 | 22 | 43 | 14 | 7 | 5 | 17 | ||||||||
| Good risk | 26 | 1 | 8 | 14 | 3 | 10 | 1 | 2 | 4 | 3 | ||||||||
| Intermediate risk | 37 | 9 | 12 | 13 | 3 | 14 | 7 | 1 | 3 | 3 | ||||||||
| Bad risk | 25 | 12 | 4 | 6 | 3 | 5 | 1 | 1 | 1 | 2 | ||||||||
| Very bad risk | 19 | 9 | 7 | 2 | 1 | 12 | 8 | 1 | 3 | 0 | ||||||||
The study group was divided into groupings of patients aged 16 to 25, 26 to 35, and 36 to 45 years. Table 2 shows that the percentages of patients who had an auto- or allo-SCT performed in those with and without a donor were quite similar in each age grouping.
Effect of donor availability on outcome
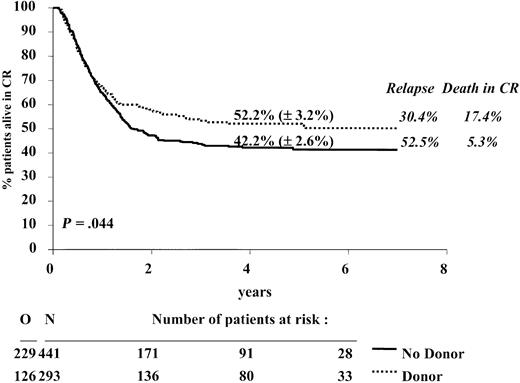
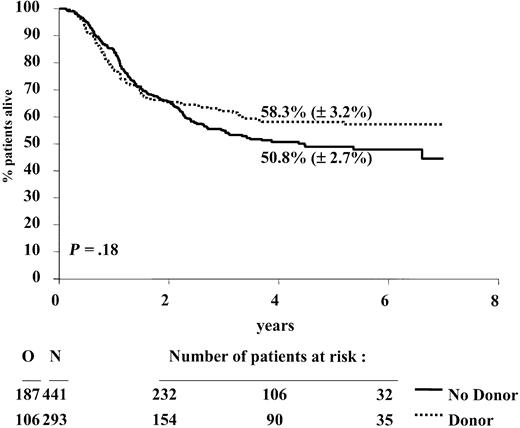
The 4-year DFS rate of the donor group was superior to that of the no donor group: 52.2% versus 42.2%, P = .044; hazard ratio = 0.80; 95% confidence interval, 0.64-0.995. In the first 9 months the curves were superimposable (hazard ratio = 0.99; 95% CI, 0.73-1.33; P = .92), but thereafter, a divergence occurred between them (hazard ratio = 0.64; 95% CI, 0.46-0.88; P = .006). The relapse incidence was 30.4% versus 52.5% (P < .0001), and the incidence of death in CR was 17.4% versus 5.3% (P < .0001), respectively. The survival from CR rate was 58.3% versus 50.8% (P = .18); hazard ratio = 0.84; and 95% confidence interval, 0.67-1.08 (Figures 1, 2, and 3).
DFS from CR according to donor availability. The estimates of the 4-year DFS rates (± SE) for the donor group (dotted line) and the no donor group (solid line) are given. The 4-year cumulative incidence of relapse and of death in CR are given in italics at the right of the graph. N indicates number of patients; O, observed number of events (relapse or death in first CR). P is determined by the log-rank test.
DFS from CR according to donor availability. The estimates of the 4-year DFS rates (± SE) for the donor group (dotted line) and the no donor group (solid line) are given. The 4-year cumulative incidence of relapse and of death in CR are given in italics at the right of the graph. N indicates number of patients; O, observed number of events (relapse or death in first CR). P is determined by the log-rank test.
Cumulative incidence of relapse and cumulative incidence of death in complete remission according to donor availability. N indicates number of patients; R, observed number of relapses; D, observed number of deaths in first CR. P is determined by the Gray test.
Cumulative incidence of relapse and cumulative incidence of death in complete remission according to donor availability. N indicates number of patients; R, observed number of relapses; D, observed number of deaths in first CR. P is determined by the Gray test.
Duration of survival from complete remission according to donor availability. N indicates number of patients; O, observed number of deaths. P is determined by the log-rank test.
Duration of survival from complete remission according to donor availability. N indicates number of patients; O, observed number of deaths. P is determined by the log-rank test.
Effect of cytogenetics and donor availability on outcome
The most important prognostic factor for the DFS (P < .0001) was initial cytogenetics: patients with good risk cytogenetics had a better outcome than those with intermediate risk cytogenetics, whereas those with bad or very bad risk cytogenetic characteristics had a far worse outcome. The outcome of patients with unknown cytogenetic examination was similar to that of those with known cytogenetic features (HR = 1.17).
Because of the slight imbalance of the distribution of patients with bad/very bad risk cytogenetics between the donor and no donor group, the Cox model shows that the difference in DFS adjusted for the initial cytogenetic features slightly increased: the estimated hazard ratio was 0.78 (P = .024) when the 2 risk groups, bad and very bad, were pooled together (Table 4, model 1) and was 0.75 (P = .012) when these 2 groups were separated. When we included in the Cox model not only variable cytogenetics (prognostic score based on binary variables corresponding to good, intermediate, bad/very bad, and unknown groups) and the donor availability but also their interaction, this interaction was marginally significant (P = .07). Therefore, the difference between the outcomes of the 2 groups might have been influenced by the cytogenetic risk group.
Results of the Cox Proportional Hazards Model regarding the disease-free survival when only cytogenetics and donor availability were considered (model 1) and when cytogenetics and the interaction between each age group and donor availability were considered (model 2)
. | Hazard ratio* . | 95% CI . | P† . |
|---|---|---|---|
| Model 1 | |||
| Cytogenetics | |||
| Good risk | 0.58 | 0.40, 0.84 | .004 |
| Intermediate | 1 | ||
| Bad/very bad | 1.63 | 1.22, 2.17 | .0009 |
| Unknown | 0.95 | 0.72, 1.25 | .70 |
| Donor vs no donor | 0.78 | 0.63, 0.97 | .025 |
| Model 2 | |||
| Cytogenetics | |||
| Good risk | 0.58 | 0.40, 0.84 | .004 |
| Intermediate | 1 | ||
| Bad/very bad | 1.65 | 1.24, 2.20 | .0007 |
| Unknown | 0.94 | 0.71, 1.24 | .67 |
| Donor vs no donor | |||
| If aged between 15 and 25 y | 0.64 | 0.43, 0.97 | .04 |
| If aged between 26 and 35 y | 0.68 | 0.48, 0.97 | .03 |
| If aged between 36 and 45 y | 0.92 | 0.70, 1.22 | .57 |
. | Hazard ratio* . | 95% CI . | P† . |
|---|---|---|---|
| Model 1 | |||
| Cytogenetics | |||
| Good risk | 0.58 | 0.40, 0.84 | .004 |
| Intermediate | 1 | ||
| Bad/very bad | 1.63 | 1.22, 2.17 | .0009 |
| Unknown | 0.95 | 0.72, 1.25 | .70 |
| Donor vs no donor | 0.78 | 0.63, 0.97 | .025 |
| Model 2 | |||
| Cytogenetics | |||
| Good risk | 0.58 | 0.40, 0.84 | .004 |
| Intermediate | 1 | ||
| Bad/very bad | 1.65 | 1.24, 2.20 | .0007 |
| Unknown | 0.94 | 0.71, 1.24 | .67 |
| Donor vs no donor | |||
| If aged between 15 and 25 y | 0.64 | 0.43, 0.97 | .04 |
| If aged between 26 and 35 y | 0.68 | 0.48, 0.97 | .03 |
| If aged between 36 and 45 y | 0.92 | 0.70, 1.22 | .57 |
A value > 1 indicates that the outcome is worse in the given category compared with the baseline.
P was determined by the Wald test.
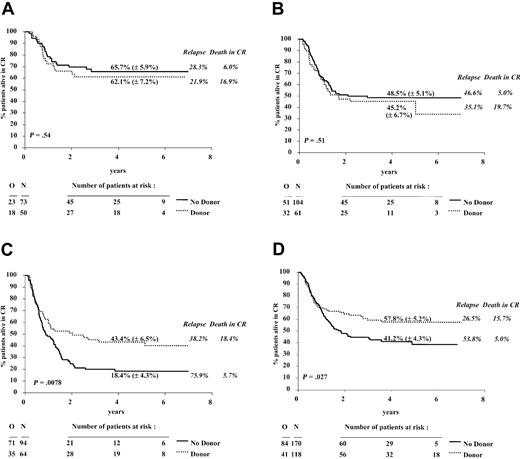
In the 446 patients with a successful cytogenetic examination the estimated hazard ratio for the DFS comparison of the donor versus no donor groups was 0.89 (P = .40), and the 95% confidence interval was 0.68 to 1.17. The relapse incidence was 32.7% versus 51.9%; the death in CR incidence was 18.5% versus 5.5%. The results in terms of DFS in each cytogenetic subgroup and according to the availability of a sibling donor, along with the 4-year estimate rates of DFS and of the cumulative incidences of relapse and of death in CR, are shown in Figure 4A-C.
DFS from CR according to donor availability in 4 cytogenetic groups. The cytogenetic groups were good risk (A), intermediate risk (B), bad/very bad risk (C), and unknown (D). The estimates of the 4-year DFS rates (± SE) for the donor group (dotted line) and the no donor group (solid line) are given. The 4-year cumulative incidences of relapse and of death in CR are given in italics. N indicates number of patients; O, observed number of events (relapse or death in first CR). P was determined by the log-rank test.
DFS from CR according to donor availability in 4 cytogenetic groups. The cytogenetic groups were good risk (A), intermediate risk (B), bad/very bad risk (C), and unknown (D). The estimates of the 4-year DFS rates (± SE) for the donor group (dotted line) and the no donor group (solid line) are given. The 4-year cumulative incidences of relapse and of death in CR are given in italics. N indicates number of patients; O, observed number of events (relapse or death in first CR). P was determined by the log-rank test.
The 4-year survival rates in the donor versus no donor group, in the cytogenetic prognostic groups (good, intermediate, and bad/very bad) were 68.1% (± 6.8%) versus 73.9% (± 5.5%), 53.4% (± 6.8%) versus 54.3% (± 5.3%), and 50.2% (± 6.7%) versus 29.4% (± 5.3%), respectively. The estimates of the hazard ratios for the comparison donor versus no donor in each of the 3 cytogenetic groups are given in Table 5. In the cytogenetic good and intermediate risk group, patients with a sibling donor did not have a better outcome (DFS and survival) than those without a sibling donor: the estimated hazard ratios were more than 1 and the lower 95% confidence interval was 0.66 or more. The reductions in the relapse incidences of the donor group were largely counterbalanced by an increase in the risk of death in CR. In the cytogenetic bad and very bad risk groups, patients with a sibling donor do have a considerably better outcome than those without such a donor: the estimated hazard ratio for the DFS and survival was 0.58 and 0.62, respectively. The increase in the TRM was largely compensated by the reduction in the relapse incidence. Therefore, for the comparison of donor versus no donor in terms of DFS, the decrease observed in the hazard ratios 1.21, 1.16, and 0.58 in the good, intermediate, and bad/very bad cytogenetic risk groups, respectively, appeared to be significant (P = .024), as indicated by the Cox model, in which the cytogenetic risk group (considered as an ordered variable), donor availability, and their interaction have been included. For survival, the interaction between these 2 variables was significant as well (P = .035).
Comparison of donor versus no donor in 3 cytogenetic groups and the 3 age groups according to different endpoints
. | Disease-free survival . | Time to relapse . | Time to death in CR . | Survival . |
|---|---|---|---|---|
| Cytogenetic groups | ||||
| Good | 1.21 (0.66, 2.25) | 0.83 (0.38, 1.78) | 3.03 (0.91, 10.06) | 1.41 (0.70, 2.82) |
| Intermediate | 1.16 (0.75, 1.81) | 0.84 (0.50, 1.41) | 4.06 (1.41, 11.68) | 1.14 (0.70, 1.86) |
| Bad/very bad | 0.58 (0.39, 0.87) | 0.42 (0.26, 0.68) | 2.70 (0.93, 7.80) | 0.62 (0.40, 0.96) |
| Age groups | ||||
| 15-25 y | 0.65 (0.41, 1.04) | 0.53 (0.32, 0.89) | 2.28 (0.64, 8.10) | 0.63 (0.37, 1.07) |
| 26-35 y | 0.69 (0.46, 1.02) | 0.48 (0.30, 0.77) | 2.63 (1.08, 6.40) | 0.70 (0.45, 1.08) |
| 36-45 y | 0.97 (0.70, 1.33) | 0.65 (0.44, 0.95) | 3.91 (1.83, 8.34) | 1.09 (0.78, 1.54) |
. | Disease-free survival . | Time to relapse . | Time to death in CR . | Survival . |
|---|---|---|---|---|
| Cytogenetic groups | ||||
| Good | 1.21 (0.66, 2.25) | 0.83 (0.38, 1.78) | 3.03 (0.91, 10.06) | 1.41 (0.70, 2.82) |
| Intermediate | 1.16 (0.75, 1.81) | 0.84 (0.50, 1.41) | 4.06 (1.41, 11.68) | 1.14 (0.70, 1.86) |
| Bad/very bad | 0.58 (0.39, 0.87) | 0.42 (0.26, 0.68) | 2.70 (0.93, 7.80) | 0.62 (0.40, 0.96) |
| Age groups | ||||
| 15-25 y | 0.65 (0.41, 1.04) | 0.53 (0.32, 0.89) | 2.28 (0.64, 8.10) | 0.63 (0.37, 1.07) |
| 26-35 y | 0.69 (0.46, 1.02) | 0.48 (0.30, 0.77) | 2.63 (1.08, 6.40) | 0.70 (0.45, 1.08) |
| 36-45 y | 0.97 (0.70, 1.33) | 0.65 (0.44, 0.95) | 3.91 (1.83, 8.34) | 1.09 (0.78, 1.54) |
Numbers are shown as estimated hazard ratio (95% CI).
Patients with an unknown cytogenetic analysis (N = 288) showed the same trends as the whole study group: a 4-year DFS rate of 57.8% versus 41.2% (Figure 4D), an estimated hazard ratio (95% CI) of 0.66 (0.45, 0.96), and a survival rate of 62.1% (± 5.2%) vs. 50.6% (± 4.7%).
Additional multivariate analyses
The multivariate Cox model showed that in addition to cytogenetic subgroup (good, intermediate, bad/very bad, unknown), the following factors were important independent prognostic factors: initial WBC count (continuous variable) (P = .0003) and FAB subtype (other subgroup versus M2 or M4E, P = .027). Adjusting for these factors, the donor group remained to show a better outcome than the no donor group (HR = 0.77, P = .019). The interaction term between the variable donor availability and a prognostic score based on cytogenetic subgroup, WBC count, and FAB subgroup was marginally significant (P = .09), suggesting that the worse the prognosis the higher the difference between donor and no donor groups. In patients with known cytogenetics, FAB subgroup and WBC count were of low prognostic importance once cytogenetics was included in the model. In patients with unknown cytogenetics, donor availability (hazard ratio = 0.61, P = .01), FAB subgroup (P = .03), and WBC count (P < .0001) were the most important independent prognostic factors for DFS.
Comparative cytogenetic grouping
The differences in terms of DFS for the comparison of donor versus no donor according to the classifications used by the EORTC/GIMEMA, SWOG/ECOG (Southwest Oncology Group/Eastern Cooperative Oncology Group), and MRC (Medical Research Council) are given in Table 6. According to all 3 classifications the outcome of the donor group was not superior to the no donor group in patients with good risk abnormalities and in patients with intermediate risk abnormalities as defined by NN or –Y alone or those comprising some additional features, such as +8, +6, del(12p).28 In patients classified as others by each of the 3 groupings, the donor group had a better outcome than the no donor group. In the intermediate group by the MRC, containing NN, –Y, and others, a slight DFS advantage in favor of the donor group was observed. Although the definition of the very bad, adverse, and unfavorable group by the 3 leukemia research groups was slightly different, the estimated hazard ratios pointed in the same direction, as they were 0.65, 0.59, and 0.64, respectively, for the EORTC/GIMEMA (n = 80), SWOG/ECOG (n = 105), and MRC (n = 30) grouping.
DFS analysis for the comparison of donor versus no donor according to the cytogenetic groups and 3 possible grouping systems
. | No donor, n . | Donor, n . | Estimated HR . | 95% CI . |
|---|---|---|---|---|
| EORTC/GIMEMA | ||||
| Favorable* | 73 | 50 | 1.21 | 0.66, 2.25 |
| NN, -Y only | 104 | 61 | 1.16 | 0.75, 1.81 |
| Others (bad)* | 56 | 22 | 0.33 | 0.15, 0.75 |
| Very bad* | 38 | 42 | 0.65 | 0.39, 1.10 |
| SWOG/ECOG | ||||
| Favorable† | 67 | 46 | 1.27 | 0.67, 2.42 |
| Intermediate‡ | 116 | 67 | 1.02 | 0.67, 1.55 |
| Others | 36 | 9 | 0.27 | 0.07, 1.16 |
| Unfavorable§ | 52 | 53 | 0.64 | 0.40, 1.02 |
| MRC | ||||
| Favorable* | 73 | 50 | 1.21 | 0.66, 2.25 |
| NN, -Y only | 104 | 61 | 1.16 | 0.75, 1.81 |
| Others | 81 | 47 | 0.55 | 0.64, 0.88 |
| Intermediate∥ | 0.83 | 0.60, 1.14 | ||
| Adverse¶ | 13 | 17 | 0.59 | 0.26, 1.36 |
. | No donor, n . | Donor, n . | Estimated HR . | 95% CI . |
|---|---|---|---|---|
| EORTC/GIMEMA | ||||
| Favorable* | 73 | 50 | 1.21 | 0.66, 2.25 |
| NN, -Y only | 104 | 61 | 1.16 | 0.75, 1.81 |
| Others (bad)* | 56 | 22 | 0.33 | 0.15, 0.75 |
| Very bad* | 38 | 42 | 0.65 | 0.39, 1.10 |
| SWOG/ECOG | ||||
| Favorable† | 67 | 46 | 1.27 | 0.67, 2.42 |
| Intermediate‡ | 116 | 67 | 1.02 | 0.67, 1.55 |
| Others | 36 | 9 | 0.27 | 0.07, 1.16 |
| Unfavorable§ | 52 | 53 | 0.64 | 0.40, 1.02 |
| MRC | ||||
| Favorable* | 73 | 50 | 1.21 | 0.66, 2.25 |
| NN, -Y only | 104 | 61 | 1.16 | 0.75, 1.81 |
| Others | 81 | 47 | 0.55 | 0.64, 0.88 |
| Intermediate∥ | 0.83 | 0.60, 1.14 | ||
| Adverse¶ | 13 | 17 | 0.59 | 0.26, 1.36 |
Favorable cytogenetics is the presence of t(8;21) or inv(16); bad is the presence of other abnormalities without good or very bad cytogenetic features; very bad is the presence of -5, 5q-, -7, 7q-, complex abnormalities, abn 3q, t(9;22), t(6;9), or abn 11q23, and absence of good cytogenetic features.
Favorable cytogenetics in this grouping system is the presence of inv(16) with/without secondary aberrations; t(8;21) without del(9q) and complex karyotype.
Intermediate cytogenetics is the presence of NN, +8, +6, -Y, del(12p) without presence of unfavorable features.
Unfavorable cytogenetics is the presence of -7, 7q-, -5, 5q-, abn (3q), 9q, 11q, 20q, 21q, 17p, t(6;9), t(9;22), or complex, defined as a clone with at least 3 unrelated abnormalities.
Intermediate is the combination of the NN, -Y only group with the other group.
Adverse cytogenetics in this grouping system is the presence of -7, -5, 5q-, abn (3q), or complex, defined as a clone with at least 5 unrelated abnormalities.
Effect of age and donor availability on outcome
Overall, the results with respect to DFS and survival were not different between the age groups (15-25, 26-35, and 36-45 years). Figure 5A-C shows the comparison of the donor and no donor groups in terms of DFS and indicates the 4-year cumulative incidence of relapse and of death in CR according to the 3 age groups.
DFS from CR according to donor availability in the 3 age groups. The age groups were 15 to 25 years (A), 26 to 35 years (B), and 36 to 45 years (C). The estimates of the 4-year DFS rates (± SE) for the donor group (dotted line) and the no donor group (solid line) are given. The 4-year cumulative incidences of relapse and of death in CR are given in italics. N indicates number of patients; O, observed number of events (relapse or death in first CR). P was determined by the log-rank test.
DFS from CR according to donor availability in the 3 age groups. The age groups were 15 to 25 years (A), 26 to 35 years (B), and 36 to 45 years (C). The estimates of the 4-year DFS rates (± SE) for the donor group (dotted line) and the no donor group (solid line) are given. The 4-year cumulative incidences of relapse and of death in CR are given in italics. N indicates number of patients; O, observed number of events (relapse or death in first CR). P was determined by the log-rank test.
As shown in Table 5, in patients aged 35 years or younger, the DFS for the patients with a sibling donor was longer than for those without a donor, because of a lower incidence of relapse (HR was approximately 0.50) and a lower increase in the TRM incidence (HR was approximately 2.5). In older patients (aged 36-45 years) the lower incidence of relapse (29.6% versus 48.3%, HR = 0.65) for the donor group was counterbalanced by a far higher incidence of death in CR (21.1% versus 5.5%, HR = 3.91). The 4-year survival rates in the donor versus no donor group, in the 3 age categories (15-25, 26-35, and 36-45 years) were 64.0% (± 6.7%) versus 50.8% (± 5.2%), 61.9% (± 5.7%) versus 49.6% (± 4.8%), and 53.4% (± 4.6%) versus 51.6% (± 4.3%), respectively.
In Table 4, model 2, the donor versus no donor comparison has been adjusted for the cytogenetics and assessed in each age group; the estimated hazard ratios were 0.64, 0.68, and 0.92 in the 3 age groups, respectively. An interaction between age (considered as an ordered categorical variable) and donor availability was detected, indicating that the older the patients the smaller the difference in terms of DFS (P = .036) and survival (P = .012) between the no donor and donor groups.
Discussion
In this study we show for the first time that using analysis by intention to treat for the patients in the EORTC-LG/GIMEMA AML-10 trial in CR1 aged younger than 46 years assigned to allo-SCT has a significantly better outcome than for those who were planned to undergo an auto-SCT. This finding seems specifically true for patients with bad or very bad risk cytogenetics. This conclusion is justified because this AML-10 trial is the first large study in which prospectively only the 2 transplantation modalities are offered at an early time point of entering CR.
Already in our previous EORTC-GIMEMA AML-8A trial, the DFS rate of the no donor group was inferior to that of the donor group; but here the no donor group was composed of patients who were randomly assigned to receive autologous bone marrow transplant (auto-BMT) or a second chemotherapy consolidation. Furthermore, the 6-year survival rate in the AML-8A trial was 8% higher for the donor group as compared with the no donor group, but, because of a limited number of deaths, the difference was not significant (P = .24).11 Similarly, Burnett et al25 reported in the recent MRC AML-10 study in which patients after 4 chemotherapy cycles continued with allo- or auto-SCT or no further treatment25 that the patients with a donor had a higher 7-year DFS rate than the patients without a donor (50% versus 42%; P = .01). However, the composition of their study group was different, as it included children, patients with acute promyelocytic leukemia, and patients aged older than 45 years, and only 24% of the patients without a donor received an auto-SC transplant.
In our current study all patients aged younger than 46 years who achieved a CR and were consolidated by one course of intensive chemotherapy were eligible for either allo- or auto-SCT, depending on the biologic availability of a sibling donor. The 2 groups, donor and no donor, were comparable with respect to sex, age, initial WBC count, FAB classification, cytogenetic distribution, and number of courses to reach CR. Only 55.8% of the patients actually received an auto-SC transplant and 68.9% received an allo-SC transplant. Although this figure might be viewed as a limitation, we are convinced, like others,18,26 that comparison on the basis of intention to treat is the only way to reliably evaluate a treatment strategy. Failure to undergo the assigned transplantation was usually because of early relapse, poor clinical condition, refusal of the patient, or failure to harvest sufficient number of stem cells. In other prospective studies the frequency to perform a transplantation is somehow comparable with ours,2,5,6,25,26 although this finding may depend on the trial design, number and intensity of chemotherapy courses before the planned SCT, and patient selection.
In the cytogenetic subgroups of our study the frequency of transplantation differed. Only 50% of patients without a sibling donor and bad/very bad risk cytogenetics did undergo a transplantation, compared with 73% of the same subgroup of patients in the donor group. This frequency was mainly because of early relapses and failure to harvest sufficient stem cells in the no donor group. In contrast, 72% of patients with good risk cytogenetics with a donor received allo-SC transplants, whereas 63% of those without a donor received auto-SC transplants. These percentages are higher than in the MRC AML-10 study (52% and 22%, respectively).25 The reasons may be the long interval between the achievement of CR and the possible transplantation date in the MRC study because of the prolonged administration of the chemotherapy courses and the high TRM rate reported among patients registered before 1994.25
The significant difference in 4-year DFS rate of 10% (95% CI, 1.9%-18.1%) in favor of the donor group was due to the lower relapse incidence (22%) despite a higher TRM (12%). In patients who received the planned treatment, the TRM incidence after allo-SCT (20%) or after auto-SCT (5%) is comparable to the data reported by different registries for patients with AML in first CR.13-15 The 4-year DFS percentages for patients who actually received the planned allo- or auto-SC transplant were approximately 10% higher than in the donor and no donor groups. Adjustment for cytogenetics led to an HR = 0.73 (P = .033). This finding indicates how biased the results are when based on the treatment given instead of the treatment planned.
Successful cytogenetic data were available in 60.8% of the patients. The unknown group comes mainly from centers at which, at that time, cytogenetics was not routinely performed but includes also patients in whom cytogenetics had failed because of insufficient material or failure of marrow cells to form enough metaphases. The unknown group achieved similar results as the whole study group with respect to DFS, relapse incidence, and TRM. These findings add to the assumption that the not cytogenetically tested patients represent a random selection of patients.
Overall DFS for the good, intermediate, bad, and very bad risk cytogenetic subgroups followed the same pattern as reported in other large studies, despite the fact that the definition of the cytogenetic subgroups is not exactly the same.27,28
In the patients with good risk the strategy to perform an allo-SCT led to a slightly lower DFS rate than the strategy to perform an auto-SCT. The lower relapse incidence in the donor group could not balance the higher TRM observed after allo-SCT. Both kinds of transplantations led to quite satisfactory DFS rates (61%-65% at 4 years). In the MRC study the 7-year DFS rate in the patients with good risk with a donor was lower (53%) than in the patients without a donor (66%) because of a high TRM rate (24%).25 A similar trend has been observed in the SWOG/ECOG study (5-year survival, 63% versus 71%).28 These data suggest that in this subgroup of patients with AML, allo-SCT may be restricted to second-line therapy.29 Also in the patients with intermediate risk (NN, –Y only), we could not detect a difference in DFS. Again, the better antileukemic activity of the treatment in patients of the donor group could not balance the higher TRM. The MRC AML-10 study identified an advantage in favor of patients with intermediate risk with a donor. This advantage might be due to their definition of intermediate risk.25,27 Their group of patients with an NN or –Y only karyotype was supplemented with those with bad risk cytogenetics, according to our definition, in which we have detected the largest difference between the 2 groups. In the SWOG/ECOG study, the results of allo-SCT group were not superior to auto-SCT in the intermediate risk group, which comprised the patients with NN and –Y and those with +8, +6, or del(12p) only.28
The situation is totally different for patients with AML with bad or very bad risk cytogenetics. Here, the strategy to perform an allo-SCT led to DFS and survival rates that were only slightly inferior to those obtained in the other cytogenetic subgroups. However, dramatically inferior results were seen in the no donor group, because of an extremely high relapse rate. Only approximately 20% of the patients with bad and very bad risk remained in first CR, and approximately 30% are alive at 4 years from CR. It is clear that auto-SCT in these patients is a highly insufficient treatment approach. This finding contrasts with results of patients in the donor group: the 4-year DFS rate was approximately 65% and 35% in patients with bad and very bad risk, respectively. This observation is in line with the SWOG/ECOG study in which patients in the allogenic bone marrow transplantation (allo-BMT) group (n = 18) had better results than those in auto-BMT (n = 20) or chemotherapy (n = 20) groups.28 In the MRC study, in patients with adverse cytogenetics, both groups, those with a donor (n = 21) or without a donor (n = 67), had very poor results: the 7-year DFS rate was 14% versus 24%, respectively. This finding may be the consequence of their application of late transplantation. Early application of an allo-SCT by stem cells from a related or unrelated donor might be indicated in this subgroup of patients with AML.
In the younger patient groups the difference between the 2 transplantation approaches was most prominent, because of a low TRM and relapse rate among those who had a sibling donor. In contrast, in patients aged 36 to 45 years, the TRM incidence in the donor group was as high as 21%, which led to similar DFS rates in the donor and no donor groups; adjustment by cytogenetics confirms these findings. Similar observations have been reported by the MRC.25
The superiority of early allo-SCT over early auto-SCT using identical conditioning regimens is likely because of a graft-versus-leukemia effect. However, the advantage of the increased antileukemic effect of allo-SCT is diminished by increased iatrogenic mortality of the transplantation procedure. However, several new developments have been introduced in the transplantation procedure, such as nonmyeloablative conditioning,30 and SCT followed by adapted doses of donor lymphocyte transfusions.31 These approaches may reduce the morbidity and mortality from conditioning and graft-versus-host disease, while maintaining the graft-versus-leukemia effect. Trials with these modified techniques in the good and intermediate risk groups of patients with AML are warranted.
Appendix
The list of the members of the EORTC or GIMEMA groups who participated in this study: Drs de Witte and Muus (Nijmegen), Dr Zittoun (Paris), Dr Labar (Zagreb), Dr Belhabri (Lyon), Drs Delarue and Varet (Paris), Dr. Sinnige (Den Bosch), Dr Bourhis (Paris), Dr Jehn (Muenchen), Dr Hagemeijer (Leuven), Dr Willemze (Lieden), Dr Vreugdenhil (Veldhoven), Drs Bron and Stryckmans† (Brussels), Dr Selleslag (Brugge), Dr De Bock (Antwerpen), Dr Berneman (Antwerpen), Dr Vermeulen (Verviers), Dr Feremans (Brussels), Dr Andrien (Liège), Dr Fillet (Liège), Dr Thyss (Nice), Dr Dreyfus (Paris), Dr. Roozendaal (Amsterdam), Drs Mandelli and Petti (Rome), Dr Gallo (Torino), Dr De Rosa (Napoli), Dr Giustolisi (Catania), Dr Liso (Bari), Dr Mirto (Palermo), Dr Leone (Roma), Dr Fioritoni (Pescara), Dr Amadori (Roma), Drs Fazi and Vignetti (Roma), Dr Rossi Ferrini (Firenze), Dr Volpe (Avellino), Dr Leoni (Ancona), Dr Broccia (Cagliari), Dr Peta (Catanzaro), Dr Mariani (Palermo), Dr Nobile (Reggio Calabria), Dr Damasio (Genova), Dr Rizzoli (Parma), Dr Mettivier (Napoli), Dr Ferrara (Napoli), Dr De Blasio (Latina), Dr Martelli (Perugia), Dr Ricciuti (Potenza), Dr Majolino (Roma), Dr Gabbas (Nuoro), Dr Lucarelli (Pesaro), Dr Bordignon (Milano), Dr Bodini (Cremona), Dr Gallamini (Cuneo), Dr Monaco (Foggia), Dr Mozzana (Gallarate), Dr Saglio (Orbassano), Dr Mazza (Taranto), and Dr Carotenuto† (San Giovanni Rotondo). † indicates deceased.
Prepublished online as Blood First Edition Paper, April 24, 2003; DOI 10.1182/blood-2002-12-3714.
Supported in part by grants from the Kay Kendall Foundation, the Leukemia Cooperative Group of the EORTC, and the National Cancer Institute (grants 5U10-CA11488-23 through 5U10-CA11488-32).
A list of the members of the EORTC and GIMEMA Leukemia Groups appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Saint Jude Children's Research Hospital for providing an SAS macro, allowing the computation of the cumulative incidences of relapse and of death in CR. We thank the data managers of the EORTC (Mr Solbu, Ms Dardenne, Ms Rodts) as well. We thank the cytogeneticists of the different institutions, in particular A. Bernheim (Villejuif), M. Mancini (Rome), and D. Olde-Weghuis (Nijmegen), for the review of karyotypes.
Contents of this study are solely the responsibility of the authors and do not represent the official views of the National Cancer Institute (Bethesda, MD).



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal