Abstract
Neoangiogenesis has been shown to play an important role in the pathogenesis of acute myeloid leukemia (AML). Autocrine and paracrine secretion of angiogenic and hematopoietic growth factors such as vascular endothelial growth factor (VEGF) and stem cell factor (SCF) in the bone marrow microenvironment may promote proliferation and survival of leukemic blasts. This concept represented the rationale for the initiation of a multicenter phase 2 trial of SU5416, a small molecule inhibitor of phosphorylation of VEGF receptors 1 and 2, c-kit, the SCF receptor, and fms-like tyrosine kinase-3 (FLT3) in patients with advanced AML. Entered into the study were 43 patients with refractory AML or elderly patients not judged medically fit for intensive induction chemotherapy; 42 patients received at least one dose of study drug. Treatment was generally well tolerated, with nausea, headache, and bone pain the most frequent treatment-related side effects. One patient had a morphologic remission (French-American-British [FAB] criteria of complete response without normalization of blood neutrophil and platelet counts) lasting for 2 months. There were 7 patients who achieved a partial response (reduction of blasts by at least 50% in bone marrow and peripheral blood) lasting 1 to 5 months. Patients with AML blasts expressing high levels of VEGF mRNA by quantitative polymerase chain reaction (PCR) had a significantly higher response rate and reduction of bone marrow microvessel density than patients with low VEGF expression consistent with the antiangiogenic effects of SU5416.
Introduction
Growth of acute myeloid leukemia (AML) depends on close interactions of leukemic blasts with cells of the bone marrow microenvironment.1 These cells include endothelial cells, fibroblasts, fat cells, and macrophages. Increased numbers of endothelial cells have been noted on bone marrow biopsies of AML patients compared with those of patients with reactive disorders resulting in elevated microvessel density (MVD) in AML.2-4 AML blasts of about half of the patients constitutively secrete vascular endothelial growth factor (VEGF), which has been shown to represent a major inducer of proliferation and activation of endothelial cells.5 Activated endothelial cells produce hematopoietic growth factors such as stem cell factor (SCF) or granulocyte-macrophage colony-stimulating factor (GM-CSF), which may be necessary for leukemic cell growth and survival in a paracrine fashion.5 AML blasts themselves express receptors for SCF (c-kit) in about half of leukemia cases and VEGF receptor-2 (VEGFR-2) in about 10% to 20% of cases.5,6 These receptors may be involved in leukemic cell proliferation and resistance to apoptosis by responding to growth stimuli provided by endothelial cells from the bone marrow microenvironment.7,8 AML may therefore represent a disease that is sensitive to a therapy directed against the effects of VEGF.
SU5416 is a potent small molecule inhibitor of phosphorylation of VEGFR-1 and -2 (inhibition constant [Ki], 0.16 μM; median inhibitory concentration [IC50], 0.1 μM in cellular phosphorylation assays).9 Due to a high degree of sequence homology among different receptor tyrosine kinases (RTKs), the related receptor c-kit is inhibited by SU5416 to a significant degree (IC50, 0.1 μM).10 FLT3 (fms-like tyrosine kinase-3, Flk2), an RTK that also belongs to the split kinase domain family is also expressed on hematopoietic cells and AML blasts. Constitutively active mutant forms of FLT3 that contain internal tandem duplications (ITD) have been identified in approximately 25% of AML patients and confer poor prognosis.11 Transfer of mutated FLT3 into murine 32D cells led to factor-independent growth and leukemogenicity in syngeneic mice.12 It has recently been shown that SU5416 inhibits cellular phosphorylation of both wild-type FLT3 and FLT3-ITD mutants (IC50, 0.1-0.25 μM).13
Since SU5416 can impede bone marrow neoangiogenesis by affecting both types of VEGF receptors and can exert direct antiproliferative effects on AML blasts by inhibition of cell surface RTKs, such as c-kit and FLT3, it represents a potential compound for testing in AML. Therefore, a multicenter phase 2 study with SU5416 was set up in patients with refractory AML using the recommended phase-2 dose of 145 mg/m2.14,15 To increase the probability of a direct effect on AML blasts, the trial was restricted to patients with c-kit–positive AML.
Patients and methods
Patients
Patients with refractory, c-kit–positive AML, with the exception of the M3 subtype, were eligible for the study protocol. Refractory AML was defined as primary resistance against at least 2 induction chemotherapy regimens or relapsed patients who were refractory against at least 1 reinduction protocol. In addition, patients 60 years or older who were judged not medically fit to endure conventional induction chemotherapy could be entered into protocol. All patients were required to have c-kit–positive AML. C-kit positivity was defined as having at least 30% c-kit–positive cells from a gated blast population either in peripheral blood or bone marrow.
The interval between prior chemotherapy or experimental therapies had to be at least 4 weeks before enrollment. No prior antiangiogenic therapies, including administration of thalidomide, was allowed. Hydroxyurea was administered to patients with high initial white blood cell count (WBC) until WBC had dropped to lower than 50 × 109/L (50 000 cells/μL), and stopped when SU5416 treatment was started. All patients had to be HIV-negative and free of leptomeningeal disease by clinical examination. Adequate renal (creatinine < 176.8 μM [2.0 mg/dL] or creatinine clearance > 1.0 mL/s [60 mL/min]), hepatic (bilirubin < 34.2 μM [2.0 mg/dL] and aspartate aminotransferase [AST] ≤ 5 × ULN [upper limit of normal]), pulmonary and cardiac function (no cardiac failure New York Heart Association [NYHA] III/IV or severe ventricular arrhythmia (Lown III/IV), and performance status (Eastern Cooperative Oncology Group [ECOG] ≤ 3) were required. Written informed consent was obtained from all patients before they were enrolled into the study. The study was approved by the ethics committees at each study site and was conducted in accordance with German and Swiss drug development regulations and the Declaration of Helsinki.
Study design
The primary end point of this study was the determination of the objective response rate. Secondary end points were response duration in responding patients, overall survival, and definition of safety and side effect profile of SU5416 in AML patients.
All patients received SU5416 at a dose of 145 mg/m2 as a 1-hour intravenous infusion twice weekly via a central venous line. To avoid allergic side effects due to the solvent cremophor, all patients received premedication with at least one dose of dexamethasone and H1 and H2 blockers. The dexamethasone dose was reduced on subsequent infusions if no allergic reactions were noted. Complete blood counts were obtained daily during the first 4 days, every 2 days for the first 2 weeks, and later twice weekly. Bone marrow and peripheral blast counts were assessed every 4 weeks to assess disease status. In case of at least a partial response (below) patients could receive additional 4-week cycles until disease progression.
Assessment of safety and response
Safety assessments included the evaluation of adverse events and vital signs, hematologic tests, biochemical tests, urinalysis, and physical examination. Toxicity was graded in accordance with the Common Toxicity Criteria of the National Cancer Institute.
We used standard criteria to define a complete hematologic response: decrease in marrow blasts to 5% or less, disappearance of blasts from the peripheral blood, absolute neutrophil count of more than 1 × 109/L (1000 per cubic millimeter), and a platelet count of more than 100 × 109/L (100 000 per cubic millimeter).16 In patients who did not have a complete hematologic response, a morphologic response was defined as absence of blasts from peripheral blood and 5% or less blasts of bone marrow nucleated cells without requiring recovery of neutrophil or platelet counts. A partial response was defined as a decrease in marrow blast percentage and in absolute peripheral blood blast counts to at least 50% of pretreatment value.
Determination of microvessel density (MVD)
Determination of MVD on bone marrow biopsies was performed as described.4 In brief, serial sections of decalcified bone marrow samples were stained with monoclonal antibodies against von Willebrand factor and thrombomodulin (both from Dako, Glostrup, Denmark) using the alkaline phosphatase antialkaline phosphatase (APAAP) technique. Stained endothelial cells were counted within hot spots identified at lower power light microscopy. At least 3 hot spots per slide were evaluated; 2 to 3 slides per sample were analyzed. All examinations were done in duplicate by 2 independent and blinded investigators. The microvessel density of a bone marrow specimen was calculated as the mean value of all independent readings and recorded as the number of microvessels per × 500 field. Microvessel counts were done before therapy and at 4, 8, and 12 weeks of therapy.
Quantitative polymerase chain reaction (PCR)
Total cellular RNA from 5 × 106 mononuclear cells from blood or bone marrow was extracted using the RNeasy Mini Kit according to the manufacturer's instructions (Qiagen, Hilden, Germany). cDNA was synthesized using the You-Prime First Strand cDNA synthesis kit (Amersham, Picataway, NJ). Reverse transcriptase (RT)–PCR was carried out on the Light Cycler Instrument (Roche, Basel, Switzerland) using the fluorescent dye SYBR Green (Roche Diagnostics, Mannheim, Germany) for relative quantification. Primer design was such that all VEGF splice variants were amplified. Primer sequences were as follows: VEGF-sense primer, 5′-CCTCCGAAACCATGAACTTT-3′; VEGF-antisense primer, 5′-TTCTTTGGTCTGCATTCACTT-3′; GAPDH sense primer, 5′-TGATGACATCAAGAAGGTGG-3′; and glyceraldehyde phosphate dehydrogenase (GAPDH) antisense primer, 5′-TTTCTTACTCCTTGGAGGCC-3′. Length of corresponding PCR products were 412 bp and 246 bp. The relative amount of the analyzed genes in the patient samples was calculated from a standard curve obtained by plotting 4 known input concentrations of plasmid containing the gene to be analyzed (log dilutions) to the PCR cycle number (Crossing Point) at which a significant increase in fluorescence is detected. The data of 2 independent analyses for each gene and sample or plasmid dilution were averaged. The calculated amount of VEGF in the patient samples was normalized to the housekeeping gene GAPDH.
Analysis of FLT3 ITD mutations
The presence or absence of an internal tandem duplication in the FLT3 mRNA was determined as decribed.17 In short, RT-PCR was performed with the FLT3 sense primer 5′-GCACATCTTGTGAGACGATCC-3′ and the FLT3 antisense primer 5′-CACCATAGCAACAATATTCAAAAATC-3′ yielding a 530-bp PCR product. After agarose gel electrophoresis, samples that migrated with higher molecular weight than the product from a control sample were scored as ITD-positive. No sequencing of the catalytic domain of FLT3 was performed.
Fluroescence-activated cell sorter (FACS) analysis
Fluorescein (FITC)– or phycoerythrin (PE)–labeled monoclonal antibodies were purchased as follows: anti–c-kit (CD117) from An-der-Grub (Kaumberg, Austria) and anti-FLT3 (CD135) from DPC Biermann (Bad Nauheim, Germany). Primary antibodies against flt-1 and flk-1 were from Sigma (Taufkirchen, Germany). FITC-labeled goat antimouse antibodies were from DAKO (Hamburg, Germany). Staining of the cells was performed as recommended by the supplier. FACS analyses were run on a FACScan using Cell Quest software, both from Becton Dickinson (Heidelberg, Germany).
Statistics
Cross tables were used to assess correlation between VEGF or c-kit expression and response. To analyze relations between changes in MVD and VEGF expression or response the Wilcoxon test was used. P values were calculated with the WinSTAT program version 3.1 from Kalmia (Cambridge, MA).
Results
Patients
Between July 2000 and August 2001, 84 patients were screened in 10 participating study centers in Germany and Switzerland for c-kit expression by central FACS analysis. Of the patients, 48 (57%) were found to have c-kit–positive AML; 43 patients were entered into the study. Patients' characteristics are shown in Table 1. The median age was 65 years and the oldest patient was 78 years. There were 6 patients who had secondary AML evolving from prior myelodysplastic syndrome; 18 patients had unfavorable cytogenetics mainly with complex aberrations; and only 3 patients had translocation (8;21) underlining the grave prognosis of the study population.
Patients' characteristics and treatment duration
Characteristic . | No. . |
|---|---|
| Patients enrolled | 43 |
| Sex | |
| Male | 26 |
| Female | 17 |
| Age, y, median (range) | 65 (27-79) |
| ECOG performance status, median (range) | 1 (0-3) |
| FAB type | |
| M0 | 5 |
| M1 | 8 |
| M2 | 9 |
| M4 | 12 |
| M5 | 3 |
| M7 | 2 |
| Not classified | 4 |
| Prior MDS | 6 |
| Prior induction therapy | |
| No | 11 |
| Yes | 32 |
| Cytogenetics | |
| Unfavorable | 18 |
| Intermediate | 14 |
| Favorable | 3 |
| Unknown | 8 |
| Patients never treated | 1 |
| No. of cycles received | |
| 1 | 17 |
| 2 | 5 |
| 3 | 1 |
| 4 | 1 |
| 5 | 1 |
Characteristic . | No. . |
|---|---|
| Patients enrolled | 43 |
| Sex | |
| Male | 26 |
| Female | 17 |
| Age, y, median (range) | 65 (27-79) |
| ECOG performance status, median (range) | 1 (0-3) |
| FAB type | |
| M0 | 5 |
| M1 | 8 |
| M2 | 9 |
| M4 | 12 |
| M5 | 3 |
| M7 | 2 |
| Not classified | 4 |
| Prior MDS | 6 |
| Prior induction therapy | |
| No | 11 |
| Yes | 32 |
| Cytogenetics | |
| Unfavorable | 18 |
| Intermediate | 14 |
| Favorable | 3 |
| Unknown | 8 |
| Patients never treated | 1 |
| No. of cycles received | |
| 1 | 17 |
| 2 | 5 |
| 3 | 1 |
| 4 | 1 |
| 5 | 1 |
FAB indicates French-American-British; and MDS, myelodysplastic syndrome.
Therapy and safety
SU5416 was administered via a central venous line at the recommended phase-2 dose of 145 mg/m2 twice weekly, which had been established in prior studies.14,15 Therapy was generally well tolerated and could be delivered on an outpatient basis in most of the patients. There were 25 patients who completed cycle 1, of whom 8 patients completed 2 to 5 cycles. There were 24 patients who received all 8 consecutive infusions in their first 4-week cycle. All patients (n = 42) who received at least one dose of SU5416 were evaluable for toxicity. The frequency and grading of adverse effects are summarized in Table 2. Most study drug–related adverse events were mild to moderate in severity and the most frequently reported adverse events included: nausea, 31%; bone/muscoloskeletal pain, 31%; headache, 26%; insomnia, 24%; vomiting, 17%; vertigo, 12%; fatigue/malaise, 12%; abdominal pain, 10%; sweating, 10%; and arthralgias, 7%. Of the patients, 21 (50%) experienced a severe adverse event, and in 10 patients this was deemed study drug–related. The occurrences of these events were mostly related to the patient's underlying disease state, the 2 most frequently reported serious adverse events were pneumonia in 14 patients (6%) and/or sepsis in 10 patients (4%). Due to a study drug–related adverse event, 4 patients (10%) discontinued treatment. Hypersensitivity reaction to SU5416 occurred in 1 patient and was not severe.
Frequency and severity of adverse events that where possibly, probably, or definitely related to SU5416 administration in patients who received at least one dose (n = 42) and seen in less than 5% of study patients
. | Number of patients experiencing events (%) . | . | . | ||
|---|---|---|---|---|---|
| Adverse event . | Grades 1 + 2 . | Grades 3 + 4 . | Total . | ||
| All events | 31 (74) | 19 (45) | 34 (81) | ||
| Bone/muscoloskeletal pain | 13 (31) | 3 (7) | 13 (31) | ||
| Nausea | 13 (31) | 1 (2) | 13 (31) | ||
| Headache | 11 (26) | 1 (7) | 11 (26) | ||
| Insomnia | 10 (24) | 0 | 10 (24) | ||
| Vomiting | 7 (17) | 0 | 7 (17) | ||
| Fatigue/malaise | 5 (12) | 3 (7) | 5 (12) | ||
| Vertigo | 5 (12) | 0 | 5 (12) | ||
| Abdominal pain | 4 (10) | 0 | 4 (10) | ||
| Diaphoresis | 4 (10) | 0 | 4 (10) | ||
| Chest pain | 3 (7) | 1 (2) | 4 (10) | ||
| Hypokalemia | 3 (7) | 0 | 3 (7) | ||
| Implant infection | 2 (5) | 1 (2) | 3 (7) | ||
| Sepsis | 0 | 3 (7) | 3 (7) | ||
. | Number of patients experiencing events (%) . | . | . | ||
|---|---|---|---|---|---|
| Adverse event . | Grades 1 + 2 . | Grades 3 + 4 . | Total . | ||
| All events | 31 (74) | 19 (45) | 34 (81) | ||
| Bone/muscoloskeletal pain | 13 (31) | 3 (7) | 13 (31) | ||
| Nausea | 13 (31) | 1 (2) | 13 (31) | ||
| Headache | 11 (26) | 1 (7) | 11 (26) | ||
| Insomnia | 10 (24) | 0 | 10 (24) | ||
| Vomiting | 7 (17) | 0 | 7 (17) | ||
| Fatigue/malaise | 5 (12) | 3 (7) | 5 (12) | ||
| Vertigo | 5 (12) | 0 | 5 (12) | ||
| Abdominal pain | 4 (10) | 0 | 4 (10) | ||
| Diaphoresis | 4 (10) | 0 | 4 (10) | ||
| Chest pain | 3 (7) | 1 (2) | 4 (10) | ||
| Hypokalemia | 3 (7) | 0 | 3 (7) | ||
| Implant infection | 2 (5) | 1 (2) | 3 (7) | ||
| Sepsis | 0 | 3 (7) | 3 (7) | ||
Response
Enrolled were 43 patients, of whom 1 never received study medication. According to the protocol, which required continuous therapy for at least 2 weeks (4 consecutive infusions) and restaging, 25 patients were evaluable for response. One patient achieved a morphologic remission, defined as absence of blasts in the peripheral blood and less than 5% in the bone marrow but without normalization of neutrophil and platelet counts for a duration of 2 months. This patient was a 29-year-old man with AML M5a with a complex karyotype who had relapsed after allogeneic bone marrow transplantation in bone marrow and with leukemic skin infiltrations. After 4 weeks of therapy with SU5416, he had a morphologic response and skin sites became pale but were not biopsied. He suffered a relapse in the bone marrow 8 weeks later.
Of the patients, 7 obtained a partial response with at least 50% reduction in bone marrow and peripheral blood blasts that lasted from 1 to 5 months. One of these was a 73-year-old male patient with untreated primary AML (M1 subtype with karyotype 47, xy,+8). During his 5 months of therapy with SU5416 he had a continuing decrease in bone marrow blasts until he achieved almost a complete remission with 6% blasts before he relapsed. Slow recovery of peripheral blood neutrophils and platelets occurred during this period. The mean response duration of all 8 responding patients was 1.6 months. All patients relapsed of their disease in spite of continuing treatment with SU5416. If analyzed on an intention-to-treat basis, which includes all patients who received at least one dose of SU5416, the overall response rate corresponds to 19%.
During the first cycle, 17 evaluable patients did not respond to SU5416 and were removed from the study, although 2 of these showed a more than 50% reduction in peripheral blood blasts. There were 17 patients who were not evaluable; of these, 13 received less than 2 weeks of continued therapy. These patients showed disease progression with rapidly rising leukocyte and blast cell counts necessitating the addition of some kind of cytoreductive chemotherapy. Another 4 patients died during the first cycle without re-evaluation of their disease status.
VEGF expression
VEGF RNA expression by leukemic blasts was studied by quantitative RT-PCR. From 27 patients, including 16 evaluable patients, sufficient pretreatment samples were available for successful amplification. Values were expressed as a ratio with the housekeeping gene GAPDH. Values ranged from 0 to 7.5. After analysis of the data, patients were separated into 2 clusters: one with low VEGF expression (range, 0-0.015) and one with high VEGF expression (range, 0.14-7.5). The mean value of the lower cluster plus 2 standard deviations did not reach the second cluster, indicating good separation of the 2 groups. VEGF expression of blasts from patients from the lower cluster was comparable with VEGF expression of normal purified CD34+ cells (data not shown). Therefore patients were classified as being VEGF-negative or -positive. Of the 16 evaluable patients, 9 were positive for VEGF expression (56%), which is comparable with previous observations from our group.5 VEGF expression was analyzed in relation to response to SU5416. All 4 patients, who achieved a partial remission and for whom VEGF expression could be assessed, were VEGF-positive compared with 5 of 12 nonresponders (P = .042) (Table 3). These results imply an association between response to therapy with SU5416 and VEGF-expressing AML.
Classification of response by VEGF expression in informative patients
. | Morphologic response/partial response . | Nonresponse . |
|---|---|---|
| VEGF-positive patients | 4 | 5 |
| VEGF-negative patients | 0 | 7 |
. | Morphologic response/partial response . | Nonresponse . |
|---|---|---|
| VEGF-positive patients | 4 | 5 |
| VEGF-negative patients | 0 | 7 |
The response rate was significantly higher in VEGF-positive AML patients (P = .042).
Microvessel density
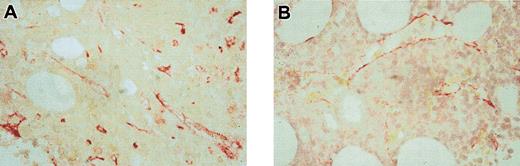
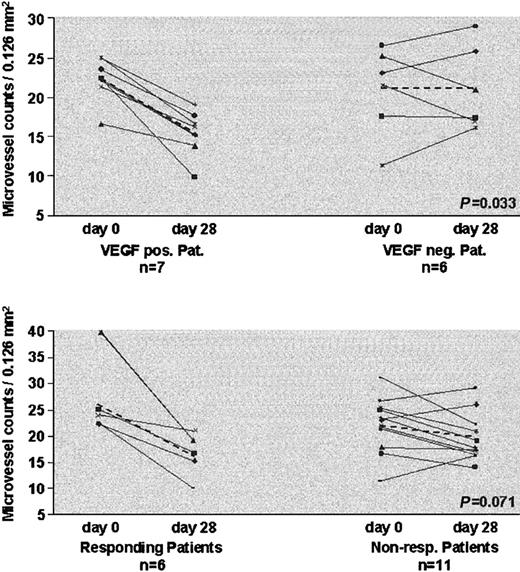
Bone marrow microvessel density was determined before and 4, 8, and 12 weeks after the start of therapy. MVD data are available from 15 of 25 patients, who received at least 4 weeks of treatment. In 12 of 15 evaluable patients a decrease in MVD was detected, underlining the effect of antiangiogenic therapy. A representative example of bone marrow biopsies taken before therapy and after 8 weeks of SU5416 in a responding patient is shown in Figure 1. We then related changes in MVD to VEGF expression and treatment outcome. All 7 patients with VEGF-positive AML had reductions in MVD compared with 3 of 6 with VEGF-negative AML (P = .033). All 6 patients who achieved a partial response to SU5416 had a decrease in MVD compared with only 8 of 11 nonresponders (P = .071) (Figure 2).
Change of MVD during therapy with SU5416 in a responding patient. Bone marrow histologies from the 74-year-old patient who was treated with SU5416 for 6 months taken prior to therapy (A) and after 8 weeks (B), showing a clear decrease in MVD under therapy. Endothelial cells were stained with an antibody against thrombomodulin (red). Original magnification, × 500.
Change of MVD during therapy with SU5416 in a responding patient. Bone marrow histologies from the 74-year-old patient who was treated with SU5416 for 6 months taken prior to therapy (A) and after 8 weeks (B), showing a clear decrease in MVD under therapy. Endothelial cells were stained with an antibody against thrombomodulin (red). Original magnification, × 500.
Analysis of microvessel density. Changes in microvessel density prior to treatment and after 4 weeks in relation to VEGF expression (top panel) and response to treatment (bottom panel). Dashed lines indicate changes of mean microvessel density.
Analysis of microvessel density. Changes in microvessel density prior to treatment and after 4 weeks in relation to VEGF expression (top panel) and response to treatment (bottom panel). Dashed lines indicate changes of mean microvessel density.
Analysis of target receptors
Prior to therapy, expression of target receptors c-kit, FLT3, VEGFR-1, and VEGFR-2 was determined by FACS analysis. Patients were required to have c-kit–positive leukemia to allow entry into the study. C-kit–positive AML was defined as at least 30% positive cells of a gated blast population. When c-kit–positive patients were grouped into cases with c-kit expression below and above the median, 5 of 11 informative patients with low c-kit expression responded compared with 1 of 12 patients with very high c-kit expression (P = .043).
Expression of VEGFR-2 was much lower, ranging from 0% to 32% positive blasts. When a cutoff value of 5% VEGFR-2–positive cells was used to ensure clear discrimination between VEGFR-2–positive and –negative AML, 6 of 16 patients with VEGFR-2–positive AML responded compared with 0 of 7 patients with VEGFR-2–negative AML (P = .059). No correlation between expression of FLT3 or VEGFR1 and response was noted.
Since ITD mutations of FLT3 represent the most common mutation in AML resulting in constitutive phosphorylation of the receptor, we retrospectively determined the occurrence of ITD mutations in 35 patients. ITD mutations were detected in 7 patients (20%). None of these patients responded to treatment with SU5416. The occurrence of FLT3 activation loop mutations was not investigated in this study.
Discussion
We conducted a phase 2 study of SU5416 in patients with c-kit–positive AML that was resistant to standard chemotherapy. Elderly patients not amenable for induction therapy were also included. Of the patients, 43 were entered into the trial, 42 received at least one dose of study drug, and 25 were evaluable for response according to the protocol because they had received at least 4 consecutive infusions in the first cycle. There were 8 patients who achieved a morphologic or partial response. If analyzed on an intent-to-treat basis, 19% of treated patients responded. Most remissions were of short duration, but some patients showed clinical benefit during therapy with SU5416. Furthermore a report of a prolonged remission due to treatment with SU5416 on a compassionate use program has recently been published indicating exquisite sensitivity of some AML cases to therapy with this drug.18
Therapy with SU5416 was usually well tolerated. Main toxicities were nausea, headache, and bone pain. The latter could be quite severe, difficult to treat, even with opioids, and did not usually respond to dose reductions. A second problem in the treatment of the patients with refractory AML was the slow recovery of neutrophil and platelet counts in responding patients, which necessitated regular blood and platelet transfusions, and the patients remained at risk for hemorrhagic and infectious complications, such as sepsis, pneumonia, or venous access site infections.
In order to establish prognostic factors, which could be used to predict response to SU5416, VEGF expression by AML blasts was determined by quantitative RT-PCR.Aclear correlation between clinical response and VEGF mRNA expression was established. Furthermore, patients who quickly progressed during the first 2 weeks of therapy were almost exclusively VEGF-negative (data not shown). This correlation lends support to the concept of pathogenetic importance of increased bone marrow neoangiogenesis in humanAML. Furthermore we showed that SU5416 treatment resulted in reduced MVD specifically in VEGF-positive AML. These observations imply that VEGF secreted by AML blasts leads to increased MVD and that blockade of VEGF receptors by SU5416 can induce regression of bone marrow capillaries. Interestingly, pretreatment MVD was not different in patients with VEGF-positive or -negativeAML. It is possible that in patients with VEGF-negativeAML, other cytokines such as fibroblast growth factor (FGF), which are not targeted by SU5416, trigger neoangiogenesis. These findings support the use of VEGF receptor antagonists as a treatment option for patients with VEGF-positive AML, either alone or in addition to chemotherapy. Further research is necessary in patients with VEGF-negative AML to evaluate alternative treatment options (eg, FGF receptor inhibitors).
The analysis of target receptors on AML blasts indicated that strong expression of c-kit negatively affected clinical response to SU5416. Similarly no remissions were observed in patients with FLT3 constitutively activated by ITD mutations. These findings imply that SU5416, in the dose regimen used, was not able to overcome the growth stimulation caused by constitutive receptor activation or high levels of receptor expression. Taken together, the main antileukemic effect of SU5416 seems to be mediated by its antiangiogenic effect and not by direct growth inhibition of leukemic blasts.
Although prolonged remission was observed in a few AML patients, the overall observed response rate was low and responses consisted mainly of partial remissions of short duration. SU5416 has a short plasma half-life and, therefore, it is possible that the dosing regimen (twice-weekly infusion) did not achieve exposures needed to enable sustained inhibition of c-kit and FLT3, which may be required for efficacy. Analysis of FLT3 phosphorylation in samples from 2 FLT3-ITD patients on this study showed phosphorylation at baseline, and weak inhibition at one hour after SU5416 infusion, which was not sustained.19 Although drug levels were not measured in this study, pharmacokinetic data are available from trials in solid tumor patients using the same dosing schedule.14 These data suggest that plasma SU5416 levels were lower at most time points than those needed to efficiently block c-kit phosphorylation of MO7e cells in vitro (data not shown).
In summary, this study gives valuable insight into the role of RTKs in AML and emphasizes the value of correlative studies as a component of clinical trials with targeted therapies. The development of alternative SU5416 dosing regimens or testing of other compounds with a similar target profile in AML is warranted.
Prepublished online as Blood First Edition Paper, July 3, 2003; DOI 10.1182/blood-2002-10-2998.
Supported by Sugen/Pharmacia.
A.-M.O., M.J., N.M.B., and P. Scigalla are employed by Sugen/Pharmacia, whose potential product was studied in the present work.
Presented in part at the European Cancer Conference (ECCO)–11 meeting in Lisboa, Portugal, in October 2001 and at the 43rd annual meeting of the American Society of Hematology in Orlando, FL, in December 2001.20
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal