Abstract
The CCAAT/enhancer binding protein-α (C/EBPα) is a transcription factor required for differentiation of myeloid progenitors. In addition to specific DNA binding, C/EBPα is also involved in protein-protein interactions, some of which (p21, Cdk2/Cdk4, E2F) appear to be required for inhibition of proliferation and possibly differentiation. To investigate the mechanisms of C/EBPα-induced granulocytic differentiation, we generated C/EBPα mutants reportedly defective in DNA binding, transactivation, and Cdk2/Cdk4 and E2F interaction and assessed their effects in a myeloid precursor cell line, primary bone marrow and C/EBPα knockout fetal liver precursor cells. We show here that the DNA binding–deficient Lys298Glu mutant, the E2F binding–deficient basic region mutant 2 (BRM-2) carrying the Ile294Ala and Arg297Ala substitutions, and the transactivation-deficient N-terminus truncated p30 mutant all fail to promote differentiation on ectopic expression in myeloid precursor cells. By contrast, ectopic expression of the Cdk2/Cdk4 interaction–deficient Δ177-191 mutant promotes differentiation and induces gene expression as effectively as wild-type C/EBPα. Thus, the integrity of the transactivation and DNA binding domains, but not of the Cdk2/Cdk4 interaction region, is necessary for C/EBPα-induced differentiation. Since the E2F binding–deficient BRM-2 mutant interacted with E2F-1 but failed to activate gene expression, our results lend support to the hypothesis that activation of gene transcription is the determining factor in C/EBPα-dependent differentiation.
Introduction
The CCAAT/enhancer binding protein-α (C/EBPα) is a member of the bZIP (basic region leucine zipper) family of transcription regulators that includes C/EBPβ, C/EBPϵ, C/EBPγ, and C/EBPδ. C/EBPα family members form homodimeric and heterodimeric transcription factors via their C-terminal leucine zipper domains1 and bind DNA as dimers via the adjacent basic regions.2 Two distinct transactivation domains located in the N-terminal region distinguish the members of this family of transcription factors. C/EBPα expression promotes granulocytic differentiation of common myeloid precursors with a concomitant block of monocytic differentiation.3 C/EBPα knock-out mice lack neutrophils and eosinophils but retain monocytes, and C/EBPα-null fetal liver cells do not form colonies in response to granulocyte colony-stimulating factor (G-CSF).4,5 Indeed, C/EBPα knock-out cells lack receptors for G-CSF, and the restoration of both interleukin 6 (IL-6) and G-CSF receptor levels enables granulocytic differentiation in vitro.6 The role of C/EBPα is, in part, independent of its effect on G-CSF receptor (G-CSFR) levels, as G-CSFR knock-out mice retain the production of mature granulocytes, whereas C/EBPα knock-out mice do not.7
Recent studies have reported C/EBPα mutations in a significant number of patients with acute myelogenous leukemia (AML). Mutations in the N-terminus lead to the production of a truncated protein that appears to function as a dominant-negative (DN) inhibitor of the DNA binding and transactivation activity of the full-length protein.8 A 30-kDa C/EBPα protein has been shown to be physiologically generated because of the use of an alternative translation initiation codon located 351 nucleotides downstream of the main AUG initiation codon.9-11 This 30-kDa product also lacks the antimitotic activity exhibited by the full-length protein.12 In another study performed in AML and myelodysplastic syndrome (MDS) patient samples, mutations affecting the bZIP domain of C/EBPα were found, but the predicted mutant proteins did not appear to have DN activity.13 It has also been demonstrated that C/EBPα expression is suppressed at the translational level in BCR-ABL–expressing cell lines and in primary bone marrow cells from patients in chronic myelogenous (CML) blast crisis.14 Ectopic expression of C/EBPα in BCR-ABL–expressing cells can overcome the block in granulocytic differentiation by promoting G-CSF–induced differentiation.14 Likewise, in an animal model of AML, C/EBPα and ϵ were shown to suppress the leukemic phenotype.15
In normal myeloid progenitors, the differentiation-inducing function of C/EBPα is thought to depend on transcription activation of many differentiation-related genes such as the G-CSFR, myeloperoxidase (MPO), and neutrophil elastase (NE) genes.16-18 Consistent with this, C/EBPα knock-out fetal liver cells lack G-CSFR expression.19
There is also evidence that C/EBP-dependent transcription repression leads to down-regulation of c-Myc expression, allowing myeloid progenitor cells to undergo granulocytic differentiation.20 In adipocyte precursor cells, the growth suppressive function of C/EBPα appears to be essential for its differentiation-inducing effect. It was initially proposed that the inhibition of proliferation was partly caused by C/EBPα's ability to activate transcription and induce posttranscriptional stabilization of p21.21,22 More recently, Wang et al23 have shown that C/EBPα interacts with and inhibits Cdk2 and Cdk4 by preventing cyclin binding, independent of C/EBPα's DNA binding activity. The Cdk2/Cdk4 interaction domain of C/EBPα is located between amino acids 175 and 188 and is responsible for its growth inhibitory ability with no apparent effect on its transcriptional activation function.23,24 Furthermore, direct repression of E2F-dependent transcription by C/EBPα has been demonstrated, and mutation of the basic region was shown to abrogate the ability of C/EBPα to induce adipocyte differentiation.25,26
To investigate whether C/EBPα induces granulocytic differentiation via transcriptional regulation of target genes (eg, NE, G-CSFR) or by inhibition of proliferation, or both, we have generated mutants defective in these functions and assessed the differentiation-inducing potential of mutant proteins in a myeloid precursor cell line, in primary marrow cells, and in C/EBPα knock-out cells. We show here that a single amino acid mutation in the DNA binding region suppresses the ability of C/EBPα to induce granulocytic differentiation, concomitant with the abrogation of C/EBPα-dependent gene induction. Deletion of the Cdk2/Cdk4 interaction region does not affect the differentiation-inducing activity of C/EBPα in all assay systems used. Mutation of the reported E2F binding site in C/EBPα's basic region also compromises the induction of differentiation; however, this mutant interacts with E2F-1 but is defective in transactivation and induction of gene expression. The p30 C/EBPα protein, which retains DNA binding but not transactivation activity, is completely defective in inducing granulocytic differentiation. These results suggest that the transactivation domain and the DNA binding/basic region of C/EBPα are necessary for granulocytic differentiation, whereas interaction with Cdk2/Cdk4 and perhaps with E2F is not. Moreover, these results lend support to the hypothesis that the transcription activation function of C/EBPα is the determining factor in C/EBPα-dependent differentiation.
Materials and methods
Cell culture
The murine IL-3–dependent 32Dcl3 myeloid precursor cells were maintained in culture in Iscoves modified Dulbecco medium (IMDM) supplemented with 10% heat-inactivated fetal calf serum (FCS; Sigma Chemical, St Louis, MO), 2 mM l-glutamine (Gibco, BRL, Gaithersburg, MD), and 10% WEHI-conditioned medium as a source of IL-3. C/EBPα–/– cells derived from the fetal liver of C/EBPα-deficient mice (kind gift of D. G. Tenen, Harvard Institutes of Medicine, Boston, MA) were maintained in IMDM supplemented with 15% FCS, 2% WEHI-conditioned medium, and 2% baby hamster kidney (BHK)–murine kit ligand (MKL)–conditioned medium (as a source of stem cell factor [SCF]). Normal murine marrow cells were obtained from the femurs of C57BL/6 mice after hypotonic lysis and enrichment for lineage-negative hematopoietic precursor cells using the standard StemSep protocol (StemCell Technologies, Vancouver, BC). Lineage-negative cells were 20% SCA1+ and 75% CD34+ and, prior to retroviral infection, were maintained for 2 days in IMDM supplemented with a cocktail of murine recombinant cytokines (2 ng/mL IL-3, 1.2 ng/mL IL-6, 10 ng/mL SCF [R&D Systems, Minneapolis, MN], 5 ng/mL granulocyte-macrophage colony-stimulating factor [GM-CSF], and 5 ng/mL Flt3/Flk2 ligand [StemCell Technologies]). 6:15 cells are an established 32D-BCR/ABL–transformed cell line that has lost endogenous C/EBPα and C/EBPβ expression14 (K.K., unpublished data, 2003).
Plasmids
ΔuORF/Δspacer-C/EBPα HA (p42 C/EBPα). This plasmid was generated using an internal ribosome entry site–green fluorescent protein (IRES-GFP)–containing bicistronic retrovirus (MigR1) carrying the hemagglutinin A (HA)–tagged rat c/ebpα cDNA lacking the upstream open reading frame (ORF) and spacer regions as previously described.14
BRM2-C/EBPα HA (Lys298Glu). This mutant contains 2 amino acid substitutions (Ile294Ala and Arg297Ala) in rat C/EBPα and was generated by site-directed mutagenesis of ΔUORF using the Quickeasy Mutagenesis system (Stratagene, La Jolla, CA).
Lys298Glu-C/EBPα HA (Lys298Glu). This mutant, previously referred to as K300E,24 which contains a single nucleotide change (892A>G corresponding to amino acid 298 of the human C/EBPα protein [kindly provided by G. J. Darlington, Baylor College of Medicine, Houston, TX]), was released from the pcDNA3.1 vector using EcoRI and subcloned into the EcoRI site of MigR1.
Δ177-191–C/EBPα HA. This deletion construct was generated by ligating the polymerase chain reaction (PCR)–amplified C/EBPα cDNA segments upstream and downstream of the nucleotide sequences corresponding to amino acid 177 and 191, respectively. The primers used were (1) a 5′ pMSCV primer that includes a XhoI restriction site, (2) a 3′ primer 5′-GAGGCC-GGCCAGCGCCAG-3′ ending at the sequence corresponding to amino acid 176, (3) a 5′ primer 5′-GCGTCTCCCGCGCACTTGGC-3′ starting at the sequence corresponding to amino acid 192, and (4) a 3′ primer corresponding to the sequence of the HA epitope followed by a stop codon and also including an EcoRI restriction site. The amplified fragments were digested with the XhoI and EcoRI restriction enzymes and subcloned into the XhoI- and EcoRI-digested MigR1 vector.
p30-C/EBPα HA. This plasmid, which encodes the N-terminal–truncated p30 C/EBPα, was made by PCR amplification starting at the nucleotides corresponding to amino acid 118 of c/ebpα cDNA using a 5′ primer (5′-ATGTCCGCGGGGGCGCACGG-3′) and a 3′ primer corresponding to the sequence of the HA epitope followed by a stop codon and including an EcoRI restriction site. The PCR product was digested with EcoRI and subcloned into the HpaI- and EcoRI-digested MigR1 vector.
MigR1-Lys298Glu-Flag (Lys298Glu). To generate this plasmid, Lys298Glu was PCR-amplified using the 5′ pMSCV primer and a 3′ primer containing the 3′ end of c/ebpα cDNA, the 24 bases Flag sequence followed by a stop codon sequence. The PCR product was digested with BamHI/EcoRV and subcloned into the BglII/HpaI sites of the MigR1 vector. Each plasmid was sequenced to verify the presence of expected mutations.
pCMV-E2F1. This plasmid was a kind gift of Dr W. G. Kaelin (Dana-Farber Cancer Center, Boston, MA).
Differentiation analysis of retrovirally transduced myeloid precursor cells
For retroviral infections, Phoenix cells (kind gift of G. P. Nolan, Stanford University School of Medicine, Stanford, CA) were transiently transfected with the indicated plasmids. The infectious supernatant was collected 48 hours later and used to infect (a 48-hour procedure) parental 32Dcl3 cells, lineage-negative marrow cells, and C/EBPα–/– fetal liver myeloid progenitor cells as described.27 Twenty-four hours later, infected cells were sorted (EPICS Profile Analyzer; Coulter, Hialeah, FL) for green fluorescent protein (GFP) expression. In 32Dcl3 cells and C/EBPα–/– cells, induction of differentiation was assessed after sorting in the presence of 10% U87-MG–conditioned medium alone (as a source of G-CSF) or 25 ng/mL recombinant G-CSF. Marrow cell differentiation was assessed in the presence of recombinant cytokines (IL-3 [2 ng/mL], IL-6 [1.2 ng/mL], SCF [10 ng/mL], GM-CSF [5 ng/mL], and Flt3/Flk2 ligand [5 ng/mL]), or in the presence of a modified cocktail lacking GM-CSF and containing a suboptimal concentration of IL-3 (0.2 ng/mL). Morphologic differentiation was monitored by May-Grünwald and Giemsa staining of cytospin preparations.
RT-PCR reactions
For reverse transcriptase (RT)–PCR detection of neutrophil elastase (NE) transcripts, total RNA was extracted using Tri-Reagent (Sigma), and 1 μg RNA was reverse-transcribed using 40 units avian myeloblastosis virus (AMV) reverse transcriptase (Roche Molecular Biochemicals, Indianapolis, IN), 200 μM of each deoxynucleoside triphosphate (dNTP) and 0.25 U/mL random hexamers (Pharmacia, Uppsala, Sweden). cDNA was used as template for PCR (Expand High Fidelity PCR System; Roche) according to the manufacturer's protocol. The oligodeoxynucleotides specific for the amplification of murine NE were upstream primer (5′-ATGGCCCTTGGCAGACTATCC-3′) and downstream primer (5′-GCCATTCTCGAAGATCCCCTG-3′). As internal control, all cDNA samples were adjusted to yield relatively equal amplification of hnRNP E1 and/or GRB2. Control PCR reactions were run without template, and duplicates of each reaction were performed without reverse transcriptase.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were obtained from retrovirus-transduced cells by allowing the cells to swell in buffer A (20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) [pH 7], 10 mM KCl, 2 mM MgCl2, 0.5% NP40, 1 mM phenylmethylsulfonylfluoride, and a protease inhibitor cocktail [Roche]) for 10 minutes on ice, homogenizing the cells by 5 strokes with a 26-gauge needle, sedimenting the nuclei by centrifugation for 3 minutes at 4000 rpm, and incubating the nuclear pellet in buffer B (buffer A plus 0.5 M NaCl) for 30 minutes at 4°C. EMSAs were performed by incubating 10 μg nuclear extracts with 50 000 cpm of double-stranded 32P–end-labeled oligonucleotide in a binding reaction mixture containing 10 mM HEPES (pH 7.9), 50 mM KCl, 2.5 mM MgCl2, 1 mM DTT (dichlorodiphenyltrichloroethane), 10% glycerol, 1 μg acetylated bovine serum albumin, and 0.5 μg poly(dI-dC) (Amersham Pharmacia Biotech, Piscatawy, NJ) at room temperature for 20 minutes. For the supershift assay, nuclear extracts were incubated with 1 μg anti-C/EBPα antibody (rabbit polyclonal; Santa Cruz Biotechnologies, Santa Cruz, CA), for 15 minutes at room temperature following the addition of radiolabeled probe (50 000 rpm). Binding reactions were resolved on a 4% nondenaturing polyacrylamide gel in 1 × Tris borate EDTA (ethylenediaminetetraacetic acid) (TBE) buffer and electrophoresed at 150 V for 2 hours. Gels were dried and exposed to autoradiography. The oligonucleotides used for the EMSAs were as follows: C/EBPα (neutrophil elastase promoter) 5′-TCGAGGCCAGGATGGGGCAATACAACCC-3′ and C/EBPα (G-CSF receptor promoter) 5′-AGGTGTTGCAATCCCCAGC-3′, both containing the binding site (underlined) for C/EBPα.
Immunoprecipitation and Western blot analysis
293T cells were transiently cotransfected with p42 C/EBPα and Lys298Glu-Flag (single transfections also performed) or with E2F-1 and p42-, BRM-2–, or Lys298Glu-C/EBPα. Cells were harvested 46 hours after transfection, washed once with 1 × phosphate-buffered saline (PBS) and lysed (107 cells/100 μL lysis buffer) in buffer containing (50 mM HEPES [pH 7.5], 150 mM NaCl, 0.5% NP40, 1 mM phenylmethyl sulfonyl fluoride [PMSF] supplemented with a cocktail of serine and cysteine protease inhibitors [complete, EDTA-free; Roche]) for 30 minutes, rotating at 4°C, and the lysates were clarified by centrifugation at 14 000 rpm for 10 minutes. Supernatants were precleared with 10 μL 1:1 slurry of protein G plus agarose (Oncogene Research Products, Boston, MA) in lysis buffer. The precleared supernatants were incubated with 5 μLAffinity Matrix HA (AFC-101P; Covance, Princeton, NJ) overnight, or the anti-HA (mouse monoclonal; Covance), the anti-Flag (M2; Sigma) antibodies with a 1:1 slurry protein G plus agarose for 4 to 5 hours rotating at 4°C. The beads were washed extensively (6 times) in lysis buffer containing 0.5% (anti-Flag), 0.05% (anti-HA), or 0.1% NP40 (anti–E2F-1), and the pellet was resuspended in 2 × protein-loading buffer. Immunoprecipitated proteins were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred onto a nitrocellulose membrane, and immunoblotted using anti-HA, anti-Flag (M2 peroxidase conjugated; Sigma), anti–2F-1 (sc-251x; Santa Cruz), or anti-C/EBPα antibodies. Proteins were detected using enhanced chemiluminescence (ECL) (Amersham, Arlington Heights, IL).
Southern blot of RT-PCR products
PCR reactions for NE were carried for 22, 25, and 28 cycles and run on a 1.5% agarose gel. The gel was soaked for 30 minutes in 0.5 M NaOH, 1.5 M NaCl; 30 minutes in 1.5 M NaCl, 1 M Tris, pH 7.5; and 15 minutes in 20 × sodium saline citrate (SSC). The PCR products were then transferred onto Hybond-N+ filter (Amersham) overnight, and the DNA was then UV-fixed to the filter. The filter was prehybridized at 50°C for 2 hours in 5 × SSC, 1 × Denhardt solution, 0.1% SDS, and 100 μg/mL sheared salmon sperm DNA. An internal NE oligonucleotide located 40 base pair (bp) upstream of the ATG (5′-CCAGGAACA-ATGCCAGTAGCAT-3′) was γ-32P-ATP-labeled, purified through a G-25 Sephadex column, and 1 × 106 cpm/mL used for hybridization overnight at 50°C in 5 × SSC, 0.1% SDS, and 100 μg/mL sheared salmon sperm DNA. The filter was washed in 2 × SSC, 0.1% SDS (3 times for 15 minutes at room temperature and for 15 minutes at 50°C), and exposed to X-AR imaging films (Eastman Kodak, Rochester, NY) at –80°C overnight.
Luciferase assay
Both 293T and NIH3T3 cells were used in this assay. Briefly, cells were transiently transfected using Fugene 6 reagent (Roche) according to the manufacturer's protocol with 50 ng reporter construct pTK-G-CSFR-luciferase (firefly) (kind gift of D. G. Tenen, Harvard Institutes of Medicine) and 100 ng of the indicated C/EBPα expression plasmid. The reporter plasmid contains 4 consensus C/EBPα binding sites from the G-CSF receptor promoter.19 As a control, a renilla reporter construct, which contains 4 mutated C/EBPα consensus binding sites, was also used. Twenty-four hours after transfection, firefly and renilla luciferase activity was recorded on a luminometer using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Results are presented as the percentage luciferase (firefly) and renilla activity relative to values obtained using p42 C/EBPα (assigned 100% luciferase/renilla activity) ± SEM, and are representative of 4 individual experiments (performed in duplicate).
Cell cycle analysis
Cells (0.2-0.5 × 106/sample) were washed in 1mL 1 × PBS/5 mM EDTA and fixed by slowly adding ice-cold 100% ethanol to give a final concentration of 70% and incubated on ice for 20 minutes. Cells were washed in PBS and incubated in 0.5 mL PBS containing 200 μg/mL DNase-free RNase A for 30 minutes at room temperature. Propidium iodide was then added to a final concentration of 50 μg/mL and incubated for at least 15 minutes at room temperature in the dark, and samples were then analyzed by Flow Cytometry (EPICS Profile Analyzer; Coulter).
Results
C/EBPα accelerates G-CSF–driven differentiation of 32Dcl3 myeloid precursor cells
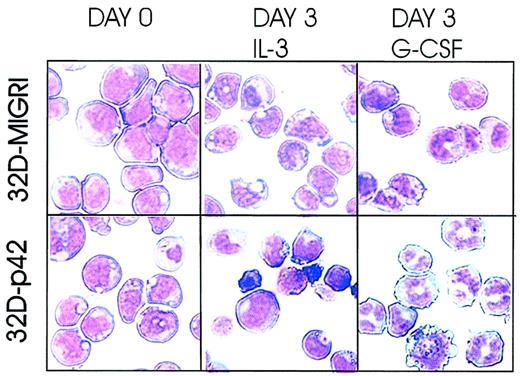
Myeloid precursor 32Dcl3 cells are IL-3 dependent for growth and undergo terminal differentiation on G-CSF treatment. To assess the effect of ectopic C/EBPα expression on G-CSF–driven differentiation, 32Dc13 cells were retrovirally infected with MigR1-p42 C/EBPα, an IRES-GFP–containing retrovirus encoding the 42-kDa wild-type C/EBPα protein, or MigR1 alone in the presence of IL-3. Twenty-four hours after infection, homogenous GFP-positive cells were selected by flow cytometry and cultured in the presence of IL-3 or G-CSF for the indicated time. In the presence of IL-3, C/EBPα was unable to induce terminal differentiation (Figure 1). By contrast, most 32D-p42 C/EBPα cells (approximately 55% in 3 different experiments) underwent terminal differentiation when cultured in the presence of G-CSF for 3 days, whereas the control 32D-MigR1 cells displayed only early signs of differentiation (Figure 1). Thus, ectopic expression of p42 C/EBPα accelerates GCSF-induced granulocytic differentiation of 32Dcl3 cells.
C/EBPα accelerates G-CSF–induced granulocytic differentiation of 32Dcl3 cells. 32Dcl3 cells were retrovirally infected with wild-type C/EBPα (p42) or the empty vector MigR1. Morphology of GFP-positive cells was assessed by May-Grünwald/Giemsa staining of cytospin preparations at day 0 (left panels) and at day 3 (middle and right panels) from liquid cultures supplemented with the indicated cytokines. Representative of 3 different experiments. Original magnification, × 40.
C/EBPα accelerates G-CSF–induced granulocytic differentiation of 32Dcl3 cells. 32Dcl3 cells were retrovirally infected with wild-type C/EBPα (p42) or the empty vector MigR1. Morphology of GFP-positive cells was assessed by May-Grünwald/Giemsa staining of cytospin preparations at day 0 (left panels) and at day 3 (middle and right panels) from liquid cultures supplemented with the indicated cytokines. Representative of 3 different experiments. Original magnification, × 40.
Transcriptional activation function of C/EBPα is necessary for granulocytic differentiation
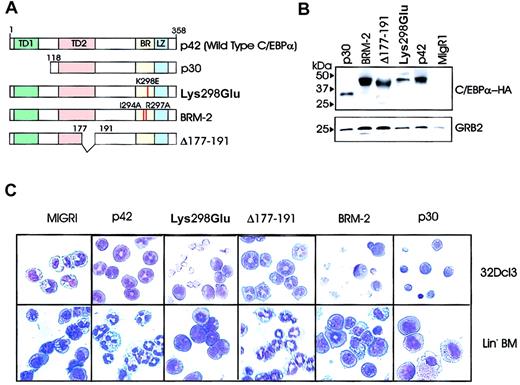
To identify the domains necessary for the differentiation-inducing function of C/EBPα, we generated several C/EBPα mutants (Figure 2A) and assessed the effects of ectopic expression in myeloid precursor cells (Figure 2B-C). The Lys298Glu-C/EBPα mutant (previously referred to as K300E24 ), containing a single amino acid mutation (Lys298Glu) in the basic region/DNA binding domain, maintains the α-helical structure but is defective in DNA binding. Basic region mutant-2 (BRM-2) has a double mutation (Ile294Ala and Arg297Ala) of residues that are on the non-DNA binding face of the basic region α-helix that, according to Porse et al,26 renders it defective in E2F binding. Δ177-191 is a C/EBPα internal deletion mutant which fails to interact with Cdk2/Cdk4.23 p30-C/EBPα encodes for an N-terminal–truncated mutant protein which lacks the transactivation domain12 (Figure 2A). Each of these mutants was subcloned into the MigR1 retrovirus.
Effects of C/EBPα on 32Dcl3 cells. (A) Schematic diagram of wild-type (p42) and C/EBPα mutants. (B) Levels of ectopic C/EBPα in retrovirally transduced 32Dcl3 cells. Morphology of 32Dcl3 cells (C, upper panels) and lineage-negative bone marrow cells (C, lower panels) retrovirally transduced with either p42, Lys298Glu, Δ 177-191, BRM-2, p30 C/EBPα, or the empty MigR1 vector. Cells were selected for GFP positivity and treated for 3 days with G-CSF (C, upper panels) or cultured for 7 days in a cocktail of cytokines without G-CSF (IL-6, Flt3/Flk2 ligand, SCF, GM-CSF, and IL-3) (C, lower panels). Original magnification, × 40.
Effects of C/EBPα on 32Dcl3 cells. (A) Schematic diagram of wild-type (p42) and C/EBPα mutants. (B) Levels of ectopic C/EBPα in retrovirally transduced 32Dcl3 cells. Morphology of 32Dcl3 cells (C, upper panels) and lineage-negative bone marrow cells (C, lower panels) retrovirally transduced with either p42, Lys298Glu, Δ 177-191, BRM-2, p30 C/EBPα, or the empty MigR1 vector. Cells were selected for GFP positivity and treated for 3 days with G-CSF (C, upper panels) or cultured for 7 days in a cocktail of cytokines without G-CSF (IL-6, Flt3/Flk2 ligand, SCF, GM-CSF, and IL-3) (C, lower panels). Original magnification, × 40.
To assess their differentiation potential, the wild-type (WT) or mutant C/EBPα retrovirus was transduced in 32Dcl3 cells grown in the presence of IL-3. Following isolation of GFP-positive cells, ectopic expression of WT or mutant C/EBPα was assessed by anti-HA Western blotting (Figure 2B), and transduced cells were cultured in the presence of G-CSF for 3 days. GFP positivity was tested in cells cultured for 24 hours and ranged from 85% to 95%. The lowest GFP positivity was typically found in cultures of p42 C/EBPα-expressing cells. p42 C/EBPα and Δ177-191 was able to induce terminal differentiation, as indicated by the appearance of polymorphonucleated neutrophils (approximately 54% and 56%, respectively) on cytospin preparations (Figure 2C, upper row), but the Lys298Glu, BRM-2, and p30 C/EBPα mutants did not (Figure 2C, upper row). To further assess the differentiation-inducing potential of these C/EBPα mutants, we performed the differentiation assay in primary murine hematopoietic precursor marrow cells depleted of lineage-committed cells (20% SCA1+ and 75% CD34+). Following lineage-negative selection, these cells were retrovirally infected with WT or mutant C/EBPα for 48 hours, selected for GFP positivity, and assessed for granulocytic differentiation. Retrovirustransduced cells were cultured in the presence of a cocktail of murine cytokines (IL-6, Flt3/Flk2 ligand, SCF, GM-CSF, and IL-3) to maintain precursor cells. In the absence of G-CSF, 80% and 77% (the average of 3 independent experiments) of WT C/EBPα- and Δ177-191–expressing cells underwent granulocytic differentiation (Figure 2C, lower row); by contrast, only 19% of MigRI-transduced cells did undergo differentiation (Figure 2C, lower row). Similar to our results in 32D cells, the Lys298Glu, BRM-2, and p30 mutants were unable to induce differentiation (< 10%), as retrovirus-transduced cells retained the morphologic features of lineage-negative precursor cells (Figure 2C, lower row). Because the Δ177-191–C/EBPα mutant is reportedly defective in Cdk2 binding and yet enables differentiation of myeloid precursor cells, it seems that DNA binding and transcription activation (not Cdk2 binding) is necessary for C/EBPα-dependent granulocytic differentiation.
The DNA binding ability of each mutant was tested in 6:15 (32D-BCR/ABL) cells, because these cells no longer express endogenous C/EBPα and C/EBPβ14 (K.K., unpublished data, 2003), thereby allowing the assessment of ectopic C/EBPα activity without any background. On retroviral infection of 6:15 cells with each of the MigR1 C/EBPα mutants and selection of GFP-positive cells in the presence of IL-3, nuclear extracts were prepared and analyzed by electrophoresis mobility shift assays (EMSAs) using radiolabeled G-CSFR (Figure 3A) and NE (Figure 3B-C) probes. p42 C/EBPα, Δ177-191, BRM-2, and p30 C/EBPα proteins bound specifically to the probes (Figure 3, lanes 4, 8, 10, and 12), whereas Lys298Glu did not (Figure 3, lane 6). Of note, p30 bound to the G-CSFR C/EBP binding site less efficiently than to the NE C/EBP binding site, as previously shown.8 No binding was detected with nuclear extracts from cells transduced with MigR1 alone (Figure 3, lane 2). Binding was confirmed by competition experiments with excess of cold oligonucleotides (data not shown). Moreover, addition of the anti-C/EBPα antibody specifically supershifted the C/EBPα-containing DNA-protein complex (Figure 3, lanes 5, 9, 11, and 13). Similar data were obtained in EMSA performed with nuclear extracts of 293T cells transfected with WT or mutant C/EBPα (Figure 3C). Thus, a single mutation (Lys298Glu) in the basic region/DNA binding domain of C/EBPα prevents DNA binding, while this activity is retained by the mutants Δ177-191 (which does not interact with Cdk2/Cdk4) and BRM-2 (which is supposedly defective in E2F binding).
DNA binding ability (EMSA) of p42 and mutant C/EBPα. EMSAs were performed with 32P-labeled oligonucleotides containing the C/EBPα binding site in the G-CSFR and NE promoters and nuclear extracts (10 μg) from 32D-BCR/ABL 6:15 cells retrovirally transduced with either MigR1, p42, and the indicated C/EBPα mutants (A-B) or C/EBPα-transfected 293T cells (C). SS C/EBPα indicates the complex supershifted by the anti-C/EBPα antibody.
DNA binding ability (EMSA) of p42 and mutant C/EBPα. EMSAs were performed with 32P-labeled oligonucleotides containing the C/EBPα binding site in the G-CSFR and NE promoters and nuclear extracts (10 μg) from 32D-BCR/ABL 6:15 cells retrovirally transduced with either MigR1, p42, and the indicated C/EBPα mutants (A-B) or C/EBPα-transfected 293T cells (C). SS C/EBPα indicates the complex supershifted by the anti-C/EBPα antibody.
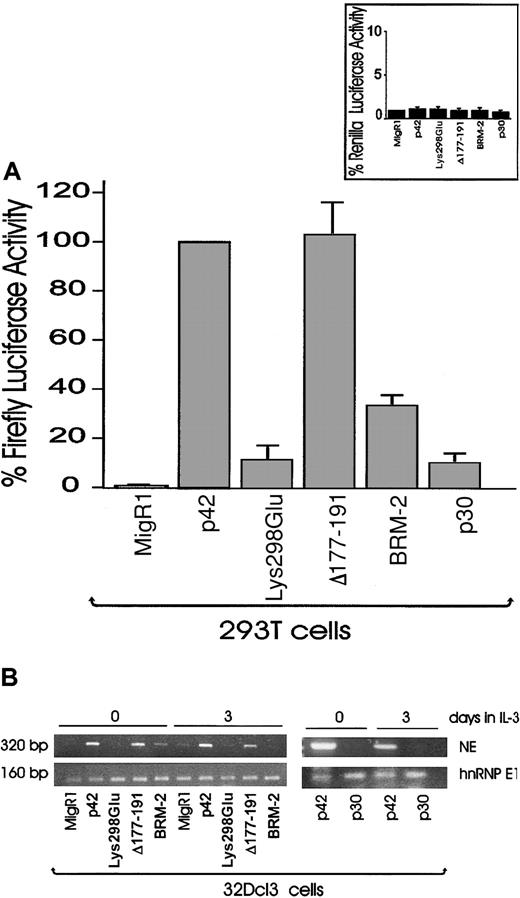
We next analyzed the transactivation potential of each of the C/EBPα mutants by using a reporter plasmid in which expression of the luciferase gene is driven by a minimal thymidine kinase promoter linked to 4 C/EBPα binding sites from the G-CSFR-5′ flanking sequence.19 We cotransfected 293T cells with WT or mutant C/EBPα along with the luciferase reporter plasmid, and 24 hours later protein extracts were assessed for luciferase activity. The assays revealed that p42 (WT) and Δ177-191 were able to transactivate luciferase expression, whereas the Lys298Glu and p30 mutants did not. The BRM-2 mutant had only a modest transactivating effect (Figure 4A, top panel). There was no transactivation in cotransfection experiments performed with a renilla reporter plasmid containing mutated G-CSFR–derived C/EBPα binding sites (Figure 4A, inset). That the p30 and Lys298Glu mutants were unable to transactivate luciferase activity is not surprising because p30 lacks the transactivation domain and Lys298Glu is deficient in DNA binding (Figures 3 and 4A). Instead, it was surprising that the BRM-2 mutant was deficient in transactivation function despite its ability to bind DNA in a sequence-specific manner. Thus, we further explored the transcription activation potential of C/EBPα mutants by assessing the expression of NE in retrovirally transduced and GFP-sorted 32Dcl3 cells. Total RNA from cells maintained in the presence of IL-3 was assessed for NE expression at day 0 and day 3. This allowed us to assess gene induction as a function of C/EBPα activity rather than as a marker of differentiation. p42 C/EBPα and the Δ177-191 mutant were capable of NE gene induction at day 0 and day 3, whereas the C/EBPα mutants Lys298Glu and p30 were not (Figure 4B). The BRM2 mutant had a modest effect at day 0, but NE transcripts were undetectable at day 3 (Figure 4B). Similar results were obtained on testing mRNA levels of G-CSFR and myeloblastin, 2 other C/EBPα-regulated genes.19,28 Thus, C/EBPα-dependent gene expression requires DNA binding and transactivation (demonstrated by the Lys298Glu, BRM-2, and p30 mutants), whereas mutation of the Cdk2 binding site does not abolish C/EBPα-dependent gene induction.
Transactivation potential and induction of NE expression by p42 and mutant C/EPBα. (A) Transactivation potential of p42 and mutant C/EBPα in 293T cells transfected with the indicated expression plasmids and the wild-type or mutant G-CSFR reporter gene. Luciferase activities were measured 24 hours after transfection and graphically represented as the percentage of luciferase activity ± SEM relative to p42 C/EBPα assigned as 100% activity. Representative of 4 different experiments. Inset shows transactivation of the mutant reporter plasmid used as control. (B) Induction of NE expression (by RT-PCR) in GFP-sorted p42- or mutant C/EBPα-expressing 32Dcl3 cells. Total RNA was isolated from cells kept in culture for the indicated time in the presence of IL-3. hnRNP E1 transcripts were detected as a loading control.
Transactivation potential and induction of NE expression by p42 and mutant C/EPBα. (A) Transactivation potential of p42 and mutant C/EBPα in 293T cells transfected with the indicated expression plasmids and the wild-type or mutant G-CSFR reporter gene. Luciferase activities were measured 24 hours after transfection and graphically represented as the percentage of luciferase activity ± SEM relative to p42 C/EBPα assigned as 100% activity. Representative of 4 different experiments. Inset shows transactivation of the mutant reporter plasmid used as control. (B) Induction of NE expression (by RT-PCR) in GFP-sorted p42- or mutant C/EBPα-expressing 32Dcl3 cells. Total RNA was isolated from cells kept in culture for the indicated time in the presence of IL-3. hnRNP E1 transcripts were detected as a loading control.
Potential mechanisms in the block of granulocytic differentiation by C/EBPα Lys298Glu and C/EBPα BRM-2 mutants
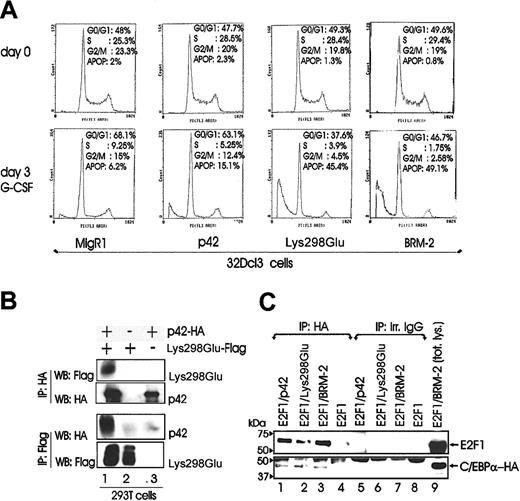
To assess potential mechanisms whereby ectopic expression of Lys298Glu and BRM-2 C/EBPα mutants interferes with G-CSF–induced differentiation of 32Dcl3 cells, we assessed the cell cycle distribution of G-CSF–treated retrovirally transduced 32Dcl3 cells. G-CSF–driven differentiation of 32Dcl3 cells transduced with MigR1 or p42 C/EBPα led to a reduction in the number of S phase cells and a concomitant increase in the number of G1 phase cells. By contrast, cells ectopically expressing the Lys298Glu DNA binding-deficient mutant underwent cell death as indicated by the fraction with sub-G1 DNA content (Lys298Glu, G1 = 37%; sub-G1 = 45%) (Figure 5A). Comparable effects on apoptosis induction were obtained by ectopic expression of BRM-2 (BRM-2, G1 = 47%; sub-G1 = 49%).
Potential mechanisms of differentiation inhibition by Lys298Glu C/EBPα. (A) DNA content of propidium iodide–stained nuclei of 32Dcl3 cells retrovirally transduced with either p42 C/EBPα, Lys298Glu, BRM-2, or MigR1 alone, selected for GFP positivity (day 0) and incubated in G-CSF (day 3). The apoptotic population is calculated as the percentage of cells with a sub-G1 DNA content. (B) Western blots show heterodimer formation of p42 and Lys298Glu C/EBPα in 293T cells transiently transfected with either HA-tagged p42 C/EBPα, Flag-tagged Lys298Glu, or together as indicated. Lane 1 of upper and lower panel is indicative of C/EBPα and Lys298Glu heterodimers. (C) Western blots show association of E2F-1 with p42 C/EBPα and the Lys298Glu and BRM-2 mutant in 293T cells transiently transfected with HA-tagged p42 or Lys298Glu or BRM-2 C/EBPα with E2F-1 individually or together as indicated.
Potential mechanisms of differentiation inhibition by Lys298Glu C/EBPα. (A) DNA content of propidium iodide–stained nuclei of 32Dcl3 cells retrovirally transduced with either p42 C/EBPα, Lys298Glu, BRM-2, or MigR1 alone, selected for GFP positivity (day 0) and incubated in G-CSF (day 3). The apoptotic population is calculated as the percentage of cells with a sub-G1 DNA content. (B) Western blots show heterodimer formation of p42 and Lys298Glu C/EBPα in 293T cells transiently transfected with either HA-tagged p42 C/EBPα, Flag-tagged Lys298Glu, or together as indicated. Lane 1 of upper and lower panel is indicative of C/EBPα and Lys298Glu heterodimers. (C) Western blots show association of E2F-1 with p42 C/EBPα and the Lys298Glu and BRM-2 mutant in 293T cells transiently transfected with HA-tagged p42 or Lys298Glu or BRM-2 C/EBPα with E2F-1 individually or together as indicated.
The induction of apoptosis in G-CSF–treated Lys298Gluexpressing 32Dcl3 cells might be due to inhibition of the wild-type C/EBPα function that would be required for differentiation of G1-arrested cells. Thus, we tested the formation of p42-Lys298Glu heterodimers by coimmunoprecipitation experiments. On coexpression of HA-tagged p42 C/EBPα and Flag-tagged Lys298Glu in 293T cells, p42 and Lys298Glu-C/EBPα were found in complex (Figure 5B), consistent with the possibility that mutant C/EBPα inhibits the activity of the endogenous C/EBPα by forming a nonfunctional heterodimer. Induction of apoptosis by BRM-2 C/EBPα may be a consequence of its reported defect in E2F interaction, which would allow free E2F-1 to induce apoptosis. Indeed, ectopic expression of E2F-1 induces apoptosis of G-CSF–treated 32Dcl3 cells.29 Thus, we tested whether E2F-1 interacts with BRM-2 C/EBPα. On coexpression of E2F-1 with p42 or BRM-2 C/EBPα, we found that E2F-1 was able to form a complex with both proteins (Figure 5C), suggesting that BRM-2 C/EBPα does not block differentiation and induce apoptosis via E2F-1. As expected, Lys298Glu C/EBPα was found in complex with E2F-1 (Figure 5C).
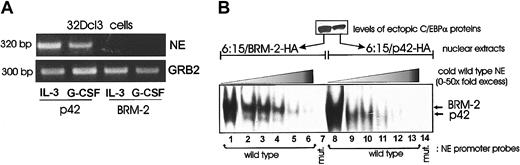
Although the BRM-2 mutant interacts with the C/EBPα binding sites in the NE promoter (as indicated by the supershift in Figure 3), its ectopic expression prevents NE mRNA up-regulation in 32Dcl3 cells treated with IL-3 or G-CSF (Figure 6A). Thus, we carried out additional EMSAs with an excess of competitor oligonucleotides to test whether p42 and BRM-2 C/EBPα bound the radiolabeled C/EBPα binding sites in the NE promoter with similar or different affinity. Such analysis suggests that there are no differences in binding affinity (Figure 6B); however, the C/EBPα–BRM-2-DNA complex had a slower mobility than that formed with WT C/EBPα. It remains to be demonstrated if this reflects an altered protein complex and if it is relevant for the block of differentiation by the BRM-2 mutant.
Gene expression and DNA binding by BRM-2 C/EBPα. (A) NE expression in GFP-sorted 32Dcl3 cells ectopically expressing p42 or BRM-2 C/EBPα. Total RNA was isolated from IL-3– or G-CSF–treated (3 days) cells. RT-PCR was performed to detect NE mRNA expression as described in “Materials and methods.” GRB2 levels are shown as a loading control. (B) EMSA of nuclear extracts (10 μg) from 6:15 cells retrovirally transduced with BRM-2 (lanes 1-7) or p42 C/EBPα (lanes 8-14) and the NE probe (wild-type or mutant) in the absence (lanes 1 and 8) or in the presence (lanes 2-6 and 9-13) of a 2- to 50-molar excess of a wild-type NE oligonucleotide used as competitor. In lanes 7 and 14, EMSAs were performed with a 32P-labeled mutant NE oligonucleotide.
Gene expression and DNA binding by BRM-2 C/EBPα. (A) NE expression in GFP-sorted 32Dcl3 cells ectopically expressing p42 or BRM-2 C/EBPα. Total RNA was isolated from IL-3– or G-CSF–treated (3 days) cells. RT-PCR was performed to detect NE mRNA expression as described in “Materials and methods.” GRB2 levels are shown as a loading control. (B) EMSA of nuclear extracts (10 μg) from 6:15 cells retrovirally transduced with BRM-2 (lanes 1-7) or p42 C/EBPα (lanes 8-14) and the NE probe (wild-type or mutant) in the absence (lanes 1 and 8) or in the presence (lanes 2-6 and 9-13) of a 2- to 50-molar excess of a wild-type NE oligonucleotide used as competitor. In lanes 7 and 14, EMSAs were performed with a 32P-labeled mutant NE oligonucleotide.
Ectopic C/EBPα expression can induce differentiation of G-CSF–treated C/EBPα knock-out cells
32Dcl3 cells express endogenous C/EBPα, which in conjunction with G-CSF–dependent signals, is instrumental for granulocytic differentiation. To further assess the effects of WT and mutant C/EBPα in a cell context in which there is no interference by endogenous C/EBPα, C/EBPα-dependent gene expression and differentiation were tested in retrovirally transduced C/EBPα knock-out (C/EBPα–/–) cells.30 For gene expression studies, total RNA was isolated from GFP-positive cells, and RT-PCR was performed to monitor possible changes in NE mRNA transcript levels. Southern hybridization of the PCR products using an internal NE probe demonstrated linear upregulation (at 22 and 25 cycles) of NE mRNAlevels in C/EBPα–/––p42-transduced cells and lack of expression in C/EBPα–/–-Lys298Glu, C/EBPα–/––BRM-2, and parental C/EBPα–/– cells (Figure 7A, top 2 panels). Amplified cDNA copies of NE mRNA transcripts were detectable on the agarose gel after 28 cycles (Figure 7A, lower panel). NE mRNAtranscripts were amplified from cells grown in the absence of G-CSF, thereby demonstrating specific C/EBPα-dependent gene induction.
Effects of WT and mutant C/EBPα on C/EBPα–/– cells. (A) NE mRNA transcripts in C/EBPα–/– cells ectopically expressing p42 or mutant Lys298Glu C/EBPα. Total RNA was extracted from GFP-sorted C/EBPα–/– cells expressing the indicated C/EBPα protein. NE transcripts were detected by RT-PCR (22, 25, and 28 cycles), and identity of the PCR products was confirmed by Southern blot hybridization using a 32P-labeled internal NE oligonucleotide probe. Panel 3 shows the ethidium bromide–stained agarose gel in which PCR products (28 cycles) were run. Amplification of GRB2 mRNA transcripts was performed as loading control. (B) Morphology (May-Grünwald/Giemsa–stained cytospins; original magnification, × 40.) of GFP-selected C/EBPα–/– cells transduced with the MigR1 or C/EBPα (wild-type or mutant) retrovirus and incubated with G-CSF for 4 days (lower panel), or left in their culture medium that contains 2% IL-3 and 2% SCF and is indicated as IL-3 (upper panel). Representative of 3 independent experiments.
Effects of WT and mutant C/EBPα on C/EBPα–/– cells. (A) NE mRNA transcripts in C/EBPα–/– cells ectopically expressing p42 or mutant Lys298Glu C/EBPα. Total RNA was extracted from GFP-sorted C/EBPα–/– cells expressing the indicated C/EBPα protein. NE transcripts were detected by RT-PCR (22, 25, and 28 cycles), and identity of the PCR products was confirmed by Southern blot hybridization using a 32P-labeled internal NE oligonucleotide probe. Panel 3 shows the ethidium bromide–stained agarose gel in which PCR products (28 cycles) were run. Amplification of GRB2 mRNA transcripts was performed as loading control. (B) Morphology (May-Grünwald/Giemsa–stained cytospins; original magnification, × 40.) of GFP-selected C/EBPα–/– cells transduced with the MigR1 or C/EBPα (wild-type or mutant) retrovirus and incubated with G-CSF for 4 days (lower panel), or left in their culture medium that contains 2% IL-3 and 2% SCF and is indicated as IL-3 (upper panel). Representative of 3 independent experiments.
The C/EBPα knock-out (C/EBPα–/–) cells were then used to further assess the role of WT and mutant C-EBPα in granulocytic differentiation, as these cells do not undergo differentiation following G-CSF treatment (Figure 7B, left panels). Thus, cells transduced with WT or mutant C/EBPα were selected by GFP positivity (day 0) and assessed for morphologic differentiation in the presence of G-CSF. Consistent with the findings in 32Dcl3 and lineage-negative myeloid precursors, C/EBPα–/–-p42 and C/EBPα–/––Δ177-191 cells underwent terminal differentiation (77% and 75%, respectively, in 3 independent experiments), whereas C/EBPα–/–-Lys298Glu and C/EBPα–/––BRM-2 cells remained undifferentiated (Figure 7B). In IL-3–supplemented cultures, only p42 C/EBPα had a modest effect (approximately 10% in 3 independent experiments). Thus, Cdk2 binding does not seem to be important for C/EBPα-dependent gene expression and differentiation, whereas abrogation of DNA binding and gene induction capability through mutation of the basic region/DNA binding domain severely compromises C/EBPα potential to induce granulocytic differentiation.
Discussion
In this paper, we have attempted to dissect the domains of C/EBPα that are required for granulocytic differentiation. Evidence suggests that the differentiation-inducing effect of C/EBPα is determined by its ability to modulate gene expression, by its growth suppressive function that appears to depend on protein-protein interactions (reviewed in McKnight31 ), or by both. We have assessed the ability of C/EBPα mutants that are unable to transactivate gene expression (p30) or bind DNA (Lys298Glu), or are defective in interaction with Cdk2/Cdk4 (Δ177-191) or E2F (BRM-2), to induce granulocytic differentiation. Our findings indicate that p42 C/EBPα and the Δ177-191 mutant were capable of inducing neutrophilic differentiation, whereas the mutants Lys298Glu, BRM-2, and the N-terminus truncated p30 were not, in 32Dcl3 cells, in lineage-negative primary bone marrow cells, and in fetal liver C/EBPα knock-out cells. Our data suggest that several requirements need to be met for C/EBPα-dependent granulocytic differentiation.
In this study, ectopic expression of wild-type C/EBPα failed to induce differentiation of 32Dcl3 cells growing in IL-3 (Figure 1). This finding contrasts with a previous study of Wang et al32 that reported induction of differentiation by the β-estradiol–regulated C/EBPα-ER chimera. This raises the possibility that the different effects of C/EBPα in 32Dcl3 cells cultured in IL-3 might, in part, depend on the properties of the plasmids used. C/EBPα has been shown to interact with Cdk2 and Cdk4, 2 proteins whose enzymatic activity is crucial for cell cycle progression. The interaction with Cdk2 prevents cyclin binding, an essential step in Cdk2 activation and G1 to S phase cell cycle progression, thereby resulting in cell cycle arrest. The inability of the Δ177-191–C/EBPα mutant to induce cell cycle arrest was not caused by a defect in its DNAbinding function.23 This finding raised the possibility that the Δ177-191 mutant might be able to induce granulocytic differentiation if inhibition of proliferation dependent on Cdk2 binding was essential for the effect. However, this mutant was as effective as wild-type C/EBPα in promoting granulocytic differentiation, in interacting with the C/EBPα binding sites of the G-CSFR and NE gene promoters, and in inducing luciferase activity from a G-CSFR promoter and NE mRNA expression in primary marrow cells and C/EBPα knock-out cells. Thus, the binding to Cdk2 and the possible growth suppression achieved by this mechanism do not seem to be required for granulocytic differentiation, although it cannot be excluded that they are important in differentiation of other cell types.
Timchenko et al21,22 have shown also that C/EBPα directly interacts with p21. Through this interaction, C/EBPα might enhance the p21 inhibitory activity on Cdk2/Cdk4, thus leading to growth arrest,21-23 although there is evidence that C/EBPα can arrest proliferation of p21–/– mouse embryo fibroblasts.33 C/EBPα-mediated growth arrest may be also enforced through proteasome-dependent degradation of Cdk4.34 Consistent with the observations that C/EBPα inhibits proliferation by its effects on several cell cycle regulators, Harris et al24 showed that the DNA binding ability of C/EBPα was not required for C/EBPα-mediated growth arrest as the Lys298Glu mutant remained able to induce growth arrest in fibroblast cell lines. We have extended these observations by assessing the effects of Lys298Glu-C/EBPα in granulocytic differentiation of hematopoietic cells. Consistent with the findings of Harris et al24 that the Lys298Glu mutant was unable to activate transcription of a reporter gene driven by C/EBPα binding sites, we demonstrated that Lys298Glu does not bind to the G-CSFR and NE promoters and does not activate C/EBPα-dependent gene expression. Moreover, the absence of C/EBPα-dependent gene induction correlated with the inability to promote granulocytic differentiation, further indicating that the DNA binding function of C/EBPα is a critical requirement for the induction of granulocytic differentiation. Therefore, although the DNA binding activity may not be required for growth arrest induced by C/EBPα, it appears to be crucial for C/EBPα-dependent gene induction and granulocytic differentiation. The inability of the Lys298Glu mutant to induce granulocytic differentiation is consistent with a dominant-negative effect. This mutant cannot bind DNA (Figure 3) but can still dimerize through its bZIP domain, as it can form homodimers and heterodimers with itself and WT C/EBPα, respectively (data not shown and Figure 5B). Therefore, it is likely that ectopically expressed Lys298Glu-C/EBPα suppresses the function of the endogenous C/EBPα via a dominant-negative effect.
Another cell cycle regulator that was shown to be inhibited by C/EBPα is E2F, as indicated by C/EBPα repression of E2F-dependent transcription.25,26 C/EBPα can directly bind E2F, but this binding does not interfere with the DNA binding function of E2F.25,26 The binding of C/EBPα does affect E2F transcription activity that can also result in repression of c-Myc, and this effect might be important for granulocytic differentiation.20 The BRM-2 mutant contains 2 amino acid mutations in the basic region that have been shown to mediate, in part, the physical association of C/EBPα with E2F.26 Porse et al26 have shown that knock-in BRM-2 mice that lack endogenous C/EBPα display abnormal adipocyte and granulocyte differentiation. The mutant failed to bind E2F in vitro and in vivo, but neither the ability to bind DNA nor the capacity to activate target gene expression in vivo were detectably affected in adipocyte tissue.26 In our studies, the BRM-2 mutant was unable to induce granulocytic differentiation in each cell culture system. This mutant interacted with E2F-1 but failed to efficiently transactivate luciferase expression from a C/EBPα target gene promoter (Figure 4A) and to induce C/EBPα-dependent gene expression (Figures 4 and 6) even if it was able to interact with C/EBPα DNA binding sites as assessed by EMSA (Figures 3 and 6B). That E2F-1 interacted with BRM-2 C/EBPα was surprising, based on the study of Porse et al.26 However, BRM-2 C/EBPα retains a region of interaction with E2F in its N-terminus,26 suggesting that this segment may be responsible for E2F-1 binding in our assay. We are presently testing whether BRM-2 C/EBPα interacts with other members (E2F-2 and E2F-3) of the E2F family, which are required for proliferation of mammalian cells35 and could interfere with myeloid cell differentiation, if constitutively active.
We do not have yet an explanation for the inability of BRM-2 C/EBPα to activate gene expression. The BRM-2 mutant did not differ from the WT protein in its affinity for C/EBPα binding sites. However, BRM-2-C/EBPα ectopically expressed in 32D-BCR/ABL 6:15 cells formed a DNA-protein complex more slowly migrating than that involving p42 C/EBPα (Figure 6B). This DNA-protein complex of altered mobility was specific because 32D-BCR/ABL 6:15 cells do not express endogenous C/EBPα and C/EBPβ, and the complex was supershifted by an anti-C/EBPα antibody. Whether formation of this altered complex is linked to the inability of BRM-2 to activate gene expression and induce differentiation is a question that can be addressed on identification of the proteins present in the BRM-2–containing complex.
C/EBPα is a lineage-determining transcription factor that modulates gene expression and suppresses proliferation by DNA binding–dependent and –independent mechanisms. Moreover, C/EBPα dysregulation and gene mutation have been implicated in the development of acute myeloid leukemias8 and in transition of chronic-phase CML to blast crisis,14 emphasizing the importance of understanding the mechanisms whereby C/EBPα functions during myeloid differentiation. By a structural-functional analysis, we have demonstrated here that the DNA binding and transactivation domains are required for the differentiationinducing function of C/EBPα. Although C/EBPα appears to suppress proliferation by interfering with the activity of several cell cycle regulators, our study does not support the importance of these mechanisms in granulocytic differentiation.
In conclusion, induction of gene expression appears to be the primary mechanism underlying C/EBPα regulation of granulocytic differentiation. However, it is not yet clear if this mechanism is also required for induction of cell cycle arrest, a function that at least in myeloid cells, is insufficient to promote differentiation independent from modulation of gene expression.
Prepublished online as Blood First Edition Paper, April 17, 2003; DOI 10.1182/blood-2003-02-0477.
Supported by grants from the National Cancer Institute (grants PO1 CA78890 and R01 CA95111) (B.C.), the E. Pardee Foundation (D.P.), and the Lauri Strauss Leukemia Research Foundation (D.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs. Daniel G. Tenen, H. Radomska, and E. Duprez for helpful discussion, sharing unpublished information, and for providing plasmids and the C/EBPα–/– cells. The human E2F-1 cDNA was a gift of Dr W. G. Kaelin.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal