Abstract
Relative quiescence is a defining characteristic of hematopoietic stem cells. Reasoning that inhibitory tone dominates control of stem cell cycling, we previously showed that mice engineered to be deficient in the cyclin-dependent kinase inhibitor, p21Cip1/Waf1 (p21), have an increased stem cell pool under homeostatic conditions. Since p21 was necessary to maintain stem cell quiescence and its absence sufficient to permit increased murine stem cell cycling, we tested whether reduction of p21 alone in human adult–derived stem cells could affect stem cell proliferation. We demonstrate here that interrupting p21 expression ex vivo resulted in expanded stem cell number and in vivo stem cell function compared with control, manipulated cells. Further, we demonstrate full multilineage reconstitution capability in cells where p21 expression was knocked down. Therefore, lifting the brake on cell proliferation by altering cell cycle checkpoints provides an alternative paradigm for increasing hematopoietic stem cell numbers. This approach may be useful for relative ex vivo human stem cell expansion.
Introduction
Relative quiescence is a defining characteristic of hematopoietic stem cells that is critical to prevent exhaustion under conditions of stress, yet severely constrains ex vivo stem cell expansion and gene therapy strategies. Hematopoietic stem cells capable of repopulating the marrow of transplant recipients are mainly in the quiescent phase of the cell cycle.1-4 Activation of stem cells by various combinations of hematopoietic growth factors as used in many current ex vivo stem cell expansion protocols generally results in a decrease of in vivo repopulating ability compared with unmanipulated hematopoietic cells.5-8 Cell differentiation and a loss of multipotentiality are often noted after cytokine treatment and cell cycle progression.
Cyclin-dependent kinase inhibitors (CKIs) participate in the regulation of the cell cycle by sequential activation or inactivation of cyclin-dependent kinases and have essential roles in arresting cell cycle progression in different systems.9-11 Of the 2 known families of CKIs, the cip/kip family, consisting of p21cip1 (p21), p27kip1 (p27), and p57kip,2 plays critical roles in the regulation of cell cycle kinetics in the hematopoietic cascade.
We demonstrated that p21 is a molecular mediator of quiescence in hematopoietic stem cells governing the restriction into entry of the cell cycle.12 Mice engineered to be deficient in p21 have increased absolute numbers of stem cells that are more actively cycling and more sensitive to exhaustion under conditions of stress. In contrast, p27 does not affect stem cell function but markedly alters progenitor cell proliferation and pool size.13 The ability of p21 to singularly regulate stem cell cycling kinetics was unanticipated given the expected redundancy of the CKI system. These findings and the recent demonstration that transforming growth factor β (TGF-β) affects primitive cell proliferation independent of p2114 suggested p21 as a target molecule to affect stem cell cycling. A proposed alternative strategy of stem cell expansion by disruption of the dominant antiproliferative tone induced by p21 was therefore tested.
We transduced CD34+ and CD34+38– cord blood cells with a vesicular stomatitis virus G-glycoprotein (VSV-G)–pseudotyped lentiviral vector containing full-length p21-antisense. Transduction of p21-antisense led to a release of cell cycle inhibition in quiescent stem cells and a relative expansion of the stem cell pool was demonstrated as measured by semiquantitative in vitro and in vivo stem cell assays when compared with empty vector–transduced cells. In contrast, p21-antisense did not alter colony-forming cell (CFC) number or differentiation potential, implying that p21 has a differentiation stage-specific function in hematopoietic stem cells and that induction of stem cell proliferation by p21-antisense is not necessarily linked to differentiation. This study validates the model that dominant inhibitory tone governs stem cell kinetics and points to p21 as an important molecular target for manipulating stem cell expansion ex vivo.
Materials and methods
Cells and cell culture
Cells were obtained from umbilical cord blood after normal full-term deliveries or from bone marrow harvests of healthy adult volunteers in accordance with St Louis University institutional review board approval. Samples were diluted in phosphate-buffered saline (PBS) and enriched for mononuclear cells by centrifugation on Ficoll/Paque (Pharmacia-Biotech, Uppsala, Sweden). CD34+ cells were enriched by immunomagnetic selection according to the manufacturer's instructions (Miltenyi Biotec, Bergisch-Gladbach, Germany) with a purity in the selected product of 95% consistently. CD34+38– cells were further enriched after staining with CD34–fluorescein isothiocyanate (FITC) and CD38–phycoerythrin (PE) (Becton Dickinson, San Jose, CA) by fluorescence-activated cell sorting (FACS Vantage; Becton Dickinson).
Human embryonic kidney–derived 293T cells and a mouse p21–/– fibroblast cell line were grown in Dulbecco modified Eagle medium (DMEM) supplemeted with 10% fetal calf serum (FCS), 100 U/mL penicillin, 100 U/mL streptomycin, and 2 mM l-glutamine (GIBCO, BRL, Rockville, MD). The human p53-deficient megakaryoblastic leukemia cell line (CMK) was grown in RPMI supplemented with 20% FCS, 100 U/mL penicillin, 100 U/mL streptomycin, and 2 mM l-glutamine (GIBCO, BRL).
Lentiviral vectors and constructs
cDNA encoding full-length p21 was subcloned as antisense into the BamH1 cloning site of the lentiviral vector pHR-CMV-GFP.15 The lentiviral vector construct resulted in a fusion gene of the p21-AS-GFP with no intervening internal ribosomal entry site (IRES). Unmanipulated lentiviral vector pHR-CMV-GFP was used as control.
Lentviral production and transduction
The lentiviral vector containing p21 antisense (p21-AS-V) or the control vector (GFP-V) were cotransfected into 293T cells with pCMV encoding the gag and pol proteins, and pCMV-VSV-G, a plasmid encoding the VSV-G, using the Geneporter lipofection method according to the manufacturer's instructions (Gene Therapy Systems, San Diego, CA). Supernatants containing pseudotyped lentiviruses were collected at 72 hours after the beginning of transfection and were used for the transduction of human CD34+ and CD34+38– hematopoietic cells.
CD34+ and CD34+38– cells were cultured in Iscoves modified Dulbecco medium (IMDM) containing 10% FCS (Sigma, St Louis, MO) (IMDM 10), 100 U/mL penicillin, 100 U/mL streptomycin, and 2 mM l-glutamine (GIBCO, BRL) supplemented with stem cell factor (SCF, 50 ng/mL), Flt-3-ligand (Flt-3-L, 50 ng/mL), thrombopoietin (TPO, 25 ng/mL), and interleukin-3 (IL-3, 10 ng/mL) (R&D Systems, Minneapolis, MN) for 24 hours on Retronectin (Takara, Otsu, Japan)–coated wells. After this prestimulation, two thirds of the culture medium was discarded and replaced with the virus containing supernatant plus Polybrene (final concentration 4 μg/mL; Sigma). The cells were then spinocculated at 1700 revolutions per minute for 30 minutes, incubated at 37°C and 5% CO2 for an additional 20 hours, then washed and plated on fresh Retronectin-coated wells in IMDM 10 plus cytokines overnight. A second transduction was performed on the following day using the same procedure. In early experiments, a mock transduction was performed, but this was not continued when substantial background GFP staining was noted. Cells were handled in the same manner regardless of their tissue source.
Flow cytometric analysis
Flow cytometry was used to estimate the transduction efficiency and content of stem cells in the transduced cell population at multiple time points after the beginning of the transduction with the optimal GFP expression noted at day 4 and shown here. Cells were stained with CD34–peridinin chlorophyll protein (PerCP) and CD38-allophycocyanin (APC) (Becton Dickinson) and incubated with propidium iodide (PI) shortly prior to the flow cytometric analysis, to distinguish between viable and dead cells.
To quantify the repopulation ability of the transplanted transduced CD34+ cord blood cells in the peripheral blood and the bone marrow of the animals that underwent transplantation (nonobese diabetic–severe combined immunodeficiency [NOD/SCID] repopulation assay), bone marrow nucleated cells were labeled with the human leukocyte antibody CD45-PerCP and differentiation markers (CD3-APC, CD14-APC, CD19-APC, CD33-APC, and CD38-APC; Becton Dickinson). The viability of the stained cells was measured by staining with PI and gating on PI-negative cells. The stained cell samples were analyzed on a FACScalibur cytometer (Becton Dickinson).
Flow cytometric analysis of cell cycle status and apoptosis
Transduced CD34+ cord blood cells were stained with CD34-PerCP and CD38-APC followed by an incubation with a DNA-dye Hoechst33342 (Hst, 1.67 μM; Sigma) and an RNA-dye, PyroninY (PY, 1 μg/mL).2 The proportion of cells in G0 (PYlow Hoechstlow) was measured in the CD34+38– cell subpopulation, representing quiescent primitive hematopoietic cells. The proportion of apoptotic cells in the CD34+38– subpopulation was measured by flow cytometric analysis after staining with CD34-PE and CD38-APC (Becton Dickinson), followed by incubation with AnnexinV–Alexa350 and 7-amino actinomycin D (7-AAD).
Western blot analysis
CMK cells (2 × 105) were transduced with p21-AS-V, p21-Sense-V and the control vector as described above and cultured for 72 hours. Then cells were split and transduced a second time. After another 72 hours of expansion, cells were sorted for GFP positivity. The GFP-positive fractions were replated and stimulated with 12-O-tetradecanoylphorbol-13-acetate (TPA, 4 nM; Sigma) for 48 hours to induce p21 expression.16 After this time, transduced cells or controls were lysed in an ELB lysis buffer. Total protein per sample (100 μg) was separated in 12% denaturating polyacralamide gel, blotted on an Immobilon-P transfer membrane (Millipore, Bedford, MA) and probed with a rabbit polyclonal antimouse p21 antibody (kind gift from Dr C. Schneider, Trieste, Italy) overnight at 1:2500 dilution. The enhanced chemiluminescence (ECL) Western blotting analysis system (Amersham Pharmacia-Biotech) based on a horseradish peroxidase–labeled secondary antirabbit antibody (1:2000 dilution) was used for detection.
Reverse transcriptase–polymerase chain reaction (RT-PCR) of transduced cells
To confirm that transduction of the p21-AS-V leads to decreased expression of p21, CMK cells (2 × 106) were transduced with p21-AS-V and the control vector followed by a stimulation with TPA (100 nM) 24 hours after the beginning of transduction. After an additional 24 hours, cells were lysed in Trizol (GIBCO, BRL) and total RNA was isolated according to the manufacturer's instructions. Total RNA (1.5 μg) was digested with DNaseI (GIBCO, BRL) for 15 minutes followed by a reverse-transcriptase reaction in a total volume of 40 μL containing 1 × RT buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 8.3; 75 mM KCl; 3 mM MgCl2), 10 mM dithiothreitol, 0.5 mM deoxynucleoside triphosphate (dNTP), 50 U RNA guard, 200 U/μL reverse transcriptase (Superscipt II, GIBCO, BRL), and 100 pmol 3′-p21 primer (5′-CGTTTTCGACCCTGAGAGAGTC-3′) for 5 minutes at 30°C, 50 minutes at 42°C, and 5 minutes at 95°C. Then, 2 μL of the sample was amplified in a 50-μL reaction containing 1 × PCR buffer (Perkin Elmer, Warrington, United Kingdom), 0.2 mM dNTP, 20 pmol of primers (p21-5′: 5′-GCGATGGAACTTCGACTTTG-3′ and the same p21-3′ primer as mentioned above), and 2.5 U Taq polymerase (Perkin Elmer) under the following conditions: 94°C for 5 minutes; 25 cycles at 94°C for 1 minute, 60°C for 1 minute, and 72°C for 1.5 minutes; extension was at 72°C for 10 minutes. Of each sample, 10 μL was loaded onto a 1.5% agarose gel and stained with ethidium bromide.
Real-time quantitative RT-PCR
Real-time quantitative reverse transcription–polymerase chain reaction was performed using TaqMan one-step RT-PCR Master Mixes on the ABI Prism 7700 sequence detector (PE Biosystems, Foster City, CA). The primer set for detection of human p21 mRNA sequences was as follows: p21 forward: 5′-AATCCCAGCTACTTGGAAGGC-3′; p21 reverse: 5′-GCTGACTGCAACCTCTGCC-3′; and p21 probe: 5′-(FAM)AGGTGGGAGGATCACTTGAACCCAGG(BHQ)-3′. Human cellular glyceraldehyde-3′-phosphate dehydrogenase (GAPDH) mRNA was used as the endogenous control for each RT-PCR reaction, and the primers and probe used to amplify and detect GAPDH were from TaqMan GAPDH Control Reagent kit (PE Biosystems). The total cellular RNA from each sample was extracted, treated with DNase 1, and purified with Qiagen RNeasy mini kit (Qiagen, Valencia, CA). An equal amount of purified RNA (50 ng) in quadruplicate from each sample was examined in each experiment. A standard curve of the amplicon being measured was run in duplicate range from 1 to 1 × 1010 copies plus a no-template control in each assay. The RNA standards were spiked with yeast tRNA (Sigma) to bring up the total RNA to 50 ng per standard curve point. The reaction was in a 50-μL volume and included an initial reverse transcription at 48°C for 30 minutes and an incubation at 95°C for 10 minutes; 40 cycles of amplification were carried out at 15 seconds at 95°C followed by 1 minute at 60°C. (A separate real-time PCR assay was used to confirm no DNA contamination in the RNA samples before the initial reverse-transcription reaction.) The amplicons from each reaction were analyzed using Sequence Detector V1.7 software (PE Biosystems). The threshold cycle (CT) value from each reaction was determined and compared with the comparative CT method.
Colony-forming assay
Transduced CD34+ and CD34+38– cells were cultured in 0.8% methylcellulose, 30% fetal bovine serum, 1% bovine serum albumin, 0.1 mM 2-mercaptoethanol, 2 mM l-glutamine on α–modified essential medium (α-MEM) semisolid matrix culture medium supplemented with cytokines (50 ng/mL SCF, 10 ng/mL IL-3, 10 ng/mL IL-6, and 4 U/mL erythropoietin [EPO]; StemCell Technologies, Vancouver, BC, Canada). Cells were plated at 500 or 600 cells/mL into 24-well plates and incubated at 37°C and 5% CO2. At day 10, colonies were scored by phase microscopy and reported as CFCs.
Long-term culture with limiting dilutions
To quantify primitive hematopoietic cells in the transduced CD34+ and CD34+38– cell population, we adapted the cobblestone area–forming cell (CAFC) assay17 with minor modifications as follows. To prepare stromal layers, human bone marrow nucleated cells were cultured at 33°C in long-term culture (LTC) medium (α-MEM with 12.5% horse serum, 12.5% fetal bovine serum, 0.2 mM I-inositol, 20 mM folic acid, 0.1 mM 2-mercaptoethanol, 2 mM l-glutamine, and 1 μM hydrocortisone; StemCell Technologies). After 4 to 8 weeks the confluent stromal layers were trypsinized, irradiated (15 Gy), and subcultured in 96-well plates at a density of 2.5 × 104 cells per well. The transduced CD34+ and CD34+38– cells were then seeded with 2-fold diluted single-cell suspensions in the same LTC medium. Half of the medium was replaced weekly and the CAFCs were scored until the sixth week.17 For those experiments in which LTC-initiating cells (LTC-ICs) were measured, the semisolid, cytokinecontaining methylcellulose medium for CFC assays was overlaid into the wells at week 5 and the colonies were counted after a further 10 days. A limiting dilution analysis software (Maxrob, kindly provided by Dr Julian Down; BioTransplant, Boston, MA) was used to calculate the frequency of LTC-ICs in the cell population.
Liquid culture
To examine the effect of p21-antisense on the differentiation and expansion of hematopoietic cells, transduced CD34+ and CD34+38– cord blood cells were cultured in IMDM 10 supplemented with 50 ng/mL SCF, 50 ng/mL Flt-3-L, and 10 ng/mL TPO. Weekly, the medium was replaced and half of the cells were taken for further analysis. To measure the proportion of primitive cells in the liquid culture, cells were stained with CD34-PerCP and CD38-APC, incubated with PI to distinguish between viable and dead cells, and analyzed by flow cytometry.
NOD/SCID repopulation assay
To evaluate the repopulation ability of the transduced human CD34+ cells, we used a NOD/SCID repopulation assay. NOD/SCID mice (Jackson Laboratories, Bar Harbor, ME) were handled under sterile conditions and maintained under microisolaters. Transduced CD34+ umbilical cord blood cells were transplanted by tail-vein injection into sublethal irradiated (3.5 Gy) 8-week-old mice along with 2 × 106 irradiated (20 Gy) nonrepopulating human bone marrow mononuclear cells. Every 2 weeks after the first month 200 μL peripheral blood was obtained from each recipient mouse by tail bleeds. The blood was stained with CD45-PerCP, CD34-APC (Becton Dickinson) antibodies, treated with a lysis buffer (ACK Lysis buffer), incubated with PI to distinguish between viable and dead cells, and analyzed by flow cytometry to detect human-derived hematopoietic progenitors (FACS-Calibur; Becton Dickinson). Mice were killed 8 to 12 weeks after transplantation. Bone marrow from femurs and tibiae of each mouse was flushed into IMDM containing 10% FCS and analyzed by flow cytometry (FACS-Calibur).
Statistical analysis
The significance of the difference between groups in the in vitro and in vivo experiments was evaluated by analysis of variance followed by a 2-tailed Student t test.
Results
Lentiviral p21-antisense expression and function in human hematopoietic cells
cDNA encoding full-length p21cip1 (p21) was subcloned in antisense orientation into the BamHI cloning site of pHR-CMV-GFP (GFP-V), a lentiviral vector that allows coexpression of subcloned cDNAs and green fluorescent protein (GFP) from a single mRNA transcript.15
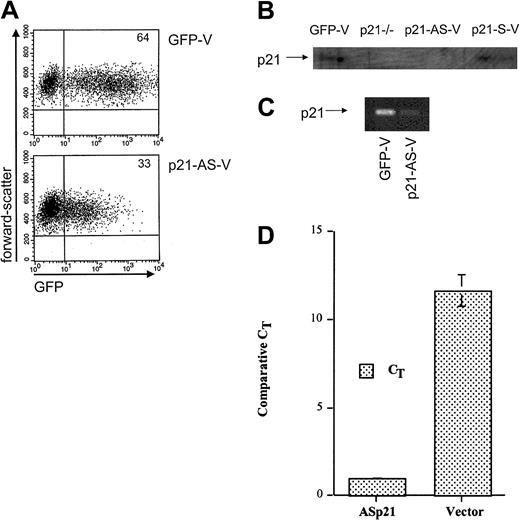
The transduction efficiency of human CD34+ and CD34+38– cord blood cells by p21-AS-V was measured by flow cytometric analysis. Independent experiments showed a transduction effi-ciency of 45% to 55% for the control vector (GFP-V) and 25% to 35% for the p21-AS-V lentiviruses 4 days after the beginning of transduction using either cord blood– or bone marrow–derived cells (Figure 1A). Mock-transfected cells did not demonstrate background GFP staining. To demonstrate the ability of the construct to reduce p21 expression in hematopoietic cells, analysis of p21-AS-V–transduced hematopoietic cells was performed. Assessing the biochemical effect of the antisense construct on p21 levels in primary cells was limited by the inability of anti-p21 antibodies to stain permeabilized cells for flow cytometric analysis with appropriate specificity. We therefore used the human hematopoietic cell line CMK, which can be induced to express p21 with TPA treatment and assessed by Western blot. The p21-AS-V–transduced cells had markedly reduced p21 levels, comparable with that seen with p21–/– fibroblast controls (Figure 1B). The reduction in p21 was shown by RT-PCR analysis to be due to reduced expression (Figure 1C).
Lentiviral transduction and expression of p21-antisense results in decreased intracellular p21. Flow cytometric analysis for GFP in transduced CD34+ cord blood cells 4 days after transduction. Cells were transduced with a lentiviral vector containing p21-antisense (p21-AS-V) or the control vector (GFP-V). Plots represent fluorescence intensity for GFP on the x-axis and cell forward scatter on the y-axis. Numbers in corners indicate percent of events in that quadrant. (B) Western blot analysis of p21–/– fibroblasts (p21–/–) or CMK cells transduced twice with control vector (GFP-V), p21-antisense (p21-AS-V), or p21-sense (p21-S-V) incubated with TPA for 48 hours, sorted for GFP expression, and then lysed for Western blot analysis. Total protein (100 μg) was used for each sample. (C) RT-PCR analysis for p21 in CMK cells transduced with a lentiviral vector containing p21-antisense (p21-AS-V) or with the control vector (GFP). Cells were stimulated with 100 nM TPA 24 hours after transduction and RNA was isolated from cells after sorting for GFP+ cells. (D) Real-time RT-PCR of primary CD34+38– cells transduced with either p21-AS-V or GFP control vector. Data are expressed as relative to GAPDH expression levels in the same population of cells measured simultaneously.
Lentiviral transduction and expression of p21-antisense results in decreased intracellular p21. Flow cytometric analysis for GFP in transduced CD34+ cord blood cells 4 days after transduction. Cells were transduced with a lentiviral vector containing p21-antisense (p21-AS-V) or the control vector (GFP-V). Plots represent fluorescence intensity for GFP on the x-axis and cell forward scatter on the y-axis. Numbers in corners indicate percent of events in that quadrant. (B) Western blot analysis of p21–/– fibroblasts (p21–/–) or CMK cells transduced twice with control vector (GFP-V), p21-antisense (p21-AS-V), or p21-sense (p21-S-V) incubated with TPA for 48 hours, sorted for GFP expression, and then lysed for Western blot analysis. Total protein (100 μg) was used for each sample. (C) RT-PCR analysis for p21 in CMK cells transduced with a lentiviral vector containing p21-antisense (p21-AS-V) or with the control vector (GFP). Cells were stimulated with 100 nM TPA 24 hours after transduction and RNA was isolated from cells after sorting for GFP+ cells. (D) Real-time RT-PCR of primary CD34+38– cells transduced with either p21-AS-V or GFP control vector. Data are expressed as relative to GAPDH expression levels in the same population of cells measured simultaneously.
To confirm that the reduction in p21 expression observed in the CMK cell line was reproduced in primary cells, we used cord blood CD34+38– cells transduced with either control or p21-AS-V and evaluated them by real-time RT-PCR. Cells exposed to the AS encoding vector demonstrated a more than 90% reduction in p21 expression relative to GAPDH control in 3 independent experiments (Figure 1D). These data support selective down-regulation of p21 in the context of the p21-AS-V in primitive primary human hematopoietic cells.
p21-antisense reduces the G0 fraction of transduced CD34+ cord blood cells
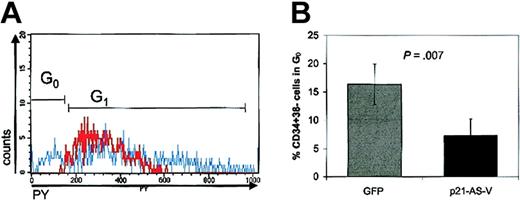
To evaluate the ability of p21-antisense to alter cell cycle kinetics in hematopoietic stem cells, we analyzed the cell cycle status of transduced CD34+ cord blood cells by simultaneously staining with DNA and RNA dyes, which allows the distinction between cells in G0 and G1.2,12 Cells determined to be in the G0/G1 phase of the cell cycle based on the Hoechst (Hst) fluorescence distribution can be further fractionated into subcompartments of varying cellular RNA content by staining with PY. Quiescent cells, in G0, have a low RNA content that increases as cells progress through G1. RNA accumulates until cells move to the S/G2+M phase during which Hst staining increases. In 6 independent experiments, transduction of p21-antisense decreased the proportion of cells in G0 in the CD34+38– subpopulation of transduced CD34+ cord blood cells (7.3% p21-AS-V vs 16.4% GFP-V; P = .007) (Figure 2A-B). These data indicate that p21-antisense promotes the entry of quiescent cells into the cell cycle, reducing the fraction of cells in G0.
p21-antisense reduces the G0 fraction of transduced CD34+ cord blood cell population. (A) Histogram of cellular RNA staining by Pyronin Y (PY) in the HoechstlowCD34+38– cell fraction of CD34+ cord blood cells transduced with a lentiviral vector containing p21-antisense (red line) or with the control vector (blue line) 4 days after beginning of transduction. PYlow cells represent cells in G0. Chart shows a representative experiment. (B) Mean ± SEM of CD34+38– cells in G0 after transduction of CD34+ cord blood cells with p21-AS-V or the control vector, GFP (n = 6).
p21-antisense reduces the G0 fraction of transduced CD34+ cord blood cell population. (A) Histogram of cellular RNA staining by Pyronin Y (PY) in the HoechstlowCD34+38– cell fraction of CD34+ cord blood cells transduced with a lentiviral vector containing p21-antisense (red line) or with the control vector (blue line) 4 days after beginning of transduction. PYlow cells represent cells in G0. Chart shows a representative experiment. (B) Mean ± SEM of CD34+38– cells in G0 after transduction of CD34+ cord blood cells with p21-AS-V or the control vector, GFP (n = 6).
p21-antisense increases the primitive hematopoietic cell compartment in transduced CD34+ and CD34+38– cord blood cells in vitro
We next sought to define the impact of p21-antisense on the differentiation status of transduced CD34+ and CD34+38– cord blood cells by in vitro functional arrays. All comparisons were performed pairwise on cells from the same cord blood sample. Comparisons on fresh, unmanipulated cells were not performed, as the intersample variability is large in cord blood. Furthermore, it should be noted that functional studies were performed on all transduced cells, not a subselected GFP-expressing fraction. GFP becomes maximally detectable 4 days following transduction and sorting would have required culture of the cells for a total of 7 days, an interval not compatible with maintaining the stem cell phenotype and function.
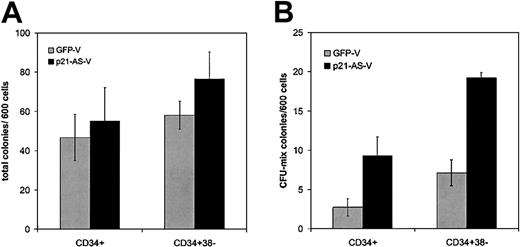
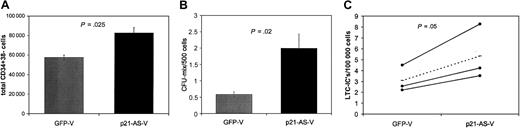
Transduced cells were analyzed for their ability to generate colonies using methylcellulose colony-forming (CFC) assays for progenitor function. Transduced cells were plated 4 days after the beginning of transduction in semisolid CFC medium. Neither CD34+ (n = 4) nor CD34+38– (n = 4) cells transduced with p21-antisense showed an altered total colony number compared with cells transduced with the control vector (Figure 3A). The trend in CFC change may have been more pronounced if only GFP+ cells were used, but all analyses were performed on unfractionated cells. Of note, however, there was a higher proportion of more primitive colony-forming unit (CFU)–mix colonies (with both erythroid and granulocyte/monocyte cells) among the colonies generated from p21-AS–expressing cells compared with those generated from controls (CD34+: 9.3 vs 2.8 colonies/600 cells, P = .02; CD34+38–: 19.2 vs 7.1 colonies/600 cells, P = .002) (Figure 3B).
p21-antisense increases primitive colony-forming cells without altering total colony number in CFC assays. CD34+ or CD34+38– cord blood cells were transduced with p21-antisense or the control vector. After transduction the cells were plated in semisolid, cytokine-containing CFC medium. Colonies were scored after 10 days. (A) Mean ± SEM of total colonies per 600 transduced cells (n = 4). (B) Mean ± SEM colonies containing mixed red and white hematopoietic cells (CFU-mix) per 600 transduced cells (n = 4).
p21-antisense increases primitive colony-forming cells without altering total colony number in CFC assays. CD34+ or CD34+38– cord blood cells were transduced with p21-antisense or the control vector. After transduction the cells were plated in semisolid, cytokine-containing CFC medium. Colonies were scored after 10 days. (A) Mean ± SEM of total colonies per 600 transduced cells (n = 4). (B) Mean ± SEM colonies containing mixed red and white hematopoietic cells (CFU-mix) per 600 transduced cells (n = 4).
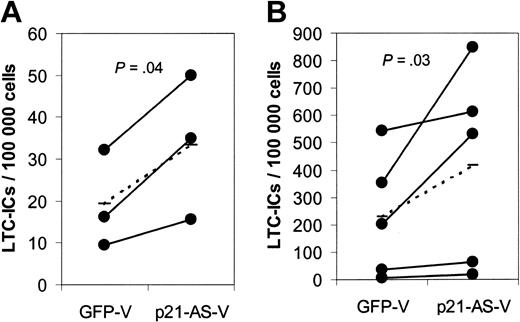
To quantify the stem cell frequency in the transduced CD34+ and CD34+38– cell population, we performed long-term cultures with limiting dilutions on primary human bone marrow stroma (LTC-IC assay). CD34+ and CD34+38– cells transduced with p21-antisense gave rise to significantly higher numbers of long-term culture-initiating cells (LTC-ICs) compared with cells transduced with the control vector, indicating a higher proportion of stem cells in the p21-antisense–transduced cell population (Figure 4, CD34+: 33.5 vs 19.3 LTC-ICs/100 000 cells (P = .04); CD34+38–: 416 vs 228 LTC-ICs/100 000 cells [P = .03]).
p21-antisense increases the frequency of long-term primitive culture-initiating cells (LTC-ICs) in transduced CD34+ and CD34+38– cord blood cells. Transduced CD34+ and CD34+38– cord blood cells were plated on irradiated human stroma in limiting dilutions. After 5 weeks the medium was replaced with semisolid CFC medium. Frequency of LTC-ICs in p21-antisense (p21-AS-V)– and control vector (GFP-V)–transduced CD34+ (A) or CD34+38– (B) cord blood cells (n = 3 CD34+ cells, n = 5 CD34+38– cells; dotted line represents average) is shown.
p21-antisense increases the frequency of long-term primitive culture-initiating cells (LTC-ICs) in transduced CD34+ and CD34+38– cord blood cells. Transduced CD34+ and CD34+38– cord blood cells were plated on irradiated human stroma in limiting dilutions. After 5 weeks the medium was replaced with semisolid CFC medium. Frequency of LTC-ICs in p21-antisense (p21-AS-V)– and control vector (GFP-V)–transduced CD34+ (A) or CD34+38– (B) cord blood cells (n = 3 CD34+ cells, n = 5 CD34+38– cells; dotted line represents average) is shown.
The results from CFC and LTC-IC assays above demonstrate that p21-AS overexpression leads to an increase of primitive hematopoietic cells in comparison with control vector–transduced cells. Thus, p21-antisense expands or preserves primitive hematopoietic cells measured by functional assays in vitro. However, these comparisons were between manipulated populations of cells. It cannot be definitively stated that the antisense-treated populations exceeded those in the fresh unmanipulated specimen.
p21-antisense expands CD34+38– cells in liquid culture
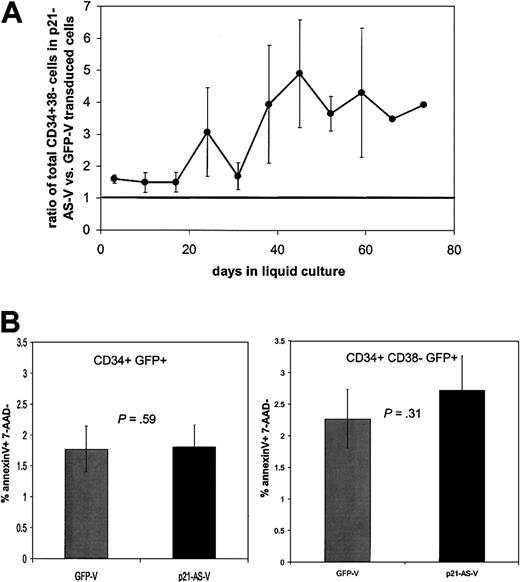
To examine the effect of p21-antisense on the differentiation and expansion of primitive hematopoietic cells, transduced CD34+ and CD34+38– cord blood cells were cultured in IMDM 10 supplemented with early-acting cytokines SCF, Flt-3-L, and TPO. The medium was replaced weekly and half of the cells were analyzed by flow cytometry to determine the proportion of CD34+38– cells in the expanded cell population. Transduction of p21-antisense led to an expansion of primitive CD34+38– subpopulation in both cultured CD34+ and CD34+38– cord blood cells in comparison with control vector–transduced cells. The relative abundance of CD34+38– cells in p21-antisense–transduced cells compared with control vector–transduced cells peaked after 5 to 6 weeks of culture in transduced CD34+38– cells (Figure 5A) and about 3 weeks in transduced CD34+ cells. These data should be interpreted with caution, however, given the known variability of CD38 expression in vitro.
p21-antisense increases the relative abundance of CD34+38– cells (in transduced CD34+ cord blood cells without alteration in apoptotic rate). CD34+ cord blood cells were transduced with p21-antisense (p21-AS-V) or the control vector (GFP-V) and maintained in culture. (A) The ratio of total CD34+38– cells in the p21-AS-V versus control over time is shown. Horizontal line indicates equivalence. (B) Apoptosis assays were performed 4 days following transduction using AnnexinV and 7-AAD. Chart shows the percentage of AnnexinV-positive cells in the CD34+GFP+7AAD– subpopulation (n = 13) (left chart) and in the CD34+38– GFP+7AAD– subpopulation (right chart).
p21-antisense increases the relative abundance of CD34+38– cells (in transduced CD34+ cord blood cells without alteration in apoptotic rate). CD34+ cord blood cells were transduced with p21-antisense (p21-AS-V) or the control vector (GFP-V) and maintained in culture. (A) The ratio of total CD34+38– cells in the p21-AS-V versus control over time is shown. Horizontal line indicates equivalence. (B) Apoptosis assays were performed 4 days following transduction using AnnexinV and 7-AAD. Chart shows the percentage of AnnexinV-positive cells in the CD34+GFP+7AAD– subpopulation (n = 13) (left chart) and in the CD34+38– GFP+7AAD– subpopulation (right chart).
p21-antisense– and control vector–transduced cells have equivalent apoptotic rates in culture
To assess whether the changes in cell abundance in liquid culture were due to altered rates of cell death, we performed AnnexinV and 7-AAD analysis. No significant differences were noted between the groups when examining either the CD34+ (left panel) or CD34+38– (right panel) cells (Figure 5B).
p21-antisense increases primitive hematopoietic cell compartment in transduced bone marrow–derived CD34+ cells
Umbilical cord blood CD34+ cells residing in G0 and G1 phases of the cell cycle show similar stem cell characteristics,18 whereas adult BM CD34+ cells in G1 have a significantly diminished stem cell capacity in comparison with BM CD34+ cells in G0.2 Therefore, we sought to validate the p21-AS–mediated expansion of primitive hematopoietic cells in adult bone marrow CD34+ cells. As was observed in cord blood cells, transduction of p21-antisense into BM CD34+ led to an increase of primitive hematopoietic cells represented by a higher total number of CD34+38– cells, a higher frequency of CFU-mix colonies, and increased LTC-ICs in comparison with control vector–transduced cells (Figure 6). These findings demonstrate the ability of p21-antisense to increase primitive hematopoietic cells in adult bone marrow cells in addition to umbilical cord blood cells.
p21-antisense increases primitive hematopoietic cells in transduced CD34+ human bone marrow cells. CD34+ human bone marrow cells were transduced with a lentiviral vector containing p21-antisense (p21-AS-V) or the control vector (GFP-V). At 4 days after transduction cells were analyzed by flow cytometric analysis and in vitro assays. (A) Transduced cells were stained for CD34 and CD38 and incubated with PI. Chart shows the mean ± SEM of total CD34+38– PI– cells after transduction. (B) Transduced cells were analyzed in a CFC assay for progenitor frequency. Chart shows mean ± SEM of CFU-mix representing primitive colony-forming cells per 500 transduced cells. (C) Transduced cells were analyzed in an LTC-IC assay for stem cell frequency. Chart shows the LTC-IC frequency per 100 000 transduced cells. Dashed line represents the average.
p21-antisense increases primitive hematopoietic cells in transduced CD34+ human bone marrow cells. CD34+ human bone marrow cells were transduced with a lentiviral vector containing p21-antisense (p21-AS-V) or the control vector (GFP-V). At 4 days after transduction cells were analyzed by flow cytometric analysis and in vitro assays. (A) Transduced cells were stained for CD34 and CD38 and incubated with PI. Chart shows the mean ± SEM of total CD34+38– PI– cells after transduction. (B) Transduced cells were analyzed in a CFC assay for progenitor frequency. Chart shows mean ± SEM of CFU-mix representing primitive colony-forming cells per 500 transduced cells. (C) Transduced cells were analyzed in an LTC-IC assay for stem cell frequency. Chart shows the LTC-IC frequency per 100 000 transduced cells. Dashed line represents the average.
p21-antisense enhances the NOD/SCID repopulation ability of transduced CD34+ cord blood cells
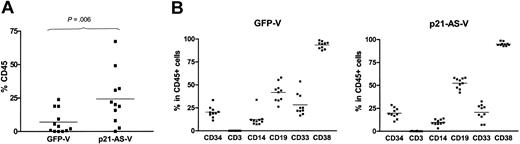
The marrow repopulating ability of p21-antisense– and control vector–tranduced CD34+ cord blood cells was assessed by transplanting equal numbers of either cell fraction into conditioned NOD/SCID recipients. In 2 separate experiments a total of 23 mice received transplants of CD34+ cord blood cells transduced with the control vector12 and p21-antisense11 using 6 × 105 to 2 × 106 cells per animal. Human cell engraftment was evaluated 8 to 12 weeks after transplantation by flow cytometric determination of human CD45+ cells in cell suspensions of the bone marrow harvested from recipient animals. The bone marrow of animals that received transplants of p21-antisense–transduced CD34+ cells showed a 3-fold higher engraftment than the controls, measured by the proportion of CD45+ cells (p21-AS-V: 24.27 ± 6.04% (n = 11) vs GFP-V: 7.01 ± 2.51% (n = 12), P = .006) (Figure 7A).
p21-antisense enhances the NOD/SCID repopulation capacity and maintains differentiative ability of transduced CD34+ cord blood cells. CD34+ cord blood cells transduced with p21-antisense (p21-AS-V) or the control vector (GFP-V) were transplanted into sublethal irradiated NOD/SCID mice along with 2 × 106 irradiated carrier cells per mouse. At 8 to 12 weeks after transplantation the bone marrow was harvested and stained for the human leukocyte marker CD45 with myeloid and lymphoid differentiation markers (CD3, CD14, CD19, CD33, CD34, and CD38) and analyzed by flow cytometric analysis. (A) Chart shows the percentage of human CD45+ cells in the bone marrow of animals that underwent transplantation (thin line represents the mean). (B) Chart shows the proportion of differentiated cells in the CD45 subpopulation in the bone marrow of animals that received transplants of p21-antisense (p21-AS-V)– or control vector (GFP-V)–transduced cells (thin line represents the mean).
p21-antisense enhances the NOD/SCID repopulation capacity and maintains differentiative ability of transduced CD34+ cord blood cells. CD34+ cord blood cells transduced with p21-antisense (p21-AS-V) or the control vector (GFP-V) were transplanted into sublethal irradiated NOD/SCID mice along with 2 × 106 irradiated carrier cells per mouse. At 8 to 12 weeks after transplantation the bone marrow was harvested and stained for the human leukocyte marker CD45 with myeloid and lymphoid differentiation markers (CD3, CD14, CD19, CD33, CD34, and CD38) and analyzed by flow cytometric analysis. (A) Chart shows the percentage of human CD45+ cells in the bone marrow of animals that underwent transplantation (thin line represents the mean). (B) Chart shows the proportion of differentiated cells in the CD45 subpopulation in the bone marrow of animals that received transplants of p21-antisense (p21-AS-V)– or control vector (GFP-V)–transduced cells (thin line represents the mean).
The bone marrow cells were further analyzed by flow cytometric analysis for the in vivo differentiation status of the engrafted transduced CD34+ cord blood cells. p21-antisense– and control vector–transduced transplanted mice BM showed no significant difference in the proportion of CD34+ cells within the CD45+ population (GFP-V: 20.34 ± 1.91%; p21-AS-V: 19.74 ± 1.79%; P = .41). Equivalent expression of CD19 and CD33, lymphoid and myeloid markers, respectively, was also noted in p21-antisense– or control vector–transduced transplanted recipient BM (Figure 7B). These data indicate that a primitive population of p21-antisense– transduced CD34+ cells is maintained and that the differentiation potential of these cells is unperturbed in this in vivo model.
Discussion
The relative quiescence of hematopoietic stem cells is accompanied by a resistance to cytokine stimulation that can be overcome by high concentrations of cytokines ex vivo, but often at the expense of potentiality. Understanding the basis for restricted cell cycle entry offers the potential for expanding stem cells by targeted reduction in inhibitory tone that we and others have attempted to accomplish. We previously demonstrated through the analysis of engineered mouse strains that the quiescence of hematopoietic stem cells is maintained in part by the CKI G1 checkpoint regulator, p21.12 The results reported here suggest that this information can be translated to human cells and that reduction in p21 expression leads to increased entry into cell cycle with a relative expansion or preservation of primitive hematopoietic cells including those with marrow repopulating potential. These data validate the model of reduced inhibition facilitating human ex vivo stem cell expansion. However, the data must be interpreted with caution regarding the comparison of manipulated cells with fresh unmanipulated stem cells. The cord blood source precluded same-donor comparisons; the variability between donors is substantial, preventing meaningful unpaired sample comparison; and the nature of the assays makes nonsimultaneous analysis suboptimal. Therefore, all analyses performed here are pair-wise assessments of cells manipulated with either control or p21-AS–encoding vectors.
The basis for the inhibition of p21 was not studied in detail, but our data indicate a more than 90% knock-down in expression of p21 without a similar effect seen on at least one control housekeeping gene, GAPDH. Use of a lentiviral construct was necessary because of the difficulty in transducing primitive quiescent human hematopoietic cells and the basis for inhibition was likely either an RNA-RNA hybrid or RNA interference (RNAi). While the former may result in broadly based RNA degradation or select, specific inhibition,19 we did not note generalized suppression of gene expression or enhanced cell cytotoxicity suggesting that an RNAi-like effect may have been active.20-23
Additional support for a restricted gene effect in our model is the consistency of results with what we previously noted in the murine p21–/– animal. In that context, reduced p21 resulted in increased cycling and number of stem cells without significant effects on more mature hematopoietic populations. Similarly, we noted that p21-antisense resulted in active cycling and relative expansion of primitive hematopoietic cells capable of repopulating bone marrow in sublethally irradiated NOD/SCID mice. We did not observe altered total cell numbers or colony-forming cells. The proportion of differentiated cells was similar in the control vector– and p21-antisense–transduced cells. These results indicate that the p21-antisense approach yields a hematopoietic phenotype comparable with the specific gene targeting by homologous recombination to create the p21-null animal.
The similar proportion of differentiated cells in the animals that received transplants of control vector– or p21-antisense–transduced cells again demonstrates the predominant function of p21 in the cell cycle regulation of the stem cell compartment. Our findings suggest that the differentiation stage–specific effects of cip/kip CDKI observed in mice may hold in human hematopoiesis.
The effects that have been seen in both the genetically modified mice and transduced human hematopoietic cells are suggestive of an effect on proliferation rather than impaired differentiation. In both settings we observed a decrease in primitive cells in G0 accompanying the change in cell numbers. To the extent that CFC size and number reflect both the state of differentiation and indicate its kinetics, differentiation was not detectably affected in the context of p21-antisense or the p21 null. These data suggest that proliferation can be dissociated from differentiation in the stem cell compartment, a critical requirement for ex vivo stem cell manipulation.
Other investigators have previously tested the ability to release the inhibition of stem cell proliferation by neutralizing antibody to TGFβ or a combined antisense approach targeting p27 in conjunction with anti-TGFβ antibody.24,25 The studies evaluating these approaches assessed progenitor cells through CFCs or CFCs plus retroviral marking in subsequently transplanted cells in the case of p27-antisense/anti-TGFβ. Neither study directly quantitated the number of stem cells or their relative abundance with manipulation. The 2 studies have diametrically opposed conclusions about the ability of TGFβ alone to affect cycling, with the findings by Dao et al25 indicating that anti-TGFβ or p27-antisense alone is not sufficient to affect primitive cell cycling. Rather, a combined effect of the 2 molecules was required. While the antisense increase in retroviral marking with p27-antisense/anti-TGFβ may reflect an increase in stem cells, it is possible that the effect of reduced expression of p27 enhanced the progenitor cell production from a limited number of stem cells. This increased “efficiency” of stem cells was documented by us using competitive transplantation of p27-null and wild-type bone marrow and was noted despite the absence of an effect of p27 on the overall stem cell number.13 Therefore, whether either TGFβ antibody or p27-antisense/anti-TGFβ expands the stem cell pool ex vivo may still be debated.
While the evidence for a stem cell expanding effect of antagonizing TGFβ or p27/anti-TGFβ is not entirely definitive, the potential for combining these approaches with p21-antisense offers a potentially attractive opportunity for enhancing the p21-antisense effect. Recent data have indicated that TGFβ does not mediate its effects through p2126 and that p21 and p27 function distinctly in primitive hematopoiesis.12,13 Thus, manipulating these distinctly acting regulators of primitive cell function may provide potentially additive effects. It is recognized that the approximately 2- to 3-fold expansion we observed with p21-antisense alone and the use of lentiviral vectors are unlikely to make this protocol clinically useful. However, they do confirm the paradigm that releasing the brake on stem cell expansion through interdiction of CDKI, can result in relative ex vivo stem cell expansion. Further refinement of methods to reduce p21 levels and combinations with either anti-TGFb, anti-p27/p15 or proliferative cytokines may be productive in achieving clinically meaningful stem cell expansion.
Prepublished online as Blood First Edition Paper, April 17, 2003; DOI 10.1182/blood-2002-10-3053.
Supported by National Institutes of Health HL44851, HL65909, AI29530, AI142851 (D.T.S.), DK02761 (T.C.), Doris Duke Charitable Foundation, Burroughs-Wellcome Trust (D.T.S.), and the Deutsche Academischer Austrauschienst (S.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Dennis M. O'Connor and Cory Johnson of the St Louis University Department of Pediatric Hematology/Oncology for their generous assistance in obtaining umbilical cord blood samples.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal