Abstract
In a search for novel growth factors, we discovered that human interleukin-20 (IL-20) enhanced colony formation by CD34+ multipotential progenitors. IL-20 had no effect on erythroid, granulocyte-macrophage, or megakaryocyte progenitors. IL-20 transgenic mice increased the numbers and cell cycling of multipotential but not other progenitors. IL-20 administration to normal mice significantly increased only multipotential progenitor cells, demonstrating that IL-20 significantly influences hematopoiesis, with specificity toward multipotential progenitors. This is the first cytokine with such specificity identified.
Introduction
Cytokines are important regulators of the growth and development of hematopoietic cells1,2 ; some are currently in clinical use. In a search for novel factors that regulate hematopoiesis, colony assays were used to screen novel secreted proteins identified through bioinformatics. One protein identified was identical to interleukin-20 (IL-20).3,4 We demonstrate that IL-20 specifically enhances the proliferation of multipotential progenitors in vitro and in vivo without effect on more lineage-restricted progenitor cells.
Study design
Protein production
Recombinant human IL-20 (rhIL-20), containing a C-terminal FLAG tag (Eastman Kodak, Rochester, NY) followed by 6 histidine residues (Flis), was produced in 293EBNA1 cells.5 We captured rhIL-20-Flis on Pharmacia Chelating Sepharose FF (Amersham-Pharmacia, Piscataway, NJ). The endotoxin level was less than 5.3 EU/mg rhIL-20.
Colony assays for human and murine progenitors
CD34+ human bone marrow or cord blood cells were purchased from BioWhittaker (Walkersville, MD). Colony assays for granulocyte-macrophage (CFU-GM), erythroid (BFU-E), and multipotential (CFU-GEMM) progenitors were as described.6,7 Megakaryocyte (CFU-Meg) progenitor assays were carried out using MegaCult-C medium (Stem Cell Technologies, Vancouver, BC, Canada).
IL-20 transgenic mice
Transgenic (TG) mice were generated by established techniques.8 Human IL-20 was overexpressed in TG mice using apolipoprotein E gene promoter. IL-20 levels in mouse serum were determined using enzyme-linked immunosorbent assay (ELISA) with antihuman rhIL-20.
rhIL-20 administration to normal mice
Female BDF1 mice (8-10 weeks of age; Harlan, Indianapolis, IN) were administered rhIL-20 (5 μg/mouse) subcutaneously twice a day for 10 days. At day 11, mice were killed, bone marrow and spleen cells were counted, cells were used for colony assays, and the proportion of progenitors in S-phase of the cell cycle was estimated.7,9,10
Results and discussion
IL-20 in vitro
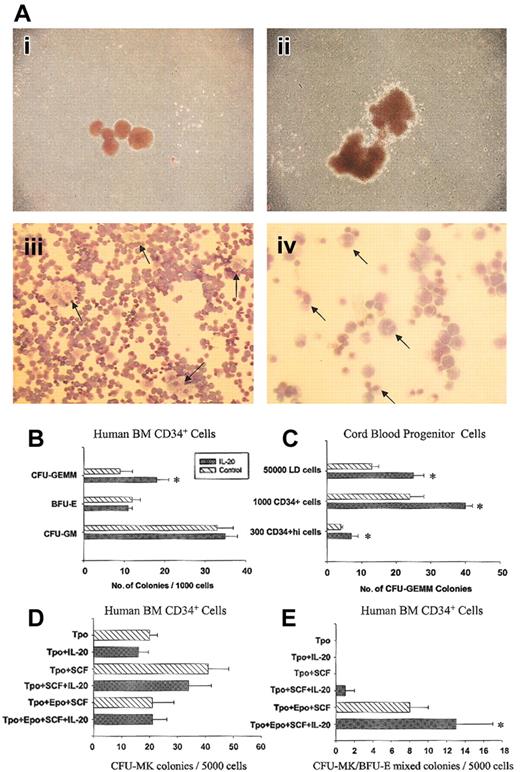
IL-20 did not stimulate colony formation, but it increased the numbers of larger-sized colonies in combination with recombinant human stem cell factor (SCF) and erythropoietin (EPO) (Figure 1Ai-ii). Colonies cultured with IL-20 contained cells with and without hemoglobin expression. Microscopic examination of 22 individual large colonies stained with Wright-Giemsa revealed mainly erythroblasts mixed with megakaryocytes. Granulocytes and monocytes were also detected (original magnification, × 400) within these colonies but were less prominent than erythroblasts and megakaryocytes (Figure 1Aiii-iv). This suggested that IL-20 enhanced CFU-GEMM.
IL-20 specifically increases multipotential progenitors in vitro. (A) IL-20 increases numbers of larger-sized colonies containing erythrocytes, megakaryocytes, granulocytes, and monocytes. Human CD34+ bone marrow cells were cultured in methylcellulose medium containing 50 ng/mL SCF and 1 U/mL EPO with or without 200 ng/mL IL-20. Colonies represented control (i) or rhIL-20–containing (ii) cultures. Aiii and iv are Wright-Giemsa–stained cells from individual colonies picked from rhIL-20–containing cultures. Arrows in panel Aiii point to megakaryocytes at × 200 original magnification. Granulocytes or monocytes were detected at higher magnification (× 400 original magnification) and are marked by arrows in panel Aiv. (B) IL-20 enhances CFU-GEMM but not BFU-E and CFU-GM numbers in human bone marrow CD34+ culture in the presence of EPO, SCF, GM-CSF, and IL-3. Increases of CFU-GEMM were also observed when IL-20 was used in combination with EPO and SCF. Combining IL-20 with G-CSF, GM-CSF, or M-CSF had no effect on CFU-GM or CFU-M. Data represent mean ± SD from triplicate experiments. Similar results were obtained from 12 individual bone marrow samples. (C) IL-20 enhances CFU-GEMMs in cord blood. Human cell studies were approved by the Institutional Review Board of Indiana University. Human cells were assessed after the recovery of low-density (less than 1.077 g/cm3) cells, magnetic-bead–separated CD34+ cells, or FACS sorted CD34+hi cells. Data represent means ± SD from triplicate experiments. Similar results were obtained in 10 different experiments. (D-E) IL-20 had no effect on CFU-Meg colonies but increased CFU-Meg/BFU-E mixed colonies. Bone marrow CD34+ cells were assayed for megakaryocyte-containing colonies in serum-free, collagen-based media containing various cytokines. The stimulus was 50 ng/mL TPO, 50 ng/mL SCF, and 2 U/mL EPO. Clusters of 3 or more CD41+ cells were scored as CFU-Meg. Colonies containing megakaryocytes and erythroid cells were scored as CFU-MIX (right panel). Each column corresponds to the mean ± SD of duplicate cultures, and each culture contains 2 repeats (n = 4). *P < .02 compared with respective controls without rhIL-20, based on 2-tailed Student t test.
IL-20 specifically increases multipotential progenitors in vitro. (A) IL-20 increases numbers of larger-sized colonies containing erythrocytes, megakaryocytes, granulocytes, and monocytes. Human CD34+ bone marrow cells were cultured in methylcellulose medium containing 50 ng/mL SCF and 1 U/mL EPO with or without 200 ng/mL IL-20. Colonies represented control (i) or rhIL-20–containing (ii) cultures. Aiii and iv are Wright-Giemsa–stained cells from individual colonies picked from rhIL-20–containing cultures. Arrows in panel Aiii point to megakaryocytes at × 200 original magnification. Granulocytes or monocytes were detected at higher magnification (× 400 original magnification) and are marked by arrows in panel Aiv. (B) IL-20 enhances CFU-GEMM but not BFU-E and CFU-GM numbers in human bone marrow CD34+ culture in the presence of EPO, SCF, GM-CSF, and IL-3. Increases of CFU-GEMM were also observed when IL-20 was used in combination with EPO and SCF. Combining IL-20 with G-CSF, GM-CSF, or M-CSF had no effect on CFU-GM or CFU-M. Data represent mean ± SD from triplicate experiments. Similar results were obtained from 12 individual bone marrow samples. (C) IL-20 enhances CFU-GEMMs in cord blood. Human cell studies were approved by the Institutional Review Board of Indiana University. Human cells were assessed after the recovery of low-density (less than 1.077 g/cm3) cells, magnetic-bead–separated CD34+ cells, or FACS sorted CD34+hi cells. Data represent means ± SD from triplicate experiments. Similar results were obtained in 10 different experiments. (D-E) IL-20 had no effect on CFU-Meg colonies but increased CFU-Meg/BFU-E mixed colonies. Bone marrow CD34+ cells were assayed for megakaryocyte-containing colonies in serum-free, collagen-based media containing various cytokines. The stimulus was 50 ng/mL TPO, 50 ng/mL SCF, and 2 U/mL EPO. Clusters of 3 or more CD41+ cells were scored as CFU-Meg. Colonies containing megakaryocytes and erythroid cells were scored as CFU-MIX (right panel). Each column corresponds to the mean ± SD of duplicate cultures, and each culture contains 2 repeats (n = 4). *P < .02 compared with respective controls without rhIL-20, based on 2-tailed Student t test.
Colony assays were performed with human bone marrow (BM) and cord blood (CB) CD34+ cells using various cytokine combinations. IL-20 (200 ng/mL), in combination with EPO and SCF, significantly enhanced BM CFU-GEMM numbers approximately 2-fold without effect on BFU-E and CFU-GM (Figure 1B). Enhancement of CFU-GEMM numbers was observed using low-density, CD34+ cells and more-purified CD34+hi CB cells (Figure 1C), without effect on BFU-E and CFU-GM (data not shown). Similar increases in CFU-GEMM colonies were observed at 100 and 50 ng/mL IL-20, but not at lower concentrations (data not shown). IL-20 did not enhance numbers of pure CFU-Meg colonies (Figure 1D), but mixed erythroid-megakaryocyte colonies were significantly increased (Figure 1E). Eighteen human BM and 10 CB CD34+ samples were assessed. IL-20 significantly enhanced multipotential progenitors in two thirds of BM and in all CB samples. The overall effect of IL-20 on CFU-GEMM of BM donors was significant. IL-20 alone did not stimulate the growth of colonies from any lineage. Pretreating cells with IL-20, washing, and culture with SCF and EPO did not enhance CFU-GEMMs.
IL-20 in vivo
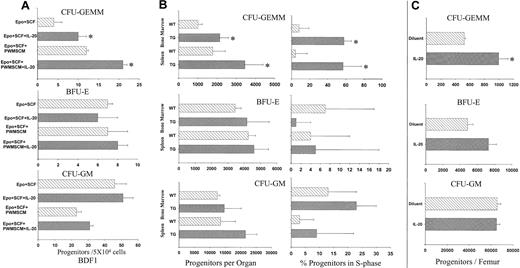
IL-20 was first tested on the colony formation of BDF-1 BM in vitro. Results on C3H/HeJ BM (not shown) were similar (Figure 2A). IL-20 increased the number of CFU-GEMM approximately 2-fold over that of EPO and SCF, or EPO, SCF, and conditioned media (PWMSCM), but it had no significant effect on BFU-E or CFU-GM.
IL-20 specifically increases numbers and cell-cycling status of mouse multipotential progenitors in vitro and in vivo. (A) IL-20 increases mouse CFU-GEMM but not CFU-GM or BFU-E in vitro. Bone marrow cells (5 × 104) from BDF-1 or C3H/HeJ mice were cultured in methycellulose medium with hemin, 1 U/mL EPO, and 50 ng/mL SCF with or without IL-20. 5% vol/vol pokeweed mitogen mouse spleen cell-conditioned medium (PWMSCM) was added to some cultures. Values represent mean ± SD of 3 triplicates. *P < .05 compared with respective controls without IL-20. (B) IL-20 TG mice had significant increases in numbers and cycling rates of CFU-GEMM, but not BFU-E or CFU-GM, in bone marrow and spleens. Data show the results (mean ± SD) of 19 to 20 animals/group. Each mouse was evaluated separately. Left panels show absolute numbers of myeloid progenitor cells per femur or spleen. Right panels show percentage of myeloid progenitor cells in S-phase. *P < .03 compared with littermate controls. (C) Administration of IL-20 to normal mice increased multipotential progenitors. The IL-20 group received 5 μg IL-20 per animal twice daily for 10 days. Control vehicle groups received a similar number of PBS injections. Data represent means ± SD for 10 animals per group. Each mouse was evaluated separately. *P < .02 compared with littermate controls.
IL-20 specifically increases numbers and cell-cycling status of mouse multipotential progenitors in vitro and in vivo. (A) IL-20 increases mouse CFU-GEMM but not CFU-GM or BFU-E in vitro. Bone marrow cells (5 × 104) from BDF-1 or C3H/HeJ mice were cultured in methycellulose medium with hemin, 1 U/mL EPO, and 50 ng/mL SCF with or without IL-20. 5% vol/vol pokeweed mitogen mouse spleen cell-conditioned medium (PWMSCM) was added to some cultures. Values represent mean ± SD of 3 triplicates. *P < .05 compared with respective controls without IL-20. (B) IL-20 TG mice had significant increases in numbers and cycling rates of CFU-GEMM, but not BFU-E or CFU-GM, in bone marrow and spleens. Data show the results (mean ± SD) of 19 to 20 animals/group. Each mouse was evaluated separately. Left panels show absolute numbers of myeloid progenitor cells per femur or spleen. Right panels show percentage of myeloid progenitor cells in S-phase. *P < .03 compared with littermate controls. (C) Administration of IL-20 to normal mice increased multipotential progenitors. The IL-20 group received 5 μg IL-20 per animal twice daily for 10 days. Control vehicle groups received a similar number of PBS injections. Data represent means ± SD for 10 animals per group. Each mouse was evaluated separately. *P < .02 compared with littermate controls.
Our IL-20 TG mice had a phenotype similar to that described by Blumberg et al.3 Approximately 50% of the TG pups died within the first few days of birth. F1 IL-20 TGs were smaller than non-TG siblings and had wrinkled skin with thickened epidermis. Serum levels of IL-20 ranged from 100 to 4000 ng/mL, depending on the TG line. Expression levels did not correlate with the severity of skin abnormalities or neonatal lethality, consistent with Blumberg et al's report that low expression in liver (fewer than 100 mRNA molecules/cell) was sufficient for neonatal lethality and skin abnormalities.3 Causes of death were not clear. TG mice surviving 5 days after birth reached body weights comparable to those of control littermates by 4 weeks. BM cells and splenocytes were analyzed for progenitor cell numbers and cycling status. IL-20 TG mice had approximately 2-fold more BM and spleen CFU-GEMM than their control littermates. CFU-GM was slightly but not significantly (P > .05) higher in IL-20 TG mice. BM or spleen cellularity was similar. The cycling status of IL-20 TG BM and spleen CFU-GEMM was greatly enhanced (Figure 2B, right panels). The cycling status of CFU-GM and BFU-E was not statistically different between TGs and littermates. This suggested that increased absolute numbers of CFU-GEMM in IL-20 TG mice were likely caused by IL-20–enhanced proliferation of multipotential progenitors. In contrast, CD34+ cells, erythroid cells, megkaryocytes, T cells, B cells, NK cells, monocytes/macrophages, and dendritic cells from BM and spleen and blood leukocytes, platelets, and neutrophils from IL-20 TG and control littermates were not significantly different (data not shown). TG mice had slightly higher erythrocyte counts than control littermates, but this was in the range of controls.
We explored whether increased multipotential progenitors in vivo were secondary to skin abnormalities observed in IL-20 TG by administering IL-20 to control mice. IL-20 enhanced BM CFU-GEMM numbers 2-fold with no significant change in CFU-GM and BFU-E (Figure 2C) compared with phosphate-buffered saline (PBS) controls. Blood cell counts were not different between IL-20– and PBS-treated mice. No obvious skin effects were seen in mice receiving IL-20. To address whether IL-20 administration stimulated the secretion of cytokines, serum cytokines were assessed using Luminex or ELISA. IL-20 administration had no effect on GM-CSF, SCF, IL-3, IL-1β, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), IL-2, IL-5, or IL-10 levels. Cytokine profiles were similar in IL-20 TG and littermate mice (data not shown). These results demonstrate that IL-20 specifically enhances multipotential progenitors in vivo, independently of IL-20 skin effects. Because IL-20 did not increase nucleated cellularity, endogenous cytokines required for further differentiation in vivo may be lacking.
Early-acting cytokines IL-3,11,12 GM-CSF,13,14 IL-11,10 and thrombopoietin (TPO)15,16 stimulate/enhance CFU-GEMM, CFU-GM, and BFU-E. To our knowledge, no other cytokine is known to enhance the proliferation of CFU-GEMM without affecting other progenitors. Bipotential (erythroid and megakaryocytic) precursors exist.17-19 IL-20 may act on these bipotential progenitors because some mixed colonies contained only erythrocytes and megakaryocytes. IL-20 binds IL-20 receptor (IL-20R) types 1 and 2. Type 1 IL-20R also binds IL-19 and IL-24; type 2 IL-20R binds IL-24.3,20 Neither IL-19 nor IL-24 had effects on CFU-GEMM (data not shown). IL-19 and IL-24 were active because they stimulated the proliferation of BaF3 cells expressing IL-20R. The mechanisms of IL-20 enhancement of multipotential progenitors remain to be determined.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-05-1419.
Several of the authors (L.L., C.D., W.Z., J.G.H., J.W.T., T.W.N., K.A.B., G.S., N.F., S.W.R., D.P.R., D.R.W., P.K.L., V.J.W., and J.R.M.) are employed by Eli Lilly, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank our colleagues at Lilly for helpful discussions: B. Grinnell, A.L. Glasebrook, A.B. Shanafelt, J.M. Beals, J.A. Jakubowski, T.D. Mckinney, K.L. Blanchard, and U. Kuchibhotla. We thank A.J. Okragly, S. Dou, C.R. Shrake, J. Manetta, G.M. Kelly, P. Atkinson, D.B. Baldwin, P.F. Grealish, Y.F. Chen, and M.A. Wiskerchen for their technical support. We thank A.B. Shanafelt for critical reading of this manuscript. We also thank C. Miller and E. Clarke from Stem Cell Technologies for providing contract assay service.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal