Abstract
Smac, second mitochondria-derived activator of caspases, promotes apoptosis via activation of caspases. Heat shock protein 27 (Hsp27) negatively regulates another mitochondrial protein, cytochrome c, during apoptosis; however, the role of Hsp27 in modulating Smac release is unknown. Here we show that Hsp27 is overexpressed in both dexamethasone (Dex)-resistant multiple myeloma (MM) cell lines (MM.1R, U266, RPMI-8226) and primary patient cells. Blocking Hsp27 by an antisense (AS) strategy restores the apoptotic response to Dex in Dex-resistant MM cells by triggering the release of mitochondrial protein Smac, followed by activation of caspase-9 and caspase-3. Moreover, AS-Hsp27 overcomes interleukin-6 (IL-6)-mediated protection against Dex-induced apoptosis. These data demonstrate that Hsp27 inhibits the release of Smac, and thereby confers Dex resistance in MM cells.
Introduction
Multiple myeloma (MM) remains fatal despite all available therapies. Initial treatment with dexamethasone (Dex) effectively induces MM cell death; however, prolonged drug exposures result in the development of de novo chemoresistance.1,2 The mechanisms mediating Dex-resistance in MM cells are multifactorial. Interleukin-6 (IL-6),3 insulin-like growth factor I (IGF-I),4 fibroblast growth factor receptor-3,5 and Bcl26 confer Dex resistance in MM cells. Dex resistance has also been linked to the bone marrow (BM) microenvironment, including extracellular matrix proteins7 and BM stromal cells. Elevated levels of heat shock protein 27 (Hsp27) transcripts have been observed in MM versus normal cells,8 and our prior oligonucleotide array study showed that Hsp27 mRNA is highly expressed in Dex-resistant MM cells versus Dex-sensitive MM cells.9,10 To date, however, the functional significance of Hsp27 in MM cells remains undefined.
Here we show that blocking Hsp27 by antisense (AS) strategy is associated with these events: (1) restoration of sensitivity to Dex and induction of apoptosis via the release of mitochondrial protein Smac (second mitochondria-derived activator of caspases), followed by activation of caspase-9 and caspase-3, as well as (2) abrogation of the IL-6-mediated protective effect against Dex-induced apoptosis. These studies provide the first evidence that Hsp27 negatively regulates Smac-mediated apoptosis and thereby confers Dex resistance in MM cells.
Patients, materials, and methods
Cell culture and reagents
Dex-sensitive MM.1S and Dex-resistant MM.1R human MM cell lines11,12 were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL). RPMI-8226 and U266 MM cell lines were obtained from the American Type Culture Collection (Rockville, MD). All cell lines were grown in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine. Drug-resistant cell lines were cultured with Dex to confirm their lack of drug sensitivity. The primary cells were freshly isolated from patients either responsive to Dex or relapsing after prior Dex therapy. Informed consent was obtained from all patients in accordance with the Helsinki protocol. Mononuclear cells were prepared from MM patient BM samples by Ficoll-Hypaque density gradient centrifugation. Tumor cells (CD138+ 97% ± 2.0%) were isolated by CD138+ selection method12 using CD138 (Syndecan-1) microbeads and the autoMACS magnetic cell sorter, according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). CD138+ myeloma cells were viable (94%-97%) for 2 to 3 weeks in vitro. Cells were treated with 5 μM Dex or eucalyptus bioflavonoids (Quercitin, 10 μM; Sigma Chemical, St Louis, MO).
Cell viability assays
Cell viability was assessed by 3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Chemicon International, Temecula, CA) assay according to manufacturer's instructions (Roche Molecular Biochemicals, Indianapolis, IN), with some modifications. Cells were seeded in 96-well plates in RPMI 1640 medium containing 5% FBS. Dex or Eucalyptus bioflavonoids were added 24 hours later and incubated for 72 hours. Cell viability was evaluated as previously described.12,13
Quantification of apoptosis
Dual-fluorescence staining with DNA-binding fluorochrome Hoechst 33342 (HO) and propidium iodide (PI) was used to quantitate the percentage of apoptotic (PI-HO+) cells using flow cytometry (The Vantage, Becton Dickinson, Franklin Lakes, NJ), as previously described.14 Apoptosis was also assessed by annexin V staining for externalization of phosphatidylserine, as in prior studies.15
Preparation of S-100 cytosolic fractions
MM.1R MM cells were washed twice with phosphate-buffered saline (PBS), and the pellet was suspended in 5 mL ice-cold buffer A (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.5, 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM EGTA [ethylene glycol tetraacetic acid], 1 mM dithiothreitol [DTT], 0.1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/mL each leupeptin, aprotinin, and pepstatin A) containing 250 mM sucrose. Cells were homogenized by douncing 3 times in a Dounce homogenizer with a sandpaper-polished pestle. Cytosolic fractions were isolated as previously described.12
Western blotting
Total cell lysates and cytosolic extracts were prepared and Western blot analysis was performed, as previously described.16 Briefly, equal amounts of proteins were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes; filters were blocked by incubation in 5% dry milk in PBST (0.05% Tween-20 in PBS), and probed with anti-Hsp27, anti-Hsp70, anti-green fluorescent protein (anti-GFP), anti-caspase-3, anti-poly(adenosine diphosphate ribose) polymerase (anti-PARP), antitubulin (Santa Cruz Biotechnology, CA), anti-cyto-c, anti-Smac (kindly provided by Dr Xiaodong Wang, University of Texas Southwestern Medical Center at Dallas), anti-caspase-3, anti-SHP2 (Santa Cruz Biotechnology), or antiactin antibodies (Sigma Chemical). Blots were then developed by enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL). The immunoblots were scanned using an LKB Produkter (Bromma, Sweden) Ultrascan XL laser densitometer and analyzed with the Gelscan software package (Bromma, Germany). Signal intensity was determined in a linear range and normalized to that for tubulin, GFP, or Hsp70. Preparation of cell lysates for PARP immunoblot analysis was performed as described using C-2-10 anti-PARP monoclonal antibody.17
Caspase activity assay
Caspase-9 activation was determined using LEHD-pNA as a substrate, as per the manufacturer's instructions (colorimetric assay kit; Biovision, Palo Alto, CA) and as previously described.18
Hsp27 expression construct and transient transfections
Hsp27 expression construct and transient transfection Hsp27 cDNA were amplified using synthesized primers, Hsp27-5 (5′-GACGTCCAGAGCAGAGTCAGCCAG-3′) and Hsp27-3 (5′-GGTGGTTGCTTGAACTTTATTTGAG-3′). Conditions for the polymerase chain reaction (PCR) were as follows: denaturing for 1 minute at 94°C, followed by 30 cycles of denaturing for 30 seconds at 94°C, and elongation for 4 minutes. The elongation temperature was 72°C for the initial 5 cycles, 70°C for the next 5 cycles, and 68°C for the last 20 cycles. The PCR product was cloned into pCR2.1 vector, and its DNA sequence was confirmed. Hsp27 cDNA was then recloned into the EcoRI site of the expression vector pTracer-SV40 (containing GFP; Invitrogen, Carlsbad, CA) in sense or AS orientation. MM.1R, MM.1S, U266, and RPMI-8226 MM cells were transiently transfected using cell line Nucleofector kit V, according to the manufacturer's instructions (Amaxa Biosystems, Cologne, Germany).19 GFP+ cells were selected by flow cytometry and treated with Dex (5 μM) for 24, 48, and 72 hours, followed by analysis for cell viability, apoptosis, and protein expression as described. Scrambled oligodeoxynucleotides (ODNs) were also used as another control.
Mitochondrial membrane potential (ΔΨm) and superoxide ( \(\mathbf{O}_{2}^{-}\) ) generation
Cells were treated with Dex (5 μM) for 24 hours, stained with lipophilic cationic dye CMXRos (Mitotracker Red; Molecular Probes, Eugene, OR) in PBS for 20 minutes at 37°C, and analyzed by flow cytometry to assay for alterations in ΔΨm, as previously described.20 Dex-treated cells were also stained with membrane permeable dye dihydroethidium (HE) followed by flow cytometric analysis, as previously described.21 Superoxide anions oxidize HE to fluorescent ethidium, which permits analysis by flow cytometry (The Vantage, FACScan, Becton Dickinson) using excitation at 480 nm and emission at 630 nm.
Results
Expression of Hsp27 in Dex-sensitive versus Dex-resistant MM cells and its functional significance
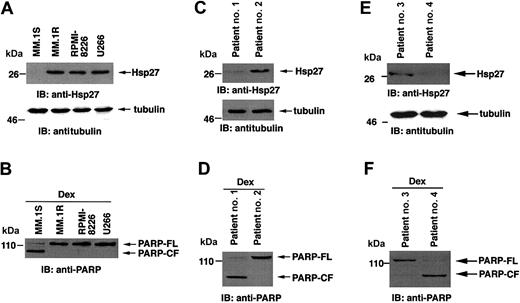
First, we determined whether Hsp27 is differentially expressed in Dex-sensitive (MM.1S) and Dex-resistant (MM.1R, U266, RPMI-8226) cells and then, whether treatment of these cells with Dex induces PARP cleavage, a signature event during apoptosis.22 Total protein lysates were subjected to immunoblot analysis with anti-Hsp27 antibody. As seen in the upper panel of Figure 1A, Hsp27 protein levels are higher in MM.1R, U266, and RPMI-8226 cells compared to MM.1S cells. The differences in Hsp27 levels are specific because no changes in tubulin levels were observed (Figure 1A, lower panel). Patterns of Hsp27 protein levels are consistent with mRNA levels, as previously shown.9 Moreover, Dex induces apoptosis in MM.1S but not in MM.1R, U266, or RPMI-8226 cells, as evidenced by PARP cleavage (Figure 1B). Similar results were obtained in Dex-sensitive (patients 1 and 4) and Dex-resistant (patients 2 and 3) patient MM cells (Figure 1C-F). Together, these findings indicate that high Hsp27 expression correlates with nonresponsiveness to Dex in MM cells; conversely, low expression of Hsp27 is associated with Dex sensitivity.
Expression of Hsp27 correlates with Dex sensitivity and Dex resistance in MM cells. (A) Total cell lysates from MM.1S, MM.1R, RPMI-8226, and U266 MM cells were prepared. Lysates were separated by 10% SDS-PAGE and analyzed by immunoblotting (IB) with anti-Hsp27 or anti-tubulin antibodies. (B) Cells were treated with 5 μM Dex for 24 hours and analyzed for apoptosis by PARP cleavage. Lysates were subjected to immunoblot analysis with anti-PARP antibody. (C,E) Expression of Hsp27 in purified patient MM cells. Lysates from patient MM cells (Dex sensitive, patients no. 1 and 4) and (Dex resistant, patients no. 2 and 3) were subjected to immunoblot analysis with anti-Hsp27 and antitubulin antibodies. (D,F) Patient MM cells were treated with 5 μM Dex for 24 hours and analyzed for apoptosis by PARP cleavage. Lysates were subjected to immunoblot analysis with anti-PARP antibody. Blots are representative of 3 independent experiments with similar results. FL indicates full length; CF, cleaved fragment.
Expression of Hsp27 correlates with Dex sensitivity and Dex resistance in MM cells. (A) Total cell lysates from MM.1S, MM.1R, RPMI-8226, and U266 MM cells were prepared. Lysates were separated by 10% SDS-PAGE and analyzed by immunoblotting (IB) with anti-Hsp27 or anti-tubulin antibodies. (B) Cells were treated with 5 μM Dex for 24 hours and analyzed for apoptosis by PARP cleavage. Lysates were subjected to immunoblot analysis with anti-PARP antibody. (C,E) Expression of Hsp27 in purified patient MM cells. Lysates from patient MM cells (Dex sensitive, patients no. 1 and 4) and (Dex resistant, patients no. 2 and 3) were subjected to immunoblot analysis with anti-Hsp27 and antitubulin antibodies. (D,F) Patient MM cells were treated with 5 μM Dex for 24 hours and analyzed for apoptosis by PARP cleavage. Lysates were subjected to immunoblot analysis with anti-PARP antibody. Blots are representative of 3 independent experiments with similar results. FL indicates full length; CF, cleaved fragment.
Inhibition of Hsp27 restores sensitivity in Dex-resistant MM cells
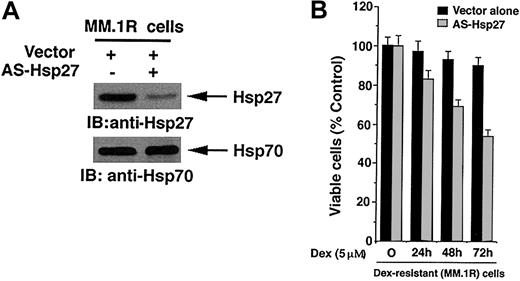
We next directly examined whether inhibition of Hsp27 affects responsiveness to Dex. MM.1R cells were transfected with anti-sense to Hsp27 (AS-Hsp27) and analyzed for alterations in endogenous Hsp27 protein expression. As seen in the upper panel of Figure 2A, the exogenous expression of AS-Hsp27 markedly reduces the expression of endogenous Hsp27, without altering cellular Hsp70 protein levels (Figure 2A lower panel). These data provide the functional specificity of AS-Hsp27.
Blockade of Hsp27 by AS-Hsp27 restores sensitivity to Dex in MM.1R cells. (A) Functional specificity of AS-Hsp27 was determined by subjecting protein lysates from AS-Hsp27-transfected or control vector-transfected cells to immunoblot analysis with anti-Hsp27 (upper panel) or anti-Hsp70 antibodies (lower panel). (B) MM.1R cells were transiently transfected with cDNA construct containing GFP-tagged AS-Hsp27 (▦) or with GFP-empty vector (▪). Following transfections, GFP+ cells were selected by flow cytometry; treated with Dex (5 μM) for 24, 48, or 72 hours, and analyzed for cell viability by MTT assay (P = .05, as determined by one-sided Wilcoxon rank sum test). Error bars represent standard deviation.
Blockade of Hsp27 by AS-Hsp27 restores sensitivity to Dex in MM.1R cells. (A) Functional specificity of AS-Hsp27 was determined by subjecting protein lysates from AS-Hsp27-transfected or control vector-transfected cells to immunoblot analysis with anti-Hsp27 (upper panel) or anti-Hsp70 antibodies (lower panel). (B) MM.1R cells were transiently transfected with cDNA construct containing GFP-tagged AS-Hsp27 (▦) or with GFP-empty vector (▪). Following transfections, GFP+ cells were selected by flow cytometry; treated with Dex (5 μM) for 24, 48, or 72 hours, and analyzed for cell viability by MTT assay (P = .05, as determined by one-sided Wilcoxon rank sum test). Error bars represent standard deviation.
To determine whether inhibition of Hsp27 in MM.1R cells restores sensitivity to Dex in these cells, AS-Hsp27-transfected cells were treated with Dex for 24, 48, and 72 hours and assessed for changes in cell viability by an MTT assay. As seen in Figure 2B, treatment of AS-Hsp27-transfected cells with Dex resulted in significant decreases in cell viability (P = .05, as determined by one-sided Wilcoxon rank sum test). Dex did not alter the viability of control vector-transfected MM.1R cells. To further provide specificity of AS-Hsp27, cells were also treated with scrambled ODNs and analyzed for effects on Dex sensitivity; no significant difference in cell viability was observed after Dex treatment of these MM.1R cells (data not shown).
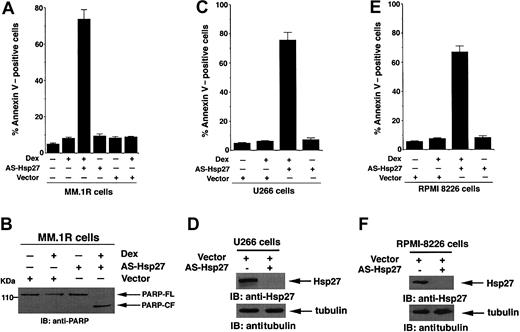
To determine whether Dex induces apoptosis in AS-Hsp27-transfected MM.1R cells, cells were analyzed using both annexin V staining and PARP cleavage activity. As seen in Figure 3A, Dex triggers apoptosis in AS-Hsp27-transfected, but not in either nontransfected or control vector-transfected MM.1R cells. Importantly, transfection of AS-Hsp27 alone does not induce apoptosis in MM.1R cells (Figure 3A). Examination of PARP cleavage, a hallmark of apoptosis, demonstrated similar results. As seen in Figure 3B, Dex induces proteolytic cleavage of PARP in AS-Hsp27-transfected, but not in control vector-transfected, MM.1R cells. To exclude the possibility that this event is specific to MM.1R cells, we performed similar experiments using other Dex-resistant MM cell lines U266 and RPMI-8226. As seen in panels C and E in Figure 3, Dex induces apoptosis in AS-Hsp27-transfected cells but not in control vector-transfected cells. Additionally, exogenous expression of AS-Hsp27 in both U266 and RPMI-8226 cells markedly reduces the expression of endogenous Hsp27 (Figure 3D,F upper panels), without altering the cellular tubulin protein levels (Figure 3D,F lower panels). These data confirm the functional specificity of AS-Hsp27. Taken together, these findings demonstrate that inhibition of Hsp27 by AS-Hsp27 restores Dex responsiveness in otherwise Dex-resistant MM cells.
Dex triggers apoptosis in AS-Hsp27-transfected Dex-resistant cells. (A,B) MM.1R cells were transiently transfected with either GFP-tagged AS-Hsp27 (WT) or with empty vector. Following transfections, GFP+ cells were selected by flow cytometry; treated with Dex (5 μM) for 72 hours, and analyzed for apoptosis by annexin V staining (A) and PARP cleavage (B). Median apoptosis was 73% ± 2% in response to Dex plus AS-Hsp27 and 8.1% ± 0.6% in response to Dex plus vector alone (P < .003; n = 3). Cells were also treated with 5 μM Dex for 24 hours and analyzed for apoptosis by PARP cleavage. Lysates were subjected to immunoblot analysis with anti-PARP antibody. Blot shown is representative of 3 independent experiments with similar results. FL indicates full length; CF, cleaved fragment. (C) U266 cells were transiently transfected with either GFP-tagged AS-Hsp27 (WT) or with empty vector. Following transfections, GFP+ cells were selected by flow cytometry; treated with Dex (5 μM) for 72 hours, and analyzed for apoptosis by annexin V staining. Median apoptosis was 74% for Dex plus AS-Hsp27 and 6% for Dex plus vector alone (P < .004; n = 3). (D) Functional specificity of AS-Hsp27 was determined by subjecting protein lysates from AS-Hsp27-transfected or control vector-transfected U266 MM cells to immunoblot analysis with anti-Hsp27 (upper panel) or anti-tubulin antibodies (lower panels). (E) RPMI-8226 cells were transiently transfected with either GFP-tagged AS-Hsp27 (WT) or with empty vector. Following transfections, GFP+ cells were selected by flow cytometry; treated with Dex (5 μM) for 72 hours, and analyzed for apoptosis by annexin V staining. Median apoptosis was 67% for Dex plus AS-Hsp27 and 9% for Dex plus vector alone (P < .005; n = 3). (F) Functional specificity of AS-Hsp27 was determined by subjecting protein lysates from AS-Hsp27-transfected or control vector-transfected U266 MM cells to immunoblot analysis with anti-Hsp27 (upper panel) or anti-tubulin antibodies (lower panel). Error bars in A, C, and E represent standard deviation.
Dex triggers apoptosis in AS-Hsp27-transfected Dex-resistant cells. (A,B) MM.1R cells were transiently transfected with either GFP-tagged AS-Hsp27 (WT) or with empty vector. Following transfections, GFP+ cells were selected by flow cytometry; treated with Dex (5 μM) for 72 hours, and analyzed for apoptosis by annexin V staining (A) and PARP cleavage (B). Median apoptosis was 73% ± 2% in response to Dex plus AS-Hsp27 and 8.1% ± 0.6% in response to Dex plus vector alone (P < .003; n = 3). Cells were also treated with 5 μM Dex for 24 hours and analyzed for apoptosis by PARP cleavage. Lysates were subjected to immunoblot analysis with anti-PARP antibody. Blot shown is representative of 3 independent experiments with similar results. FL indicates full length; CF, cleaved fragment. (C) U266 cells were transiently transfected with either GFP-tagged AS-Hsp27 (WT) or with empty vector. Following transfections, GFP+ cells were selected by flow cytometry; treated with Dex (5 μM) for 72 hours, and analyzed for apoptosis by annexin V staining. Median apoptosis was 74% for Dex plus AS-Hsp27 and 6% for Dex plus vector alone (P < .004; n = 3). (D) Functional specificity of AS-Hsp27 was determined by subjecting protein lysates from AS-Hsp27-transfected or control vector-transfected U266 MM cells to immunoblot analysis with anti-Hsp27 (upper panel) or anti-tubulin antibodies (lower panels). (E) RPMI-8226 cells were transiently transfected with either GFP-tagged AS-Hsp27 (WT) or with empty vector. Following transfections, GFP+ cells were selected by flow cytometry; treated with Dex (5 μM) for 72 hours, and analyzed for apoptosis by annexin V staining. Median apoptosis was 67% for Dex plus AS-Hsp27 and 9% for Dex plus vector alone (P < .005; n = 3). (F) Functional specificity of AS-Hsp27 was determined by subjecting protein lysates from AS-Hsp27-transfected or control vector-transfected U266 MM cells to immunoblot analysis with anti-Hsp27 (upper panel) or anti-tubulin antibodies (lower panel). Error bars in A, C, and E represent standard deviation.
Overexpression of Hsp27 confers Dex-resistance in MM cells
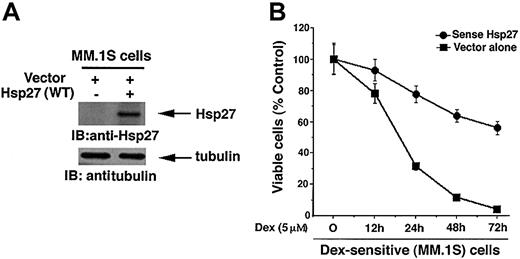
Given that Hsp27 protein levels are significantly lower in Dex-sensitive MM.1S cells vis-à-vis Dex-resistant MM.1R cells, we next asked whether exogenous expression of Hsp27 wild-type (WT) would confer Dex resistance in Dex-sensitive MM.1S cells. As seen in the upper panel of Figure 4A, transfection of Hsp (WT), but not control vector, led to a marked increase in the Hsp27 levels. Expression Hsp27 (WT) does not alter the endogenous tubulin protein levels (Figure 4A, lower panel). Importantly, as seen in Figure 4B, Hsp27 (WT)-transfected cells survive significantly longer than control vector-transfected MM.1S cells after treatment with Dex (P = .04, as determined by Wilcoxon rank sum test). Together, these data demonstrate the ability of Hsp27 to confer Dex resistance in MM cells; conversely, inhibition of Hsp27 restores sensitivity to Dex.
Expression of Hsp27 (WT) in Dex-sensitive MM.1S cells increases resistance to Dex. (A) Functional specificity of Hsp27 (WT) was determined by subjecting protein lysates from Hsp27 (WT)-transfected or control vector-transfected MM.1S cells to immunoblot analysis with anti-Hsp27 (upper panel) or anti-tubulin antibodies (lower panels). Blots are representative of 3 independent experiments with similar results. (B) MM.1S cells were transiently transfected with cDNA expression construct containing GFP-tagged Hsp27 (WT) (•) or with empty vector alone (▪). GFP+ cells were selected by flow cytometry, treated with Dex (5 μM) for 12, 24, 48, or 72 hours, and analyzed for cell viability by an MTT assay. Median viability was 30% (24 hours), 10% (48 hours), and 4% (72 hours) after Dex treatment of empty vector-transfected cells and 79% (24 hours), 63% (48 hours), and 54% (72 hours) after Dex treatment of Hsp27 (WT)-transfected MM.1S cells (P = .04, as determined by one-sided Wilcoxon rank sum test). Error bars represent standard deviation.
Expression of Hsp27 (WT) in Dex-sensitive MM.1S cells increases resistance to Dex. (A) Functional specificity of Hsp27 (WT) was determined by subjecting protein lysates from Hsp27 (WT)-transfected or control vector-transfected MM.1S cells to immunoblot analysis with anti-Hsp27 (upper panel) or anti-tubulin antibodies (lower panels). Blots are representative of 3 independent experiments with similar results. (B) MM.1S cells were transiently transfected with cDNA expression construct containing GFP-tagged Hsp27 (WT) (•) or with empty vector alone (▪). GFP+ cells were selected by flow cytometry, treated with Dex (5 μM) for 12, 24, 48, or 72 hours, and analyzed for cell viability by an MTT assay. Median viability was 30% (24 hours), 10% (48 hours), and 4% (72 hours) after Dex treatment of empty vector-transfected cells and 79% (24 hours), 63% (48 hours), and 54% (72 hours) after Dex treatment of Hsp27 (WT)-transfected MM.1S cells (P = .04, as determined by one-sided Wilcoxon rank sum test). Error bars represent standard deviation.
Effect of inhibition of Hsp27 on Dex sensitivity in the patient MM cells
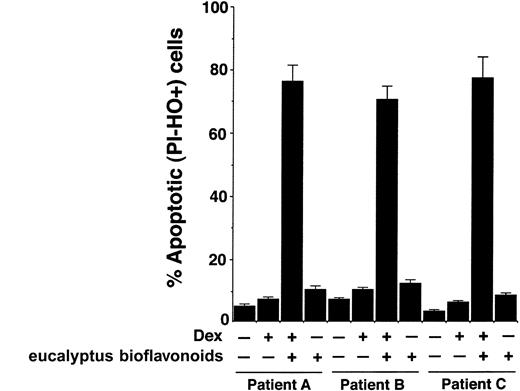
To confirm whether our in vitro findings in MM cell lines are reproducible in patient MM cells, we examined the effect of inhibition of Hsp27 on Dex sensitivity in purified patient MM cells. Tumor cells were purified from BM aspirates by CD138+ selection using CD138 microbeads and the autoMACS magnetic cell sorter. Due to lack of adequate transfection efficiency in primary patient cells, we used a known biochemical inhibitor of Hsp27, eucalyptus bioflavonoids. MM cells were obtained from patients refractory to Dex therapy (patients A-C, Figure 5), treated with Dex, Dex plus eucalyptus bioflavonoids, or eucalyptus bioflavonoids alone for 48 hours, and analyzed for both apoptosis and cell viability.
Inhibition of Hsp27 sensitizes Dex-refractory patient MM cells to Dex. Purified MM cells (CD138+) from Dex-refractory patients (patients A-C) were treated with Dex, eucalyptus bioflavonoids, or Dex plus eucalyptus bioflavonoids for 48 hours, and analyzed for apoptosis by flow cytometric analysis using dualfluorescence staining with DNA-binding fluorochrome Hoechst 33342 (HO) and propidium iodide (PI). PI-- and HO+ stained cells represent apoptotic cells. Error bars represent standard deviation.
Inhibition of Hsp27 sensitizes Dex-refractory patient MM cells to Dex. Purified MM cells (CD138+) from Dex-refractory patients (patients A-C) were treated with Dex, eucalyptus bioflavonoids, or Dex plus eucalyptus bioflavonoids for 48 hours, and analyzed for apoptosis by flow cytometric analysis using dualfluorescence staining with DNA-binding fluorochrome Hoechst 33342 (HO) and propidium iodide (PI). PI-- and HO+ stained cells represent apoptotic cells. Error bars represent standard deviation.
As seen in Figure 5, treatment of cells with Dex plus eucalyptus bioflavonoids triggers a significant increase in percentage of apoptotic cells (PI-HO+ cell population), whereas neither Dex nor eucalyptus bioflavonoids alone induces apoptosis in these cells (median percent apoptotic cells for Dex plus eucalyptus bioflavonoids, 76.3% ± 4.3%; Dex, 7.7% ± 0.6%; and eucalyptus bioflavonoids, 9%; P < .003; n = 3). Examination of cell viability by an MTT assay showed similar results; treatment of cells with Dex plus eucalyptus bioflavonoids induced a significant loss in cell viability, whereas neither Dex nor eucalyptus bioflavonoids alone decreased cell viability (median viability for Dex plus eucalyptus bioflavonoids, 33% ± 1.3%; Dex, 97.3% ± 2.1%; and eucalyptus bioflavonoids, 95%; P < .003). Taken together, these results suggest that inhibition of Hsp27 in Dex-refractory patient cells renders these cells sensitive to Dex therapy.
Inhibition of Hsp27 enables Dex to trigger loss of mitochondrial membrane potential (ΔΨm) and generation of superoxide anions ( \(\mathbf{O}_{2}^{-}\) )
We next examined the molecular mechanism whereby Hsp27 modulates Dex-induced responses in MM cells. In this context, a recent study showed that Hsp27 protects against apoptosis through its interaction with cytosolic cytochrome c, thus establishing a link between Hsp27 and mitochondria signaling.23
Stress-induced apoptosis correlates with mitochondria-related events: loss of ΔΨm24 and generation of reactive oxygen species (ROS), including
Dex induces alterations in mitochondrial membrane potential (ΔΨm) and superoxide (
Dex induces alterations in mitochondrial membrane potential (ΔΨm) and superoxide (
Because loss of ΔΨm is associated with
Hsp27 negatively regulates the release of mitochondrial protein Smac and activation of downstream caspase cascade
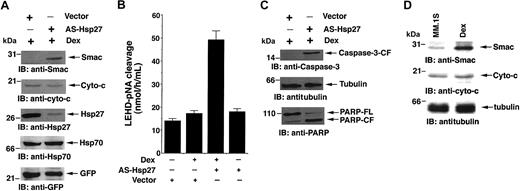
Alteration of ΔΨm leads to the release of mitochondrial apoptogenic proteins cyto-c and Smac, with subsequent activation of caspase-9 and caspase-3.26,32 We therefore next examined whether Dex treatment of AS-Hsp27-transfected cells induces the release of Smac and cyto-c. As seen in the first panel of Figure 7A, Dex-induces Smac release in AS-Hsp27-transfected MM.1R cells. No increase in the cytosolic cyto-c was observed (Figure 7A second panel). Expression of endogenous Hsp27, but not of Hsp70, is markedly reduced by exogenous AS-Hsp27, confirming the functional importance of Hsp27 and specificity of AS-Hsp27 (Figure 7A, third and fourth panels). Immunoblotting with anti-GFP antibody demonstrated equal transfection efficiency (Figure 7A fifth panel). As a negative control, transfection of AS to Hsp70 does not sensitize MM.1R cells to Dex or trigger Smac release (data not shown). Furthermore, treatment of AS-Hsp27-transfected MM.1R cells with Dex induces caspase-9, caspase-3, and PARP cleavage, without similar changes in control vector-transfected cells (Figure 7B and C, respectively). Importantly, neither Dex nor AS-Hsp27 alone induces caspase-9, caspase-3, or PARP cleavage. In contrast, treatment of AS-hsp27-transfected MM.1R cells with Dex does not activate caspase-8 (data not shown). Taken together, these findings demonstrate that the combination of AS-Hsp27 and Dex triggers apoptosis in MM.1R cells via Smac greater than caspase-9 greater than caspase-3 greater than PARP.
AS-Hsp27 induces the release of mitochondrial protein Smac and activates downstream caspase cascade. (A) Ectopic expression of AS-Hsp27 in MM.1R cells restores apoptosis in response to Dex via Smac release. MM.1R cells were transfected with GFP vector alone or GFP-AS-Hsp27. GFP+ cells were isolated and treated with Dex (5 μM). Cytosolic extracts from these cells were separated by SDS-PAGE and analyzed by immunoblotting with anti-Smac and anti-cyto-c antibodies (first and second panels, respectively). Expression and functional specificity of AS-Hsp27 was determined by immunoblotting with anti-Hsp27 and anti-Hsp70 antibodies (third and fourth panels, respectively). As a control for equal transfections, filters were reprobed with anti-GFP antibody (fifth panel). Blots are representative of 3 independent experiments with similar results. (B) Dex triggers caspase-9 activation. MM.1R cells were transfected with GFP vector alone or GFP-AS-Hsp27. GFP+ cells were isolated and treated with Dex (5 μM) for 48 hours and harvested. Caspase-9 activation was determined by subjecting cytosolic extracts for protease activity using LEHD-pNA as substrate. Results are representative of 3 independent experiments (P < .005; n = 3). Error bars represent standard deviation. (C) Dex triggers caspase-3 and PARP cleavage in AS-Hsp27-transfected MM.1R cells. Cytosolic extracts from the cells were subjected to immunoblot analysis with anti-caspase-3 (upper panel), anti-tubulin (middle panel), or anti-PARP (lower panel) antibodies. Blots are representative of 3 independent experiments with similar results. (D) MM.1S cells treated with Dex (5 μM) for 24 hours; cytosolic extracts were prepared and subjected to immunoblot analysis with anti-Smac (upper panel), anti-cyto-c (middle panel), or anti-tubulin (lower panel) antibodies. Blots are representative of 3 independent experiments with similar results.
AS-Hsp27 induces the release of mitochondrial protein Smac and activates downstream caspase cascade. (A) Ectopic expression of AS-Hsp27 in MM.1R cells restores apoptosis in response to Dex via Smac release. MM.1R cells were transfected with GFP vector alone or GFP-AS-Hsp27. GFP+ cells were isolated and treated with Dex (5 μM). Cytosolic extracts from these cells were separated by SDS-PAGE and analyzed by immunoblotting with anti-Smac and anti-cyto-c antibodies (first and second panels, respectively). Expression and functional specificity of AS-Hsp27 was determined by immunoblotting with anti-Hsp27 and anti-Hsp70 antibodies (third and fourth panels, respectively). As a control for equal transfections, filters were reprobed with anti-GFP antibody (fifth panel). Blots are representative of 3 independent experiments with similar results. (B) Dex triggers caspase-9 activation. MM.1R cells were transfected with GFP vector alone or GFP-AS-Hsp27. GFP+ cells were isolated and treated with Dex (5 μM) for 48 hours and harvested. Caspase-9 activation was determined by subjecting cytosolic extracts for protease activity using LEHD-pNA as substrate. Results are representative of 3 independent experiments (P < .005; n = 3). Error bars represent standard deviation. (C) Dex triggers caspase-3 and PARP cleavage in AS-Hsp27-transfected MM.1R cells. Cytosolic extracts from the cells were subjected to immunoblot analysis with anti-caspase-3 (upper panel), anti-tubulin (middle panel), or anti-PARP (lower panel) antibodies. Blots are representative of 3 independent experiments with similar results. (D) MM.1S cells treated with Dex (5 μM) for 24 hours; cytosolic extracts were prepared and subjected to immunoblot analysis with anti-Smac (upper panel), anti-cyto-c (middle panel), or anti-tubulin (lower panel) antibodies. Blots are representative of 3 independent experiments with similar results.
These data are consistent with our prior study showing that Dex-induced apoptosis in MM.1S (Dex-sensitive) cells is associated with release of Smac, but not cyto-c18 (Figure 7D). The present findings further show that (1) Hsp27 confers Dex resistance and (2) blocking Hsp27 restores Dex-sensitivity via Smac-mediated apoptotic signaling in these cells.
Blocking Hsp27 disables IL-6-mediated protective effects against Dex-induced apoptosis
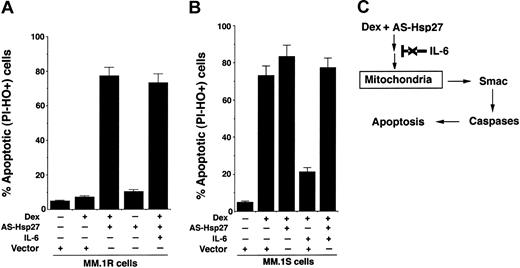
We and others have shown that IL-6 protects against Dex-induced apoptosis.18,33-36 We therefore next examined whether IL-6 similarly prevents Dex-induced apoptosis in AS-Hsp27-transfected MM.1R cells. As seen in Figure 8A, IL-6 does not block Dex-triggered apoptosis in AS-Hsp27-transfected cells. We next determined in MM.1S Dex-sensitive cells (1) whether inhibition of Hsp27 enhances the anti-MM activity of Dex, and (2) whether IL-6 protects against Dex plus AS-Hsp27-triggered apoptosis. MM.1S cells were transfected with AS-Hsp27 and control vector; cells were then treated with Dex, in the presence or absence of IL-6, and analyzed for apoptosis. As seen in Figure 8B, AS-Hsp27 increases Dex-induced apoptosis in MM.1S cells. Importantly, IL-6 fails to protect against AS-Hsp27 plus Dex-induced cell death in these cells (Figure 8B). As a control, IL-6 prevents Dex-induced apoptosis in control vector-transfected MM.1S cells (Figure 8B). These data show that AS-Hsp27 not only overcomes Dex resistance, but also abrogates the protective effect of IL-6 against Dex-induced apoptosis.
IL-6 does not protect against Dex-induced apoptosis in AS-Hsp27-transfected cells. (A) MM.1R cells were transfected with GFP vector alone or GFP-AS-Hsp27. Following transfections, GFP+ cells were selected by flow cytometry, treated with Dex (5 μM) in the presence or absence of IL-6 (100 ng/mL) for 48 hours, and analyzed for apoptosis by dual-fluorescence staining with DNA-binding fluorochrome Hoechst 33342 (HO) and propidium iodide (PI). PI-- and HO+-stained cells represent apoptotic cells. Median percent apoptotic cells: Dex plus vector = 6.4%; Dex plus AS-Hsp27 = 78.6% ± 5.6%; AS-Hsp27 = 9.3%; and Dex plus AS-Hsp27 plus IL-6 = 76.3% ± 2.1%. Results are mean ± SD from 3 independent experiments; P < .003. (B) MM.1S cells were transfected with GFP vector alone or GFP-AS-Hsp27. Following transfections, GFP+ cells were selected by flow cytometry; treated with Dex (5 μM) in the presence or absence of IL-6 (100 ng/mL) for 48 hours, and analyzed for apoptosis, as described. Median percent apoptotic cells: Dex plus vector = 76.2% ± 3.5%; Dex plus AS-Hsp27 = 84.6% ± 5.6%; Dex plus vector plus IL-6 = 20.4% ± 1.2%; Dex plus AS-Hsp27 plus IL-6 = 73.4%. Results are mean ± SD from 3 independent experiments; P < .003. (C) Schema showing the mechanism whereby inhibition of Hsp27 enables Dex to trigger apoptosis via Smac release in Dex-resistant cells. Dex-induced apoptotic signaling in AS-Hsp27-transfected cells is not blocked by IL-6.
IL-6 does not protect against Dex-induced apoptosis in AS-Hsp27-transfected cells. (A) MM.1R cells were transfected with GFP vector alone or GFP-AS-Hsp27. Following transfections, GFP+ cells were selected by flow cytometry, treated with Dex (5 μM) in the presence or absence of IL-6 (100 ng/mL) for 48 hours, and analyzed for apoptosis by dual-fluorescence staining with DNA-binding fluorochrome Hoechst 33342 (HO) and propidium iodide (PI). PI-- and HO+-stained cells represent apoptotic cells. Median percent apoptotic cells: Dex plus vector = 6.4%; Dex plus AS-Hsp27 = 78.6% ± 5.6%; AS-Hsp27 = 9.3%; and Dex plus AS-Hsp27 plus IL-6 = 76.3% ± 2.1%. Results are mean ± SD from 3 independent experiments; P < .003. (B) MM.1S cells were transfected with GFP vector alone or GFP-AS-Hsp27. Following transfections, GFP+ cells were selected by flow cytometry; treated with Dex (5 μM) in the presence or absence of IL-6 (100 ng/mL) for 48 hours, and analyzed for apoptosis, as described. Median percent apoptotic cells: Dex plus vector = 76.2% ± 3.5%; Dex plus AS-Hsp27 = 84.6% ± 5.6%; Dex plus vector plus IL-6 = 20.4% ± 1.2%; Dex plus AS-Hsp27 plus IL-6 = 73.4%. Results are mean ± SD from 3 independent experiments; P < .003. (C) Schema showing the mechanism whereby inhibition of Hsp27 enables Dex to trigger apoptosis via Smac release in Dex-resistant cells. Dex-induced apoptotic signaling in AS-Hsp27-transfected cells is not blocked by IL-6.
Discussion
In the present study, we demonstrate the following: (1) Hsp27 confers Dex resistance in MM cells; (2) blocking Hsp27 by AS to Hsp27 restores sensitivity to Dex in Dex-resistant MM cells; (3) inhibition of Hsp27 enables Dex to trigger apoptosis via mitochondrial apoptotic pathway involving the loss of ΔΨm, generation of
Our prior study using oligonucleotide arrays showed that Hsp27 transcripts are up-regulated in Dex-resistant MM.1R cells compared to Dex-sensitive MM.1S MM cells.9 In the current study, we show that Hsp27 protein levels are also similarly high in MM.1R cells versus MM.1S cells. Our results further demonstrate that Dex resistance in MM cells is associated with high Hsp27 expression. These findings are consistent with various other studies that link between Hsp27 and chemoresistance in different cell types. For example, higher than normal levels of Hsp27 expression are commonly detected in breast,37 prostate,38 ovarian,39 and oral squamous carcinoma cancers,40 as well as in Hodgkin disease.41 Together, these data suggest a role of Hsp27 in conferring Dex resistance in MM cells.
We next determined the functional significance of Hsp27 expression in MM cells. Blockade of Hsp27 expression using an AS strategy restores Dex sensitivity in otherwise Dex-resistant MM cells. Conversely, ectopic expression of wild-type Hsp27 in Dex-sensitive MM cell renders these cells resistant to Dex. Furthermore, treatment with the biochemical inhibitor of Hsp27 eucalyptus bioflavonoids markedly increases Dex sensitivity in primary patient MM cells, confirming our in vitro observations in MM cell lines. Our data are consistent with a recent report demonstrating a similar role of Hsp27 in cisplatin-resistant human ovarian cancer cells19 and doxorubicin-resistant colorectal cancer cells.42 Taken together, these findings demonstrate that Hsp27 confers Dex resistance in MM cells.
The mechanisms mediating the cytoprotective function of Hsp27 are unclear. Multiple studies demonstrate a potential involvement of Hsp27 in negatively regulating mitochondrial apoptotic signaling,43 thereby allowing increased drug resistance and survival. Mitochondria harbor 2 key modulators of apoptosis, cyto-c and Smac.44,45 During stress, these mitochondrial proteins are released to cytosol where they activate the caspase cascade and cause subsequent cell death.25 Prior studies showed that Hsp27 inhibits cyto-c release and prevents related cell death.23,46,47 In the present study, we further demonstrate that Hsp27 also inhibits the release of another mitochondrial protein, Smac, thereby allowing increased survival and resistance to Dex in MM cells. Blocking Hsp27 using an AS strategy restores sensitivity to Dex in Dex-resistant MM cells via activation of Smac-mediated caspase-9/caspase-3 apoptotic signaling. These findings, coupled with other studies, confirm that Hsp27 mediates its antiapoptotic function, at least in part, by blocking mitochondria-initiated apoptotic pathways.
Given that Hsp27 is linked to mitochondria, its localization within the cell is of central importance. In this context, a recent study reported that a significant pool of Hsp27 resides in the mitochondria48,49 and functions in a manner similar to another mitochondria-resident protein Bcl2.50,51 Indeed, both these proteins prevent the release of cyto-c and block apoptosis.43 Preliminary examination of the Bcl2 and Hsp27 protein levels in Dex-resistant versus Dex-sensitive MM cells shows differential expression patterns of Hsp27, but not of Bcl2 (data not shown). These results indicate that Hsp27, but not Bcl2, is likely to facilitate Smac release and confer Dex resistance in MM cells. Alternatively, Hsp27 may influence the phosphorylation of Bcl2 and coordinate the release of Smac. Further studies are required to establish the role of Bcl2 vis-à-vis Hsp27 in MM cell survival and drug resistance.
It has been shown in various studies that Hsp27 interferes with apoptotic signaling pathways upstream of mitochondrial cyto-c release via modulation of the Bcl2 family member Bid.23 A recent study also showed that Hsp27 blocks cyto-c release by maintaining the integrity of actin network: Hsp27 prevents the translocation of proapoptotic factors from the actin cytoskeleton to mitochondria, where they can trigger cyto-c release.23,52,53 In the present study, blocking Hsp27 allows Dex to induce Smac release; however, the underlying mechanism mediating this event remains to be defined. Our data show that Hsp27 inhibition also prevents both loss of ΔΨm and generation of
Having shown that Hsp27 confers Dex resistance in MM cells, we next examined whether Hsp27 also coordinates with other antiapoptotic factors to promote the development of Dex resistance in these cells. Adhesion of MM cells to bone marrow stromal cells (BMSCs) triggers secretion of factors such as IL-6 from BMSCs, which mediate MM growth, survival, and drug resistance.54 Moreover, a high serum level of IL-6 contributes to clinical chemoresistance and treatment failure.55 We and others have also shown that IL-6 protects against Dex-induced apoptosis.33-35 We therefore asked whether IL-6 blocks Dex-induced apoptosis in AS-Hsp27-transfected cells. IL-6 failed to prevent Dex-induced apoptosis in AS-Hsp27-transfected MM.1S or MM.1R cells. These data show that inhibition of Hsp27 not only overcomes Dex resistance, but also abrogates the protective effects of IL-6 against Dex-induced apoptosis.
Finally, an in vivo relevance of our in vitro studies was derived from the examination of purified patient MM cells. Inhibition of Hsp27 also markedly enhanced Dex sensitivity in patient MM cells. These results confirmed that inhibition of Hsp27 in Dex-refractory patient cells renders these cells sensitive to Dex therapy and suggest a potential therapeutic approach for Dex-resistant MM. Previous reports demonstrating that synthetic Smac peptides can enhance the apoptotic activity of chemotherapeutic agents,56 coupled with our present studies showing Hsp27 inhibition sensitizes Dex-resistant cells via release of mitochondrial Smac, provide the preclinical rationale for use of Smac peptides or AS-Hsp27 or both to overcome clinical Dex resistance in MM cells.
Prepublished online as Blood First Edition Paper, July 10, 2003; DOI 10.1182/blood-2003-05-1417.
Supported by National Institutes of Health grants 50947 and CA 78373, SPORE P50 CA100707-01, P01 CA078378-06, a Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.), the Myeloma Research Fund, and the Cure Myeloma Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ”advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 6. Dex induces alterations in mitochondrial membrane potential (ΔΨm) and superoxide (\batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(\mathbf{O}_{2}^{-}\) \end{document}) generation in AS-Hsp27-transfected MM.1R cells. (A) MM.1R cells were transiently transfected with either GFP-tagged AS-Hsp27 (WT) or with empty vector. Following transfections, GFP+ cells were selected by flow cytometry; treated with Dex (5 μM) for 24 hours, incubated with CMXRos for the last 20 minutes, and analyzed by flow cytometry to assay for alterations in ΔΨm. Results are mean ± SD of 3 independent experiments (P < .003). (B) AS-Hsp-27 or vector-transfected MM.1R cells were treated with Dex (5 μM) for 24 hours, harvested, stained with membrane permeable dye dihydroethidium (HE) for the last 15 minutes, and analyzed by flow cytometry. Results are mean ± SD of 3 independent experiments (P < .005). Superoxide anions oxidize HE to fluorescent ethidium, permitting analysis by flow cytometry (The Vantage, FACScan, Becton Dickinson) using excitation at 480 nm and emission at 630 nm.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/102/9/10.1182_blood-2003-05-1417/6/m_h82135161006.jpeg?Expires=1769159339&Signature=XEkLEBI8JRNr0bCwk9ETUz8jjY52GEwsHKYyc-cDpiHqcWzAaai4MOKfL24tt0dRdff2QFb8afOCewBvijCUXCD5qFbJfZjxWYrFN5y3Emx6dBVZSP2KgEu41veunReCq5BgrFQqPgPgq5aSVukaA004aFU7oO7IoFl950ugkuJM~7qwoks9k5fYkicdvdTm5DXbyco1AxfjDnBttHMn-rPIrOdsxHAFZz-HpQOpkmgbCyE5boVdetOiffvwP54ycrzY6ER5KEcvpzo8nR8-OHfoJz4dPO3bJuUVeg47w-QkvF~0JPMuLR4UfuGB8AtG7WdlDaaYJXbH0wJoI6a6yw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal