Abstract
Imatinib mesylate, a competitive inhibitor of Abl tyrosine kinase, is highly effective for the early stages of chronic myelogenous leukemia (CML), but remissions induced in advanced-phase CML and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia tend to be relatively short-lived. Therefore, the search for agents that enhance the anti-Ph+ effect of imatinib mesylate is warranted. We investigated the combined effects of imatinib mesylate and the third-generation bisphosphonate zoledronate (ZOL) on Ph+ leukemias, because ZOL inhibited the prenylation of Ras-related proteins downstream of Bcr/Abl. First, we identified the potency of ZOL in vitro against human leukemic cell lines, including 2 Ph+ and a P-glycoprotein–overexpressing leukemic cell line. ZOL was also effective in vivo because as it prolonged the survival of nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice who were given xenografts with Ph+ BV173 leukemic cells. Next, we showed the in vitro synergistic effects with ZOL and imatinib mesylate for Ph+ cell lines. ZOL combined with imatinib mesylate showed synergistic effects in vivo that prolonged the survival of mice inoculated with BV173. ZOL only minimally inhibited the growth of normal hematopoietic progenitors in vitro, and mice receiving ZOL or imatinib mesylate or both tolerated these treatments well. These findings indicate that ZOL is a potent antileukemic agent that augments synergistically the anti-Ph+ leukemia activity of imatinib mesylate.
Introduction
Imatinib mesylate, a selective inhibitor of Abl tyrosine kinase, has significant and rapid antileukemic activity against chronic myelogenous leukemia (CML) and Philadelphia-positive (Ph+) acute lymphoblastic leukemia (ALL). However, refractoriness and early relapse have frequently been reported, particularly in patients with advanced-stage disease.1-3 Several mechanisms of refractoriness and relapse have been reported, including point mutations within the Abl kinase domain,4,5 Bcr-Abl gene amplification, Bcr-Abl mRNA overexpression, and increased drug efflux via the P-glycoprotein (P-gp)–mediated process,6-8 which all lead to reactivation of the Bcr-Abl kinase. Levels of Bcr-Abl mRNA in peripheral blood (PB) and bone marrow (BM) after imatinib mesylate administration have been reported to be predictive of good response achievement and prolonged survival.9 These findings suggest that therapeutic agents that can augment the anti-Ph+ leukemia activity of imatinib mesylate may be mandatory to overcome resistance and relapse. To improve response rates and prolong survival, a number of preclinical and clinical evaluations of combinations of imatinib mesylate and other commonly used antileukemic agents have been investigated.10 Treatment of imatinib-resistant Ph+ leukemia is challenging. However, the effects of combination therapy with imatinib mesylate against Ph+ leukemias are still controversial.
Ras signaling pathways are frequently overly active in Ph+ leukemias. Therefore, targeting one of the Bcr-Abl downstream signaling proteins essential for Bcr-Abl–mediated leukemogenesis, in addition to Bcr-Abl, was intriguing. Several lines of evidence have implicated the Ras/mitogen-activated protein kinase (MAPK) signaling pathway as an important molecular target.11-13 Farnesyl transferase inhibitors (FTIs) reportedly block Ras signaling by preventing farnesylation.11,14-16 However, even when farnesyl transferase is inhibited, Ras can be transferred to the membrane by an alternative pathway using geranylgeranyl transferase-1 to successfully transduce Bcr-Abl signals.17
Bisphosphonates (BPs), developed primarily to treat bone diseases, may also act as anticancer drugs by inhibiting the activation of Ras proteins through suppression of both geranylgeranylation and farnesylation.18-21 Second- and third-generation BPs inhibit the proliferation of some cancer cells by preventing posttranslational prenylation of Ras-related proteins. One such novel third-generation BP is zoledronate (ZOL), a heterocyclic imidazole. Compared to one of the second-generation BPs, pamidronate (PAM), ZOL is more effective by 87-fold at increasing rat femoral trabecular calcium content and by 940-fold in thyroparathyroidectomized rat model.22 ZOL may be a potent anti-Ph+ leukemia agent for combined therapeutic use with imatinib mesylate.
First, we evaluated the effects of ZOL alone on the growth of human leukemic cell lines including a line overexpressing P-gp. ZOL alone showed significant inhibitory effects on leukemic cell growth in vitro and in vivo. Next, we assessed the combined effects of ZOL and imatinib mesylate in 2 Ph+ cell lines in vitro and found significant synergistic suppression of growth. ZOL combined with imatinib mesylate significantly prolonged the survival of nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice who received xenografts with Ph+ leukemic cells, indicating that ZOL has antileukemic activity and augments synergistically the anti-Ph+ leukemia effects of imatinib mesylate.
Materials and methods
Reagents and cell lines
Imatinib mesylate and ZOL (1-hydroxy-2-(1H-imidazole-1-yl) ethylidenebisphosphonic acid) were obtained from Novartis Pharma (Basel, Switzerland). PAM (3-amino-1-hydroxy-propylidene-1.1-bisphosphonate) and hydrochloride daunorubicin (DNR) were obtained from Nippon Kayaku (Tokyo, Japan) and Sigma Aldrich (Tokyo, Japan), respectively. The human leukemic cell lines BV173, K562, HL60, NALM6, and SEM were obtained from American Type Culture Collection (Manassas, VA). BV173 and K562 are pre-B and erythroleukemic cell lines, respectively, established from the blastic phase of Ph+ CML. HL60 was established from an acute myelogenous leukemia and NALM6 from a pre-B cell ALL. SEM is a biphenotypic acute leukemia. Cells were maintained at 37°C in a fully humidified atmosphere of 5% CO2 in air as suspension cultures in RPMI 1640 medium (Gibco BRL, Paisley, Scotland; BV173, K562, HL60, and NALM6) or, for SEM, basal Iscove medium (Gibco BRL) supplemented with 10% heat-inactivated fetal calf serum (FCS; Hyclone, Logan, UT). The P-gp–overexpressing multidrug-resistant (MDR) K562/D1-9 cell line was established previously and maintained in suspension cultures in RPMI 1640 medium with 10% heat-inactivated FCS and 0.1 μM DNR.23 Cells undergoing exponential growth were used in the experiments.
Growth inhibitory effects of imatinib mesylate, ZOL, and PAM in vitro
Cell proliferation was determined by the trypan blue dye exclusion method and a modified methyl-thiazol-diphenyl-tetrazolium (MTT) assay with the SF reagent (Nacalai Tesque, Kyoto, Japan). Leukemic cell lines were seeded in a flat-bottomed 96-well plate (Greiner Labortechnik, Hamburg, Germany) at 2 × 104 cells in 100 μL medium per well and incubated with various concentrations of imatinib mesylate, ZOL, or PAM for 48 hours. K562/D1-9 cells were cultivated under similar conditions, with various concentrations of ZOL or DNR for 72 hours. The means of 5 values were calculated. Values for inhibitory concentration of 50% (IC50) were obtained using the nonlinear regression program CalcuSyn (Biosoft, Cambridge, United Kingdom).
Western blot analysis
Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then electroblotted onto a nitrocellulose membrane (Amersham Biosciences, Tokyo, Japan). The membranes were saturated with 5% (wt/vol) nonfat dry milk in TBST (25 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 7.8, 140 mM NaCl, 0.1% [vol/vol] Tween 20) and then incubated overnight with goat polyclonal anti-Rap1A antibody (diluted 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) and mouse monoclonal anti-Ras antibody (diluted 1:1000; Becton Dickinson, San Jose, CA). The membranes were washed thoroughly with TBST and incubated for 1 hour with antigoat IgG coupled to horseradish peroxidase (Santa Cruz Biotechnology) for Rap1A and antimouse rabbit IgG coupled to peroxidase (Sigma Aldrich) for Ras. Detection was performed with enhanced chemiluminescence kits (Amersham Biosciences).
Cell-cycle analysis and induction of apoptosis
Untreated BV173 cells or BV173 cells treated with ZOL for 12 or 24 hours were analyzed for alterations in the cell cycle by double staining with fluorescein isothiocyanate (FITC)–conjugated antibromodeoxyuridine (BrdUrd; Becton Dickinson) and propidium iodide (PI; Sigma Aldrich). These cells were also evaluated for apoptosis using TUNEL with PI counterstaining as previously described.24
Toxicity of ZOL in normal hematopoietic progenitors
Normal hematopoietic progenitors from leukapheresis blood were obtained from 5 healthy donors who provided cells with mobilized granulocyte colony-stimulating factor (G-CSF); all 5 gave informed consent. Cells (1 × 105) were exposed to 0, 10, 20, or 50 μM ZOL in a 1-mL standard methylcellulose culture (MethoCult GF H4434; StemCell Technologies, Vancouver, BC, Canada) for 12 days. The numbers of granulocyte-macrophage colony forming unit (CFU-GM)–derived colonies were counted under an inverted microscope.25 Three dishes were prepared for each group.
Combined effects of imatinib mesylate and ZOL in vitro
After an IC50 was obtained for each drug, the antileukemic activity of imatinib mesylate in combination with ZOL was assayed in a single 96-well plate. Both Ph+ leukemic cell lines, BV173 and K562 cells, and other Ph– cell lines were treated with 6 concentrations (0.25, 0.5, 0.75, 1.0, 1.5, or 2.0 × IC50) of both compounds. Both compounds were also treated as single agents on the determination of the combined effects. The fraction affected (Fa) (ie, Fa of 0.25 would equal 25% viable cells) and the combination index (CI) were calculated with CalcuSyn (Biosoft).26 This method provides quantification of synergism (CI < 1) and antagonism (CI > 1) at different dose and effect levels. CI calculations were made under the assumption that the mechanisms of the drugs were not mutually exclusive.
In vivo effects of imatinib mesylate, ZOL, and their combination
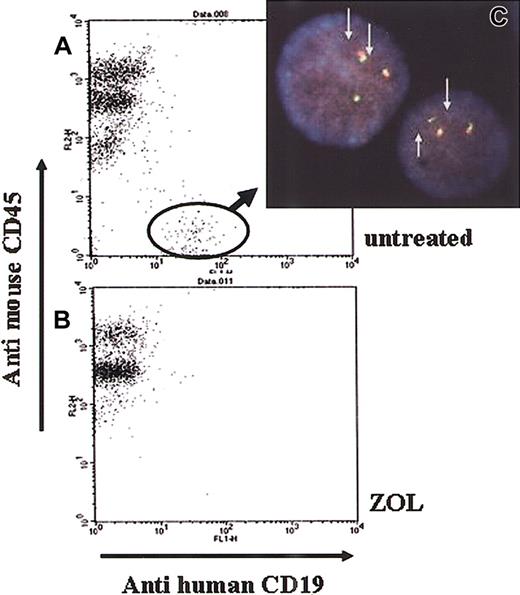
Approval was obtained from the institutional review board at Kyoto University Hospital for these studies. Male NOD/SCID mice 6 to 8 weeks of age (Japan Clea, Osaka, Japan) were sublethally irradiated (2 Gy) and administered 1 × 106 BV173 cells on day 0. Seven days after injection of BV173 cells, 3 mice were killed and BM cells were collected. After we confirmed the engraftment of human leukemic cells in BM by reverse transcription–polymerase chain reaction (RT-PCR; data not shown), administration of ZOL or imatinib mesylate or both was initiated on day 8. The RT-PCR methods for detection of b2a2 transcripts were described elsewhere.27 Mice were divided into 5 groups as follows: (1) untreated mice; (2) mice treated with 80 μg/kg ZOL once a week for 6 weeks; (3) mice treated with 80 μg/kg ZOL 3 sequential days a week for 10 weeks; (4) mice treated with 120 mg/kg/d imatinib mesylate from day 8 to 17; and (5) mice treated with 80 μg/kg ZOL 3 sequential days a week for 10 weeks and with 120 mg/kg/d imatinib mesylate from day 8 to 17. Each group contained 6 mice. Imatinib mesylate was dissolved in deionized distilled water and 60 mg/kg imatinib mesylate was given orally by gavage twice a day. ZOL was administered intravenously via the tail vein. After 10 weeks of injection, PB was collected via the orbital plexus under anesthesia. Leukemic cells in PB were determined to have a Bcr-Abl fusion gene by fluorescence in situ hybridization analysis as described previously6,28 and the percentages of human leukemic cells in PB were determined by flow cytometry after double staining with FITC-conjugated antihuman CD19 antibody (Becton Dickinson) and phycoerythrin-conjugated antimouse CD45 antibody (Becton Dickinson). For survival analysis, death was determined either by spontaneous death or elective killing due to pain or suffering according to established criteria. To determine whether the death was accompanied with leukemic progression or not, we performed a flow cytometric analysis of PB and BM as well as a microscopic analysis of the liver and spleen in each mouse. At 40 weeks, all surviving mice were humanely killed and their PB and BM were analyzed by flow cytometry.
Statistical analysis
The influence of ZOL on normal hematopoietic progenitors was compared by the Student t test. To analyze the in vivo efficacy, survival curves were drawn using the Kaplan-Meier method and compared with the log-rank test. P values were derived from 2-sided tests and P < .05 was considered statistically significant.
Results
Inhibition of cell growth by imatinib mesylate, ZOL, or PAM
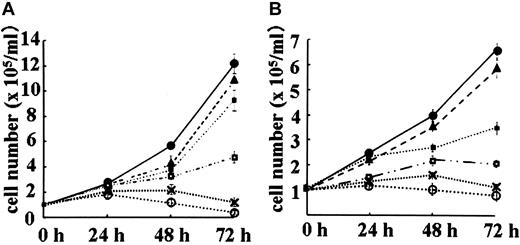
Figure 1A represents the inhibitory growth effect of ZOL in BV173, the most sensitive cell line, and Figure 1B represents the effect of ZOL in the HL60 cell line, the worst-responding cell line. Likewise, in the BV173 cell line and the HL60 cell line, the growth of other 3 leukemic cell lines was inhibited by ZOL in a concentration-dependent manner (data not shown). PAM concentrations exceeding 300 μM were required to inhibit the growth of the 5 lines to similar degrees. Imatinib mesylate inhibited the growth of the 2 Ph+ cell lines, BV173 and K562, at lower concentration, whereas a rather higher dose of imatinib mesylate was needed to inhibit the growth of Ph– cell lines, HL60, NALM 6, and SEM. The IC50 values of these compounds are summarized in Table 1.
Effect of ZOL on BV173 leukemic cell growth. BV173 cells were exposed to 0 μM (•), 1 μM (▴), 5 μM (▪), 10 μM (□), 20 μM (x), or 30 μM (○) ZOL (A), whereas HL60 cells (B) were exposed to 0 μM (•), 5 μM (▴), 25 μM (▪), 50 μM (□), 100 μM (x), or 150 μM (○), respectively. Cells were counted by the trypan blue dye exclusion method. Data are presented as means ± SDs of at least 3 independent experiments.
Effect of ZOL on BV173 leukemic cell growth. BV173 cells were exposed to 0 μM (•), 1 μM (▴), 5 μM (▪), 10 μM (□), 20 μM (x), or 30 μM (○) ZOL (A), whereas HL60 cells (B) were exposed to 0 μM (•), 5 μM (▴), 25 μM (▪), 50 μM (□), 100 μM (x), or 150 μM (○), respectively. Cells were counted by the trypan blue dye exclusion method. Data are presented as means ± SDs of at least 3 independent experiments.
The IC50 values of the leukemic cell lines for imatinib mesylate, ZOL, and PAM
. | ZOL, μM . | PAM, μM . | Imatinib mesylate, μM . |
|---|---|---|---|
| BV173 | 24.1 | >300 | 0.907 |
| K562 | 60.9 | >300 | 0.219 |
| HL60 | 87.2 | >300 | 15.8 |
| NALM6 | 73.4 | >300 | 15.1 |
| SEM | 26.1 | >300 | 39.4 |
. | ZOL, μM . | PAM, μM . | Imatinib mesylate, μM . |
|---|---|---|---|
| BV173 | 24.1 | >300 | 0.907 |
| K562 | 60.9 | >300 | 0.219 |
| HL60 | 87.2 | >300 | 15.8 |
| NALM6 | 73.4 | >300 | 15.1 |
| SEM | 26.1 | >300 | 39.4 |
Values represent the mean of at least 3 independent experiments.
Effect of ZOL on small GTP-binding protein prenylation
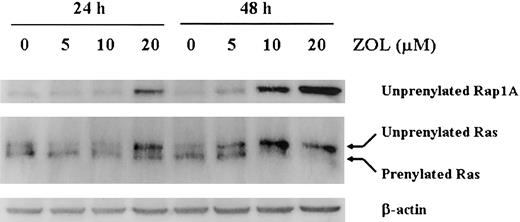
BV173 cell lysates were analyzed by Western blotting using an antibody that specifically recognizes the unprenylated form of Rap1A, a substrate of geranylgeranyl transferase (GGTase I).29 Unprenylated Rap1A was absent in untreated BV173 cells but accumulated markedly after treatment for 24 hours with 5, 10, or 20 μM ZOL. Unprenylated Rap1A levels were even greater after 48 hours of treatment with ZOL. As previously described,29 the anti-Ras antibody recognized both a slower migrating band, indicating the unprenylated form Ras, and a faster migrating band, indicating the prenylated form Ras. Unprenylated Ras was faintly stained in untreated BV173, whereas it accumulated after ZOL treatment in dose- and time-dependent manner. In contrast, prenylated Ras was gradually decreased after ZOL treatment. After 20 μM ZOL treatment for 48 hours, prenylated Ras was almost diminished (Figure 2).
ZOL prevents the prenylation of Rap. Cell lysates from BV173 cells treated with 5, 10, or 20 μM ZOL for 24 and 48 hours were subjected to Western blotting using an antibody specific for the unprenylated form of Rap1A, an antibody for Ras, and for β-actin. The data shown are representative of 3 independent experiments.
ZOL prevents the prenylation of Rap. Cell lysates from BV173 cells treated with 5, 10, or 20 μM ZOL for 24 and 48 hours were subjected to Western blotting using an antibody specific for the unprenylated form of Rap1A, an antibody for Ras, and for β-actin. The data shown are representative of 3 independent experiments.
Effects of ZOL on the cell cycle and induction of apoptosis
After exposure for 12 or 24 hours, ZOL decreased the percentage of cells in G2/M phase and increased the percentage between the G0/G1 and G2/M phases lacking appropriate BrdUrd incorporation that were expected to undergo apoptosis (Figure 3A-C). A TUNEL assay with PI counterstaining revealed that ZOL induced leukemic cells into apoptosis from S phase to G2/M boundary (Figure 3D-F).
ZOL alters the cell cycle and induces apoptosis in BV173 cells. The effect of ZOL was evaluated by cell cycle analysis using flow cytometry. BV173 cells were exposed to different concentrations of ZOL for 12 or 24 hours. ZOL at 20 μM decreased the percentage of cells in G2/M phase and increased the proportion of cells between G0/G1 and G2/M without appropriate BrdUrd incorporation (indicated by arrows in panels A-C). A TUNEL assay with PI counterstaining revealed that ZOL induces widespread apoptosis from the S phase to G2/M boundary (D-F). The data shown are representative of 3 independent experiments.
ZOL alters the cell cycle and induces apoptosis in BV173 cells. The effect of ZOL was evaluated by cell cycle analysis using flow cytometry. BV173 cells were exposed to different concentrations of ZOL for 12 or 24 hours. ZOL at 20 μM decreased the percentage of cells in G2/M phase and increased the proportion of cells between G0/G1 and G2/M without appropriate BrdUrd incorporation (indicated by arrows in panels A-C). A TUNEL assay with PI counterstaining revealed that ZOL induces widespread apoptosis from the S phase to G2/M boundary (D-F). The data shown are representative of 3 independent experiments.
Toxicity of ZOL in normal hematopoietic progenitors
The number of CFU-GM–derived colonies without ZOL from 5 healthy individual donors was 78 ± 14/1 × 105 cells at day 12. When normal progenitors were treated with 10, 20, or 50 μM ZOL, the CFU-GM–derived colonies were 90% ± 4.1%, 87% ± 5.4%, and 83% ± 6.7% of the control, respectively. These percentages are the mean ± SE among the 5 individuals. Colony formation derived from erythroid burst-forming units (BFU-Es) was also not suppressed by ZOL treatment. Thus, ZOL at in vitro dosages up to 50 μM does not suppress the growth of normal hematopoietic progenitors.
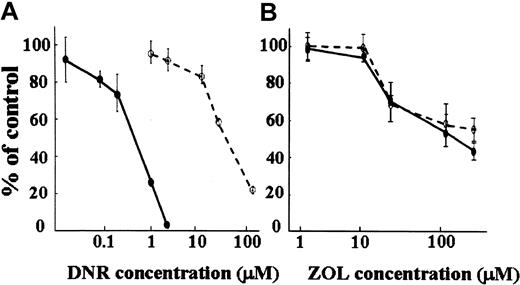
Effect of ZOL on the P-gp–overexpressing K562/D1-9 cell line
The K562/D1-9 cell line is 75 times more resistant to DNR than its parental K562 cell line (Figure 4A). However, this K562/D1-9 cell line was as sensitive to ZOL as the parental cell line (Figure 4B), indicating that ZOL does not involve the P-gp–related MDR system.
Effect of ZOL on P-gp–overexpressing MDR K562/D1-9 cells. Growth inhibitory effects of ZOL were determined by the trypan blue dye exclusion method. The MDR K562/D1-9 cell line (○) was 75 times more resistant to DNR than its parental K562 (•) cell line (A). The K562/D1-9 cell line is as sensitive to ZOL as the parental cell line (B).
Effect of ZOL on P-gp–overexpressing MDR K562/D1-9 cells. Growth inhibitory effects of ZOL were determined by the trypan blue dye exclusion method. The MDR K562/D1-9 cell line (○) was 75 times more resistant to DNR than its parental K562 (•) cell line (A). The K562/D1-9 cell line is as sensitive to ZOL as the parental cell line (B).
In vitro combined effects of ZOL and imatinib mesylate
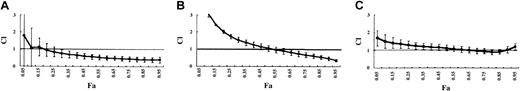
The data are plotted as CI versus Fa. At growth inhibition levels exceeding Fa 0.2 and Fa 0.55, imatinib mesylate plus ZOL acted synergistically (CI < 1.0) in Ph+ leukemic cell lines, BV173 (Figure 5A) and K562 (Figure 5B). At Fa 0.80, the CIs of BV173 and K562 were 0.443 ± 0.136 and 0.664 ± 0.142, respectively, revealing significant synergism between imatinib mesylate and ZOL at inhibiting the growth of Ph+ leukemic cell lines. In contrast, as shown in Figure 5C representing the result of HL60, imatinib mesylate plus ZOL showed at most the additive but not synergistic combination effects in other Ph– cell lines.
The combined effect of ZOL and imatinib mesylate on leukemic cell proliferation. CI is plotted as a function of the fraction affected (Fa), which represents the percentage of growth inhibition and was evaluated using the modified MTT assay (0.5 = 50%). This allows the combination of multiple equipotent drug concentrations to be analyzed for synergistic (CI < 1), additive (CI = 1), or antagonistic (CI > 1) effects. Panel A represents the result in BV173; panel B represents the result in K562; and panel C represents the result in HL60. Data points show the means of 3 independent experiments. Bars represent 1 SD.
The combined effect of ZOL and imatinib mesylate on leukemic cell proliferation. CI is plotted as a function of the fraction affected (Fa), which represents the percentage of growth inhibition and was evaluated using the modified MTT assay (0.5 = 50%). This allows the combination of multiple equipotent drug concentrations to be analyzed for synergistic (CI < 1), additive (CI = 1), or antagonistic (CI > 1) effects. Panel A represents the result in BV173; panel B represents the result in K562; and panel C represents the result in HL60. Data points show the means of 3 independent experiments. Bars represent 1 SD.
In vivo effects of ZOL alone and in combination with imatinib mesylate
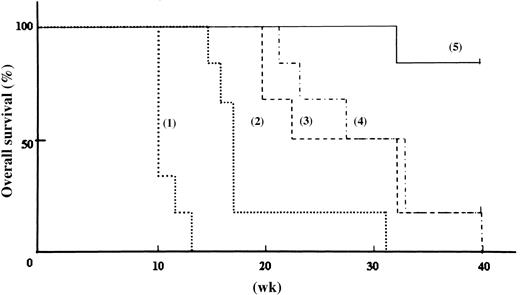
Because in vitro assay of ZOL alone or in combination with imatinib mesylate showed significant effects against leukemia cell growth, we also investigated the in vivo effects of ZOL. Ten weeks after transplantation, all untreated NOD/SCID mice injected with BV173 cells (group 1) had human leukemic cells with Bcr-Abl fusion genes in their PB (Figure 6C). The average percentage of leukemic cells in the PB was 4.0% ± 0.6% (Figure 6A). All untreated mice died 12 weeks after transplantation. Of the mice in the group 2 administered the lower does of ZOL (80 μg/kg/wk for 6 weeks), one died of an unknown cause, whereas human leukemic cells were not detected in the remaining 5 mice at the 15th week. However, human leukemic cells were detected in the PB of 4 of the 5 mice at week 16 and these mice died within 2 weeks. The last mouse in group 2 died at week 31 (Figure 7). The log-rank test for overall survival showed statistically significant differences between groups 1 and 2 (P < .001). Mice in group 3 were administered a higher dose of ZOL (240 μg/kg/wk for 10 weeks) and leukemic cells were detected in the PB of 2 mice at week 19; these mice died at week 20. Five of the 6 mice died by week 32, whereas the last survived until the 40th week. The log-rank test revealed that the higher dose of ZOL was significantly more effective than the lower dose (P = .014). Mice of group 4 received imatinib mesylate alone; 5 of the 6 mice died before the 33rd week and the sixth mouse died at the 40th week. Interestingly, the combination of ZOL and imatinib mesylate was substantially more efficacious than imatinib mesylate alone. The combination treatment administered to group 5 mice prolonged survival compared to survival of mice that received the higher dose of ZOL alone (group 3; P = .017) and that received imatinib mesylate alone (group 4; P = .006). Of the mice in group 5 receiving combination therapy, only one mouse that did not appear to have leukemic cells died of an unknown cause at week 32, whereas the remaining 5 mice survived until week 40. Flow cytometric analyses identified the presence of circulating leukemic cells in the PB a few weeks before death in all mice that died by the 40th week except one in group 2 and one in group 5. In these mice, autopsy revealed hepatosplenomegaly and microscopic examinations identified leukemic invasion in the BM, liver, and spleen (data not shown). PB and BM cells of all the mice that survived until the 40th week showed no apparent leukemic cell invasion. These data clearly indicate that ZOL alone is a potent antileukemic agent not only in vitro but also in vivo that significantly enhances the effects of imatinib mesylate in vivo.
In vivo effect of ZOL on the emergence of leukemic cells in the blood of engrafted mice. Ten weeks after leukemic cell engraftment, all untreated NOD/SCID mice (group 1) had human leukemic cells (A), whereas none of the ZOL-treated mice (groups 2 and 3) had human leukemic cells in their PB (B). Fluorescein in situ hybridization analysis revealed that the human cells in the untreated mice had 2 Bcr-Abl fusion genes (arrow), indicating they were Ph+ leukemic cells (C). Original magnification × 400. The data shown are representative of 6 mice.
In vivo effect of ZOL on the emergence of leukemic cells in the blood of engrafted mice. Ten weeks after leukemic cell engraftment, all untreated NOD/SCID mice (group 1) had human leukemic cells (A), whereas none of the ZOL-treated mice (groups 2 and 3) had human leukemic cells in their PB (B). Fluorescein in situ hybridization analysis revealed that the human cells in the untreated mice had 2 Bcr-Abl fusion genes (arrow), indicating they were Ph+ leukemic cells (C). Original magnification × 400. The data shown are representative of 6 mice.
In vivo effect of ZOL alone and in combination with imatinib mesylate on survival in engrafted mice. Each line represents the survival ratio of 5 groups of mice. Administration of ZOL/imatinib mesylate was initiated on day 8. Mice were divided as follows: (1) untreated mice; (2) mice treated with 80 μg/kg ZOL once a week for 6 weeks; (3) mice treated with 80 μg/kg ZOL 3 sequential days a week for 10 weeks; (4) mice treated with 120 mg/kg/d imatinib mesylate from day 8 to 17; and (5) mice treated with 80 μg/kg ZOL 3 sequential days a week for 10 weeks and with 120 mg/kg/d imatinib mesylate from day 8 to 17. Each group contained 6 mice.
In vivo effect of ZOL alone and in combination with imatinib mesylate on survival in engrafted mice. Each line represents the survival ratio of 5 groups of mice. Administration of ZOL/imatinib mesylate was initiated on day 8. Mice were divided as follows: (1) untreated mice; (2) mice treated with 80 μg/kg ZOL once a week for 6 weeks; (3) mice treated with 80 μg/kg ZOL 3 sequential days a week for 10 weeks; (4) mice treated with 120 mg/kg/d imatinib mesylate from day 8 to 17; and (5) mice treated with 80 μg/kg ZOL 3 sequential days a week for 10 weeks and with 120 mg/kg/d imatinib mesylate from day 8 to 17. Each group contained 6 mice.
Discussion
The aim of this study was to identify a novel antileukemic therapeutic partner for imatinib mesylate for treatment of Ph+ leukemias. The Ras/MAPK pathway is crucial for cellular growth and proliferation of human leukemias, and Ras functions in the downstream signal transduction pathway of the Bcr/Abl oncogenic protein.13,30,31 Effective Ras-related signaling requires the attachment of Ras-related proteins to the plasma membrane. This event is initiated by the enzyme farnesyl protein transferase. FTIs specifically prevent farnesylation and have recently been reported to be antileukemic both in vitro and in vivo, suggesting that blocking Ras binding to the plasma membrane may be a good antileukemic therapeutic target.14-16 However, even when farnesyl protein transferase is inhibited, Ras can be transferred to the membrane by an alternative pathway using geranylgeranyl transferase-1. N-Ras and K-Ras become available as substrates for geranylgeranyl transferase-1 when farnesyl protein transferase is inhibited by FTIs,17 and the FTI SCH66336 induces apoptosis in fibroblasts transformed with farnesylated but not geranylgeranylated H-Ras.32 The growth-suppressing effect of FTIs and imatinib mesylate on Ph+ leukemia cells has still not been definitively determined to be additive or synergistic.10,11
ZOL may inhibit the mevalonate pathway and consequently inhibit protein prenylation in Caco-2 cells, as determined by characterization of Rap1A.33 ZOL inhibits the mevalonate pathway and depletes the cellular pools of both geranylgeranyl pyrophosphate and farnesyl pyrophosphate, thereby inhibiting both geranylgeranylation and farnesylation.34 We focused on ZOL in this study because it appears to exert stronger antileukemic activities than FTIs.
First, we assessed the antileukemic effects of ZOL alone and defined its in vitro and in vivo potency. ZOL blocked the prenylation of Ras proteins in a dose- and time-dependent manner and induced leukemic cells into apoptosis from the S phase to G2/M boundary. To verify that ZOL truly blocks the mevalonate pathway in leukemic cells, we determined whether the addition of geranylgeraniol, an intermediate of the mevalonate pathway, could reverse the inhibitory effects of ZOL.35 However, evidence of reversal of leukemic cell growth inhibition was not obtained because geranylgeraniol per se was cytotoxic to the leukemic cell lines used in this study (data not shown). The addition of geranylgeraniol has been reported to reverse the inhibitory effects of BP in some cell lines, but not in leukemia cell lines.35,36
We compared the antileukemic efficacy of ZOL with the second-generation BP, PAM. ZOL has been reported to be 2 to 3 orders of magnitude more potent in bone resorption assays than PAM.21 Our study indicated that the cytotoxic effects of ZOL were also significantly greater than PAM (Figure 1; Table 1). To investigate the utility of ZOL for the treatment of leukemias refractory to other anticancer drugs, we evaluated its ability to suppress the growth of an MDR cell line. One of the underlying mechanisms of MDR is the cellular overproduction of P-gp, which acts as an efflux pump for various anticancer drugs.37 Imatinib mesylate is also involved in P-gp–related MDR system.8 The P-gp–overexpressing cell line K562/D1-9 was as sensitive to ZOL as was the parental K562 cell line. Simultaneously, K562/D1-9 has the characteristics of overexpression of protein kinase C and the loss of topoisomerase II, which are related to MDR in leukemia.23,38 Thus, ZOL is not involved in multifactorial MDR systems and may represent a promising means of overcoming MDR, although its mechanisms of escape remain to be clarified. Taken together, these findings indicate that ZOL alone has antileukemic effects in vitro.
Next, we assessed the combined effects of ZOL and imatinib mesylate. Recently, a number of conventional chemotherapeutic agents including interferon-α and cytosine arabinoside, and newly developed agents such as FTIs, AS203, and MAPK inhibitors, have been reported to act synergistically or additively with imatinib mesylate in vitro, but these effects are still controversial and little in vivo evidence exists.10,11,39-43 As shown in Figure 5, ZOL plus imatinib mesylate produced dramatic antiproliferative effects against Ph+ leukemia cell lines in vitro. Likewise, combined ZOL and imatinib mesylate treatment significantly prolonged the survival of leukemic mice compared to the survival of mice treated with imatinib or ZOL alone (Figure 7). These data clearly indicate that ZOL alone is a potent antileukemic agent that also significantly augments the effects of imatinib mesylate both in vitro and in vivo. Imatinib mesylate exerts its effect against Ph+ leukemia via G1/S cell cycle blockage. However, imatinib mesylate does not induce complete G1/S arrest and a proportion of leukemic cells in the S phase survive.44 ZOL may induce Ph+ leukemic cells into apoptosis from S phase to G2/M boundary. The cell cycle–specific activity might contribute to the synergistic effects of ZOL and imatinib mesylate.
The clinical utility of imatinib mesylate in combination with ZOL will depend on its safety. In a clinical trial, 16 mg ZOL was given every 28 days repeatedly to patients with bone metastasis of prostate carcinoma with relative safety.45 This human dose level is equivalent to approximately 80 μg/kg ZOL administered weekly in mice. In the present study, we injected each mouse with 80 μg/kg (2 μg/body) or 240 μg/kg (6 μg/body) ZOL per week with or without 120 mg/kg imatinib mesylate. Mice showed no apparent significant adverse effects and tolerated these doses well. In addition, ZOL did not significantly reduce the number of CFU-GM–derived and BFU-E–derived colonies in vitro. Although the pharmacokinetics and pharmacodynamics of ZOL may differ between humans and mice, these findings suggest that ZOL may be of value in clinical trials.
Therapeutically effective serum concentrations of ZOL may be difficult to achieve in vivo. ZOL has high affinity for mineralized bone and rapidly localizes to bone. In the present study, 20 to 30 μM ZOL was required in vitro over 24 to 48 hours to induce apoptosis in BV173 cells. According to a previous study evaluating ZOL efficacy for the treatment of osteoporosis,46 peak serum concentrations were in the range of 1 to 3 μM and maintained for only a few hours, indicating that sufficient serum concentrations for antileukemic activity may not be readily obtained. However, the concentration of BP in bone tissue reached as high as 800 μM in osteoclast bone.47 Moreover, it is known that ZOL concentrations in BM are much higher that those in other organs,48 because BPs incorporated in BM osteoclasts disrupt osteoclasts and release BPs.49 Thus, ZOL may directly promote apoptosis in BM tumor cells. Although we have not directly measured ZOL concentrations in BM, ZOL clearly has antileukemic effects in vivo.
In conclusion, ZOL is a third-generation BP with antileukemic activity that acts synergistically with imatinib mesylate both in vitro and in vivo, indicating that imatinib mesylate plus ZOL treatment may be a promising therapeutic strategy for Ph+ leukemias. Recently, the Food and Drug Administration approved ZOL for treatment of not only osteoporosis but also cancer-related bone complications.50 Efficacy and safety of imatinib mesylate plus ZOL therapy for Ph+ leukemias should be verified in early-phase clinical trials.
Prepublished online as Blood First Edition Paper, May 22, 2003; DOI 10.1182/blood-2003-01-0305.
Supported in part by a Research Fellowship from the Alexander von Humboldt Foundation (S.K.); Grants-in-Aid 13218032, 15025232, and 13671048 for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (T.M.); grant 14130101 from the Ministry of Health, Labor, and Welfare of Japan (T.M.); and by the Shimizu Foundation for Immunology (T.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal