Abstract
Minor histocompatibility antigens (mHAs) recognized by donor T cells play a central role as immunologic targets of graft-versus-host disease (GVHD) and graft versus leukemia after allogeneic hematopoietic stem cell transplantation (HSCT). Men who have undergone sex-mismatched allogeneic HSCT are at high risk for GVHD because of immune responses directed against mHAs encoded by genes on the Y chromosome (termed H-Y antigens). We hypothesized that the immunogenicity of mHAs results in a coordinated response involving B cells as well as T cells. To test this, we measured antibody responses to a well-characterized H-Y antigen, dead box RNAhelicase Y (DBY), and its homolog, DBX, in 150 HSCT patients. Using Western blot and enzyme-linked immunosorbent assay (ELISA), we found that 50% of male patients who received stem cell grafts from female donors developed antibody responses to recombinant DBY protein. Antibodies to DBY were also detected in 17% of healthy women, but not in healthy men. Antibody responses were directed primarily against areas of amino acid disparity between DBY and DBX. These studies demonstrate that the immune response to mHA includes the generation of specific antibodies and suggests that the serologic response to these antigens may also be useful in the identification of new mHAs.
Introduction
Minor histocompatibility antigens (mHAs) have traditionally been defined as peptides derived from normal cellular proteins presented by major histocompatibility complex (MHC) class I and class II molecules.1,2 Following allogeneic hematopoietic stem cell transplantation (HSCT), recipient mHAs are recognized by donor T cells and contribute to both the development of graft-versus-host disease (GVHD) and graft versus leukemia (GVL).3-6 Thus far, human mHAs have been defined by allo-reactive T-cell clones.7-14 This has resulted in the identification of several MHC class I– or class II–restricted mHA and the demonstration that T-cell recognition is dependent on the presence of amino acid polymorphisms within these peptides or in adjacent regions. These genetic polymorphisms distinguish recipient from donor but do not generally affect the function of the protein containing the substituted amino acid. Despite improvements in methods for identification of T-cell antigens, the requirement for T-cell recognition has limited the identification of new mHAs. We hypothesized that the immunogenicity of mHAs results in a coordinated response involving both B- and T-cell immunity to the target antigen. To test this hypothesis, we studied a well-defined mHA termed dead box RNA helicase Y (DBY)12 and determined whether patients developed specific antibody responses to this antigen following allogeneic HSCT.
DBY is a 660–amino acid protein encoded in the nonredundant portion of the Y chromosome and shares 91% identity with its X homolog, DBX.15 In men, both DBY and DBX are ubiquitously expressed in all tissues, including peripheral blood cells, spleen, liver, gut, and skin.12,15 Primary structure analysis of DBY and DBX reveals the presence of Asp-Glu-Ala-Asp (DEAD) motifs associated with putative RNA helicases. Peptide epitopes binding human leukocyte antigen (HLA) class II were initially identified in the murine homolog of DBY with the use of skin graft rejection assays.16,17 Subsequently, a human DQB5 HLA class II–restricted peptide epitope has also been identified.12
Using both Western blotting and enzyme-linked immunosorbent assay (ELISA), we demonstrate the presence of specific antibody for DBY but not DBX in 50% of male patients who engraft with hematopoietic stem cells from female donors. Antibodies specific for DBY are also found in 17% of healthy women. These experiments demonstrate that H-Y mHAs elicit high-titer and specific antibody responses after allogeneic HSCT and in healthy individuals. These antibody responses may facilitate the identification of new mHAs and may also play a role in the pathogenesis of GVHD.
Patients, materials, and methods
Samples from patients and healthy donors
Plasma samples were obtained from 150 patients 6 to 24 months after allogeneic HSCT. All patients had hematologic malignancies and received marrow or peripheral blood stem cells from HLA-matched donors, either related or unrelated. HSCT patient characteristics are reported in Table 1 in various donor and patient sex combinations.
Characteristics of hematopoietic stem cell transplantation patients and donors
. | HSCT donor → patient sex . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | F → M n = 60 . | M → M n = 39 . | F → F n = 28 . | M → F n = 23 . | |||
| Patient age, y | |||||||
| Median | 47 | 48 | 44 | 42 | |||
| Range | 19-66 | 21-62 | 22-60 | 22-58 | |||
| Donor age, y | |||||||
| Median | 42 | 36 | 39 | 38 | |||
| Range | 2-70 | 21-64 | 22-59 | 16-63 | |||
| Donor relation, no. (%) | |||||||
| Unrelated | 15 (25) | 18 (46) | 16 (57) | 8 (35) | |||
| Related | 45 (75) | 21 (54) | 12 (43) | 15 (65) | |||
| HSC source, no. (%) | |||||||
| Bone marrow | 46 (77) | 23 (59) | 21 (75) | 18 (78) | |||
| PBSCs | 14 (23) | 16 (41) | 7 (25) | 5 (22) | |||
| HSC processing, no. (%) | |||||||
| Unmanipulated | 37 (62) | 25 (64) | 24 (86) | 19 (83) | |||
| T-cell depleted | 23 (38) | 14 (36) | 4 (14) | 4 (17) | |||
| Conditioning regimen, no. (%) | |||||||
| Myeloablative | 48 (80) | 27 (69) | 22 (78.6) | 20 (87) | |||
| Nonmyeloablative | 12 (20) | 12 (31) | 6 (21.4) | 3 (13) | |||
. | HSCT donor → patient sex . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | F → M n = 60 . | M → M n = 39 . | F → F n = 28 . | M → F n = 23 . | |||
| Patient age, y | |||||||
| Median | 47 | 48 | 44 | 42 | |||
| Range | 19-66 | 21-62 | 22-60 | 22-58 | |||
| Donor age, y | |||||||
| Median | 42 | 36 | 39 | 38 | |||
| Range | 2-70 | 21-64 | 22-59 | 16-63 | |||
| Donor relation, no. (%) | |||||||
| Unrelated | 15 (25) | 18 (46) | 16 (57) | 8 (35) | |||
| Related | 45 (75) | 21 (54) | 12 (43) | 15 (65) | |||
| HSC source, no. (%) | |||||||
| Bone marrow | 46 (77) | 23 (59) | 21 (75) | 18 (78) | |||
| PBSCs | 14 (23) | 16 (41) | 7 (25) | 5 (22) | |||
| HSC processing, no. (%) | |||||||
| Unmanipulated | 37 (62) | 25 (64) | 24 (86) | 19 (83) | |||
| T-cell depleted | 23 (38) | 14 (36) | 4 (14) | 4 (17) | |||
| Conditioning regimen, no. (%) | |||||||
| Myeloablative | 48 (80) | 27 (69) | 22 (78.6) | 20 (87) | |||
| Nonmyeloablative | 12 (20) | 12 (31) | 6 (21.4) | 3 (13) | |||
PBSCs indicates peripheral blood stem cells.
Plasma samples were also obtained from 72 patients prior to HSCT and from 65 healthy donors. Samples were cryopreserved and stored at –70°C until use. Approval for these studies was obtained from the Dana-Farber/Harvard Cancer Center institutional review board for these studies, and individual informed consent for sample collection and in vitro studies was obtained from all patients and donors.
Preparation of recombinant proteins
Full-length DBY, and DBX cDNA were reverse transcribed from male peripheral blood mononuclear cells and polymerase chain reaction (PCR)–amplified with primers derived from GenBank sequence AF000985 and NM 024005. They are as follows: DBY 5′ forward primer, 5′-CACCATGGGTCATGTGGTGGTGAAAAATGAC-3′; DBY 3′ reverse primer, 5′-GTTGCCCCACCAGTCAACCCCCT-3′; DBX 5′ forward primer, 5′-CACCATGGGTCATGTGGCAGTGGAAA-3′; DBX 3′ reverse primer, 5′-GTTACCCCACCAGTCAACCC-3′.
Each gene was topo cloned (Invitrogen, Carlsbad, CA), and expressed with C-terminal V5 epitope tag and 6 histidine residues in Escherichia coli (pET-Dest42) and female-derived 293 cell line (pcDNA-Dest40). Both DBY and DBX formed inclusion bodies when synthesized in E coli and were solubilized in 6 M guanidine and subsequently purified by nickel affinity chromatography in the presence of 6 M urea. Before elution with 500 mM imidazole, DBY and DBX proteins were renatured by removing 6 M urea over a 12-hour linear gradient into 500 mM sodium chloride, 20 mM sodium phosphate, pH 6.0, and 20% glycerol. Protein p24 is encoded by the second open reading frame (ORF), proteolytically processed from the Gag-pol polypeptide in vivo, and begins with proline. To facilitate translation, methionine and glycine were incorporated at the N-terminus of p24 by PCR amplification of HIV HXB2 Gag-pol ORF with primers 5′-CACCATGGGACCTATAGTGCAGAACATCCAG-3′ and 5′-CAAAACTCTTGCCTTATGGCCG-3′. The p24 was expressed in E coli (pET-Dest42) and was purified in similar fashion.
Western blotting
Purified E coli–derived p24, DBY, and DBX proteins (0.5 μg per lane) were separated by sodium dodecyl sulfate–polyacrylamide get electrophoresis (SDS-PAGE) and Coomassie stained. The same p24, DBY, and DBX samples were blotted with anti-V5, or plasma samples diluted 1:500. Human female 293 cells were transfected with pcDNA-DEST40 expressing either mock (empty vector), DBX, or DBY genes. Samples of total cell lysates (5 μg per lane) were prepared 24 hours after transfection, separated by SDS-PAGE, and Coomassie stained. These total cell lysates were Western blotted and detected with anti-V5 or plasma samples diluted 1:500.
ELISA for antibodies to recombinant DBY and DBX proteins
Purified DBY, DBX, and HIVp24 proteins were diluted to 5.0 mcg/mL in carbonate binding buffer before coating 96-well ELISA plates (NUNC Scientific, Rochester, NY) with 50 μL (0.25 μg antigen) per well.18 Tris-buffered saline with 0.5% Tween 20 (TBST)–washed ELISA plates were blocked with 2% nonfat dry milk powder in TBST for 2 hours before patient plasma (diluted 1:250) was incubated with 50 μL per well overnight at 4°C. Antibodies were detected with goat antihuman immunoglobulin G (IgG) conjugated to alkaline phosphatase (Pierce, Rockford, IL). Each assay was developed 30 minutes before measuring the absorbance at 450 to 550 nm. Since all patients and donors were previously screened for antibody to HIVp24 and were known to be negative, reactivity with recombinant HIVp24 was used as a negative control and was subtracted from each patient's DBY and DBX measurement. ELISA values for p24 were low in all samples (98% had fewer than 0.05 absorbance [Abs]) and were assumed to reflect the level of nonspecific background binding in the assay.
ELISA for antibodies to DBY and DBX peptides
Plasma from male patients with female donors and 47 healthy donors were tested for antibody response to 93 individual DBY peptides (New England Peptides, Fitchburg, MA) (http://hiv-web.lanl.gov/content/hiv-db/PEPTGEN/PeptGenSubmitForm.html. Accessed October 2, 2003). Each peptide was solubilized 10 mg/mL in 100% dimethyl sulfoxide (DMSO) and diluted into carbonate coating buffer. Then, 0.5 μg peptide was incubated overnight in each well. TBST-washed ELISAplates were blocked with 2% nonfat dry milk powder in TBST for 2 hours before patient plasma (diluted 1:250) was incubated overnight at 4°C. Antibodies were detected with goat antihuman IgG conjugated to alkaline phosphatase (Pierce). Each assay was developed 60 minutes before measuring the absorbance 450 to 550 nm.
Statistical methods
Several statistical approaches were explored to establish cut-off points for DBY and DBX ELISA values. These approaches included taking the 95th or 99th percentile value, taking the mean +2 standard deviations (SD) value from the log-transformed distribution, a classification tree, and the receiver operator characteristic (ROC) curve. The suggested cut-off points from these approaches lie between 0.06 and 0.1 Abs. Because of the exploratory nature of the study and to avoid false positives, an OD of 0.1 was established as the cut-off value for determination of positive antibody reactivity for both DBY and DBX. For 2 × 2 table analysis, a 2-sided Fisher exact test and a 2-sided exact McNemar test were performed. Multiple comparisons are not adjusted, owing to the exploratory nature of the study. Kappa statistic was used to describe the agreement between 2 categorical measurements on the same cohort of patients. Kappa ranges from –1 (no agreement) through +1 (perfect agreement). The smoothing spline curve estimation technique was used to explore the pattern of development of antibody response to recombinant DBY protein after allogeneic HSCT.
Results
Patients develop antibodies specific for DBY after HSCT
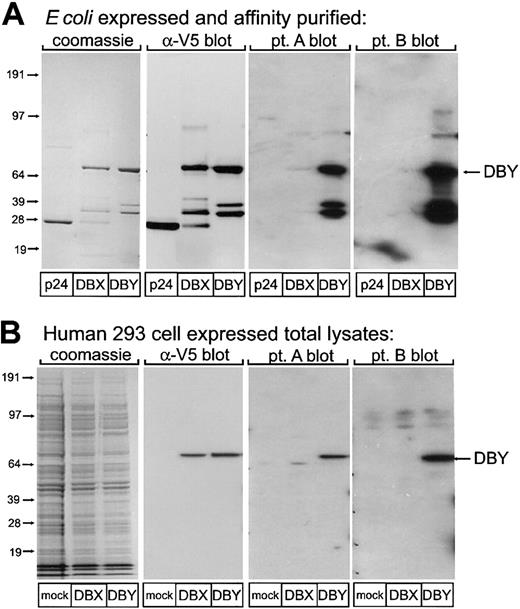
In contrast to T cells, which recognize mHAs as 8– to 12–amino acid peptides presented by specific HLA alleles, mHA-specific antibodies could potentially target any native or denatured epitope of the cellular protein from which the HLA-restricted epitope has been derived. We therefore expressed full-length recombinant DBY protein in both E coli and 293 female human embryonal kidney cells. The homologous DBX protein and HIV protein p24 were also synthesized by means of similar methods to provide specificity controls. All patients and donors were previously screened for antibody to HIVp24 and were known to be negative. Western blotting experiments shown in Figure 1 were used to detect the presence of antibodies specific for these proteins. Coomassie stains of E coli–derived affinity-purified DBY, DBX, and control p24 protein demonstrate equal loading (Figure 1A). Immunoblotting for the V5 tag demonstrates the presence of full-length recombinant DBY and DBX as well as several presumed proteolytic fragments. Plasma from 25 male HSCT patients collected at least 6 months after transplantation was tested for reactivity against this panel of purified recombinant proteins by Western blotting. Ten patient samples demonstrated the presence of antibodies specific for DBY without recognition of DBX or p24. Two representative blots, labeled patients A and B, demonstrate the presence of antibodies specific for DBY fragments as well as the full-length protein. Figure 1B shows Coomassie staining of wholecell lysates of human 293 cells transiently transfected with DBY, DBX, or empty vector control. Immunoblotting for the V5 tag demonstrates the presence of full-length DBY and DBX in these lysates. Immunoblotting 293 cell lysates with plasma from the same 25 male HSCT patients confirmed the selective reactivity with DBY but not DBX in the same 10 male patients (representative blots for patients A and B are shown). Thus, some male HSCT patients develop serologic responses to DBY, and these experiments demonstrate the specificity of the antibody response to recombinant DBY produced in bacteria as well as in human cells.
Reactivity of patient plasma with recombinant human DBY and DBX proteins. (A) Recombinant p24, DBY, and DBX proteins produced in E coli were probed in Western blots with antibodies specific for the V5 epitope tag and plasma obtained from 2 male patients after allogeneic HSCT. The location of the full-length recombinant DBY protein is indicated by the arrow. (B) Recombinant DBY and DBX were expressed in human female 293 cells, and total cell lysates were probed in Western blots with antibodies specific for the V5 epitope tag and plasma obtained from the same male patients shown in panel A. Results with DBY and DBX are compared with 293 cells transfected with an empty vector (mock transfectants). The location of the full-length recombinant DBY protein is indicated by the arrow.
Reactivity of patient plasma with recombinant human DBY and DBX proteins. (A) Recombinant p24, DBY, and DBX proteins produced in E coli were probed in Western blots with antibodies specific for the V5 epitope tag and plasma obtained from 2 male patients after allogeneic HSCT. The location of the full-length recombinant DBY protein is indicated by the arrow. (B) Recombinant DBY and DBX were expressed in human female 293 cells, and total cell lysates were probed in Western blots with antibodies specific for the V5 epitope tag and plasma obtained from the same male patients shown in panel A. Results with DBY and DBX are compared with 293 cells transfected with an empty vector (mock transfectants). The location of the full-length recombinant DBY protein is indicated by the arrow.
DBY antibody develops in male patients with female stem cell donors and some healthy female donors
To facilitate detection and quantitative measurement of antibodies specific for DBY, an ELISA was developed. The specific reactivity of each plasma sample was also tested against recombinant DBX and HIVp24 protein produced in E coli and purified in an identical fashion. Since all patients and donors were known to be negative for antibody to HIV, p24 ELISA values were assumed to reflect the level of nonspecific background binding in the assay and were subtracted from each patient's DBY and DBX measurement. Plasma samples collected from 150 HSCT patients between 6 months and 2 years after transplantation, 72 samples collected before transplantation, and 65 samples from healthy donors were tested for the presence of specific antibody to these proteins (Table 2).
Antibody responses to recombinant DBY and DBX measured by ELISA
Group . | Individuals tested . | Donor sex . | DBY positive, no. (%) . | DBX positive, no. (%) . |
|---|---|---|---|---|
| 1 | Male after HSCT | Female | 30/60 (50) | 2/60 (3) |
| 2 | Male after HSCT | Male | 2/39 (5) | 1/39 (3) |
| 3 | Male before HSCT | — | 3/48 (6) | 0/48 (0) |
| 4 | Female after HSCT | Female | 3/28 (11) | 1/28 (4) |
| 5 | Female after HSCT | Male | 4/23 (17) | 3/23 (13) |
| 6 | Female before HSCT | — | 6/24 (25) | 0/24 (0) |
| 7 | Healthy male | — | 0/30 (0) | 0/30 (0) |
| 8 | Healthy female | — | 6/35 (17) | 0/35 (0) |
Group . | Individuals tested . | Donor sex . | DBY positive, no. (%) . | DBX positive, no. (%) . |
|---|---|---|---|---|
| 1 | Male after HSCT | Female | 30/60 (50) | 2/60 (3) |
| 2 | Male after HSCT | Male | 2/39 (5) | 1/39 (3) |
| 3 | Male before HSCT | — | 3/48 (6) | 0/48 (0) |
| 4 | Female after HSCT | Female | 3/28 (11) | 1/28 (4) |
| 5 | Female after HSCT | Male | 4/23 (17) | 3/23 (13) |
| 6 | Female before HSCT | — | 6/24 (25) | 0/24 (0) |
| 7 | Healthy male | — | 0/30 (0) | 0/30 (0) |
| 8 | Healthy female | — | 6/35 (17) | 0/35 (0) |
With the 2-sided McNemar test, P < .001 for the comparison of DBY versus DBX in male patients after HSCT with female donors; P = .03 for the comparison of DBY versus DBX in healthy females. For DBY comparisons by 2-sided Fisher exact test, P < .001 for group 1 versus 2, group 1 versus 4, group 1 versus 7; P = .64 for group 2 versus 4; and P = .03 for group 7 versus 8.
Antibody responses to DBY were detected in 30 of 60 male patients with female donors, but only 2 of these patients had detectable antibody responses to DBX (P < .001, McNemar test). In contrast, only 2 of 39 male patients who received stem cells from male donors developed antibody responses to DBY. None of the 30 healthy men tested positive for either DBY or DBX. Only 3 of 48 male patients had DBY antibodies before transplantation, and none had DBX responses. Thus, the high frequency of DBY-specific antibody in male patients who received stem cells from female donors differs significantly in comparison with male patients with male stem cell donors (P < .001) and healthy men (P < .001).
Antibody responses to DBY were also detected in 11% to 17% of female patients after stem cell transplantation. In contrast to male patients, the presence of antibodies to DBY was also detected in 25% of female patients prior to transplantation, and the presence of antibodies after transplantation was not associated with the sex of the stem cell donor. In contrast to healthy men, 6 of 35 healthy women had detectable antibodies to DBY with no DBX reactivity (P = .03), suggesting that exposures other than allogeneic transplantation can stimulate immunity to HY antigens. In women, allogeneic transplantation does not appear to significantly increase the frequency of antibodies to DBY above the 17% detected in healthy female donors. Like men, few women develop antibodies to DBX under any circumstance.
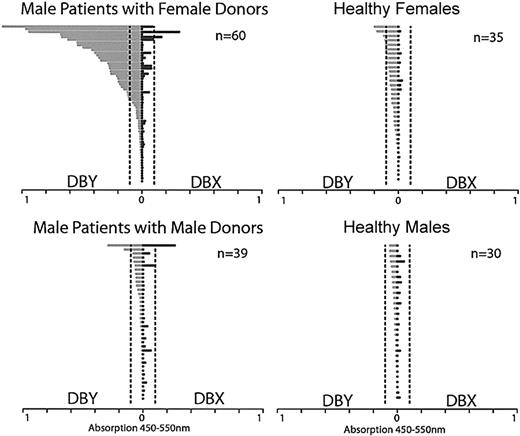
To examine the magnitude of antibody responses to DBY and DBX, we compared results of ELISA for 99 male patients and 65 healthy donors (Figure 2). All samples were tested at 1:250 dilution. The anti-DBY response was clearly greatest in male patients with female donors. Although antibody responses were also present in some healthy women and male patients with male donors, the level of reactivity was substantially less in these individuals. The reactivity of these samples with DBY was confirmed by Western blot (data not shown). Antibodies to DBX were detected primarily in those patients who also had DBY responses, and the magnitude of any DBX response was less than the corresponding response to DBY.
Antibody response to recombinant DBY and DBX in male patients after allogeneic HSCT and in healthy donors. The magnitude of DBY reactivity by ELISA is shown on the left in light gray bars, and the corresponding DBX reactivity is shown on the right in dark gray bars for each individual. Dotted vertical lines indicate the lower limit of values considered positive for the assay (0.1).
Antibody response to recombinant DBY and DBX in male patients after allogeneic HSCT and in healthy donors. The magnitude of DBY reactivity by ELISA is shown on the left in light gray bars, and the corresponding DBX reactivity is shown on the right in dark gray bars for each individual. Dotted vertical lines indicate the lower limit of values considered positive for the assay (0.1).
Antibody response to recombinant DBY develops after allogeneic HSCT
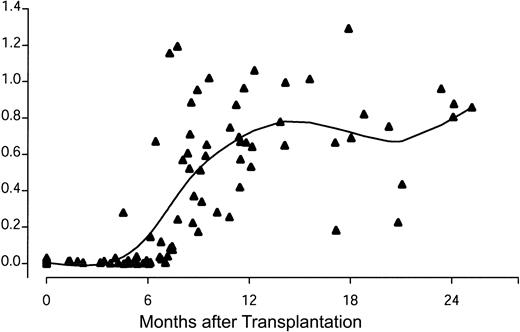
When examined prior to HSCT, only 6% of 48 male patients had detectable antibody to DBY (Table 2). Twenty-three male HSCT patients with female donors had serum samples measured for DBY antibody by ELISA both before transplantation and 6 months to 1 year after transplantion. None of these 23 patients had anti-DBY antibodies prior to HSCT, but 13 (57%) developed anti-DBY antibodies after HSCT (P < .001, McNemar test). This observation suggested that antibody responses to DBY occurred as a result of allogeneic HSCT. The temporal development of anti-DBY antibody was explored by measurement of anti-DBY IgG by ELISA in serial samples from 12 of these male patients with female donors who developed DBY antibody (Figure 3). DBY antibodies were not detectable in any of these patients prior to stem cell transplantation or in the first 3 months after HSCT. DBY-specific antibodies developed between 4 and 8 months after HSCT and persisted at relatively high levels for at least 2 years.
Development of antibody response to recombinant DBY protein after allogeneic HSCT. Serial samples from 12 male patients were tested for antibody to DBY by ELISA. Individual samples from these patients were previously known to be DBY positive 6 to 12 months after HSCT. A smoothing spline curve is superimposed on the scatterplot.19
Development of antibody response to recombinant DBY protein after allogeneic HSCT. Serial samples from 12 male patients were tested for antibody to DBY by ELISA. Individual samples from these patients were previously known to be DBY positive 6 to 12 months after HSCT. A smoothing spline curve is superimposed on the scatterplot.19
Epitope specificity of the antibody response to DBY
To further characterize the specificity of the antibody response to the DBY protein, we synthesized 93 peptides corresponding to the amino acid sequence of DBY. Peptides were 17 to 21 amino acids in length and overlapped one another by 5 amino acids over the entire 660–amino acid sequence of DBY. Sixty samples from male HSCT patients with female donors and 47 healthy donors were tested for reactivity with each of these 93 peptides by ELISA. As shown in Table 3, the presence of antibodies to full-length recombinant DBY protein was highly correlated with the presence of antibodies to DBY peptides. Of the 30 patient samples that were positive for antibodies to DBY protein, 23 (77%) were also positive for at least 1 DBY peptide. Conversely, only 4 (13%) of 30 patient samples that did not react with recombinant DBY protein demonstrated reactivity with any DBY peptides (P < .001). A similarly high correlation between these 2 independent assays was found in serum samples from healthy donors (P = .003). These results demonstrate that the antibody response to DBY was directed against distinct amino acid sequences and that further characterization of DBY peptide epitopes could be used to further define the DBY antibody response in vivo.
Correlation of antibody response to DBY protein and DBY peptides
. | DBY peptide positive . | DBY peptide negative . |
|---|---|---|
| Male patients with female donors, n = 60* | ||
| DBY protein positive | 23 | 7 |
| DBY protein negative | 4 | 26 |
| Healthy donors, n = 47† | ||
| DBY protein positive | 4 | 2 |
| DBY protein negative | 3 | 38 |
. | DBY peptide positive . | DBY peptide negative . |
|---|---|---|
| Male patients with female donors, n = 60* | ||
| DBY protein positive | 23 | 7 |
| DBY protein negative | 4 | 26 |
| Healthy donors, n = 47† | ||
| DBY protein positive | 4 | 2 |
| DBY protein negative | 3 | 38 |
Weighted Kappa statistic = 0.63. P < .001 from an exact test.
Weighted Kappa statistic = 0.55. P = .003 from an exact test.
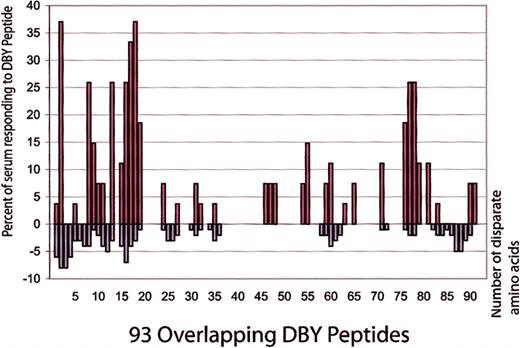
Twenty-seven samples from male patients with female donors were reactive with at least 1 DBY peptide by ELISA. These samples were reactive with 36 different DBY peptides. A mean of 4 peptides were recognized by individual patient samples, but plasma from 3 patients reacted with more than 10 DBY peptides. The frequency with which each individual peptide was found to be reactive with patient samples is shown in Figure 4 as a percentage of the 27 DBY peptide–reactive samples (red bars). For comparison, Figure 4 also shows the number of disparate amino acids between DBY and DBX for each peptide (blue bars). Antibodies reactive with DBY peptides are preferentially directed against regions of DBY with amino acid disparity compared with DBX. Although DBY and DBX are 91% identical, there are 57 disparate amino acids, and 82% of the anti-DBY responses measured by ELISA were directed against disparate peptides. In contrast, only 18% of the anti-DBY responses were directed against peptides that were identical in DBY and DBX.
Reactivity of patient plasma with DBY peptides. Plasma samples from 27 male patients with female donors were found to have developed antibodies to DBY peptides after allogeneic HSCT. The left axis (red bars) plots the frequency of recognition for each DBY peptide as a percentage of the 27 DBY peptide–positive samples. The right axis (blue bars) indicates the number of disparate amino acids comparing DBY and DBX for each peptide.
Reactivity of patient plasma with DBY peptides. Plasma samples from 27 male patients with female donors were found to have developed antibodies to DBY peptides after allogeneic HSCT. The left axis (red bars) plots the frequency of recognition for each DBY peptide as a percentage of the 27 DBY peptide–positive samples. The right axis (blue bars) indicates the number of disparate amino acids comparing DBY and DBX for each peptide.
Comparison of antibody responses to homologous DBY and DBX peptides
To further characterize the specificity of antibody responses to DBY, we synthesized homologous DBX peptides for 10 DBY peptides that were frequently recognized by patients after HSCT. Plasma samples from 27 male patients with female donors known to be reactive with DBY peptides were retested against both DBY and DBX peptides under identical conditions. As shown in Table 4, these patient samples primarily recognized only DBY peptides. In this analysis, 62 assays were positive for DBY peptides, but only 3 were positive for the homologous DBX peptide. Remarkably, there are several examples of antibodies from male HSCT patients directed against DBY peptides (DBY 9, 20, 71, and 76) that differed from DBX by only a single amino acid. For example, all 5 samples that were reactive with DBY 76 failed to recognize DBX 76, and only 1 of 3 DBY 71 positive sera recognized DBX 71. Only 2 samples from 30 healthy male donors had previously been found to be reactive with any of the 93 DBY peptides. One was reactive with DBY peptides 71 and 77, and the second was reactive only with DBY peptide 77. In contrast to the male HSCT patients with female donors, these 2 healthy donor sera reacted equally with the homologous DBX peptides (data not shown).
Specificity of antibody response to DBY peptides
Peptide . | Amino acid sequence . | No. male patients with female donors with reactivity to each peptide,*n = 27 . |
|---|---|---|
| DBY 2 | KNDPELDQQLANLDLNSEK | 10 |
| DBX 2 | ENALGLDQQFAGLDLNSSD | 0 |
| DBY 8 | EASKGFHDKDSSGWSCSK | 7 |
| DBX 8 | EATRGFYDKDSSGWSSSK | 0 |
| DBY 11 | AYSSFGSR-DSRGKPGYF | 2 |
| DBX11 | AYSSFGSRSDSRGKSSFF | 0 |
| DBY 13 | GYFSERGSGSRGRFDDR | 7 |
| DBX 13 | SFFSDRGSGSRGRFDDR | 0 |
| DBY 16 | DGIGNR-ERPGFGRFERSGH | 7 |
| DBX 16 | DGIGSRGDRSGFGKFERGGN | 0 |
| DBY 17 | PGFGRFERSGHSRWCDK | 7 |
| DBX 17 | SGFGKFERGGNSRWCDK | 0 |
| DBY 71 | VAARGLDISNVRHVINF | 3 |
| DBX 71 | VAARGLDISNVKHVINF | 1 |
| DBY 76 | RTGRVGNLGLATSFFNEK | 5 |
| DBX 76 | RTGRVGNLGLATSFFNER | 0 |
| DBY 77 | LGLATSFFNEKNMNITK | 7 |
| DBX 77 | LGLATSFFNERNINITK | 1 |
| DBY 78 | FFNEKNMNITKDLLDLLV | 7 |
| DBX 78 | FFNERNINITKDLLDLLV | 1 |
Peptide . | Amino acid sequence . | No. male patients with female donors with reactivity to each peptide,*n = 27 . |
|---|---|---|
| DBY 2 | KNDPELDQQLANLDLNSEK | 10 |
| DBX 2 | ENALGLDQQFAGLDLNSSD | 0 |
| DBY 8 | EASKGFHDKDSSGWSCSK | 7 |
| DBX 8 | EATRGFYDKDSSGWSSSK | 0 |
| DBY 11 | AYSSFGSR-DSRGKPGYF | 2 |
| DBX11 | AYSSFGSRSDSRGKSSFF | 0 |
| DBY 13 | GYFSERGSGSRGRFDDR | 7 |
| DBX 13 | SFFSDRGSGSRGRFDDR | 0 |
| DBY 16 | DGIGNR-ERPGFGRFERSGH | 7 |
| DBX 16 | DGIGSRGDRSGFGKFERGGN | 0 |
| DBY 17 | PGFGRFERSGHSRWCDK | 7 |
| DBX 17 | SGFGKFERGGNSRWCDK | 0 |
| DBY 71 | VAARGLDISNVRHVINF | 3 |
| DBX 71 | VAARGLDISNVKHVINF | 1 |
| DBY 76 | RTGRVGNLGLATSFFNEK | 5 |
| DBX 76 | RTGRVGNLGLATSFFNER | 0 |
| DBY 77 | LGLATSFFNEKNMNITK | 7 |
| DBX 77 | LGLATSFFNERNINITK | 1 |
| DBY 78 | FFNEKNMNITKDLLDLLV | 7 |
| DBX 78 | FFNERNINITKDLLDLLV | 1 |
Measured by ELISA. Amino acids that differ compared with DBY and DBX are shown in bold.
Discussion
The present studies demonstrate that human H-Y mHAs elicit a specific high-titer antibody response after allogeneic HSCT. Using DBY as a model H-Y mHA, we found that 50% of male patients with female donors develop antibodies to this antigen after transplantation. Because mHAs have previously been defined only as targets of T-cell immunity,20-23 we undertook an extensive series of experiments to ensure the validity of these observations. These included the demonstration of specific antibody reactivity with recombinant DBY protein produced in mammalian cells as well as E coli, the testing of more than 150 patient and 65 healthy samples by ELISA, and the further confirmation of reactivity against synthetic DBY peptides. In each of these experimental approaches, we demonstrated that the antibody response that developed in vivo could distinguish between DBY and the highly homologous DBX.
Although no studies have previously demonstrated the development of antibody responses to defined H-Y antigens, early studies of transplant organ rejection also focused on the recognition of transplantation antigens by antibodies in animal models. In 1955, Eichwald and Silmser24 reported that murine syngeneic skin grafts from male donors were rejected by female recipients 20 to 30 days after transplantation. In contrast, skin grafts in all other sex combinations survived. These experiments indicated the presence of immunogenic Y chromosome–encoded antigens, and further experiments in 1971 demonstrated the generation of antibodies in sensitized female mice that were capable of killing male cells.24,25 Subsequent studies demonstrated the presence of MHC-restricted T-cell responses to these antigens.26-28 These results were extended to humans when H-Y–specific cell-mediated cytotoxicity was identified in a female patient with aplastic anemia who rejected a bone marrow graft from her HLA-identical brother.29 In this patient, graft rejection was also associated with the development of IgM antibodies that were selectively cytolytic for male cells.30,31 Thus, this single patient developed both humoral and cellular immune responses to H-Y antigens. However, the targets of both B- and T-cell immunity were selectively expressed on the surface of male cells, and no previous reports have described specific antibody responses to soluble proteins encoded by H-Y genes. Despite these previous reports supporting our hypothesis that patients can develop coordinated responses involving both B- and T-cell immunity to mHAs, these antigens have exclusively been defined as T-cell epitopes.
It has been clinically recognized that donor/recipient sex-mismatch is a significant risk factor for the development of GVHD following allogeneic HSCT.29,32-34 This is presumably due to the immunogenicity of widely expressed H-Y antigens. In addition to DBY,12,17 several other H-Y antigens, including SMCY,7,35 UTY,10,13 and DFFRY,11,36 have been found to elicit specific T-cell responses from HLA-identical women. Female donor T cells have not been exposed to unique peptides encoded on the Y chromosome, and thymic maturation has not resulted in the deletion of T cells capable of recognizing these H-Y antigens. As a result, female donor T cells are able to respond to peptides derived from H-Y antigens after allogeneic transplantation and initiate GVHD. Women can also be exposed to these antigens through pregnancy or blood transfusion. The clinical significance of such exposure has been suggested by large studies, which show a significant association with donor parity and GVHD after transplantation.33,37 In this context, our studies demonstrate that male recipients of female stem cells also develop high-titer antibody responses to H-Y antigens such as DBY after allogeneic HSCT. Moreover, 17% of healthy female donors and 25% of female patients also had detectable DBY antibodies. Although the magnitude of the antibody response was less in these individuals than in male patients with female stem cell donors, these responses were specific for DBY, as none of these 19 individuals had detectable antibody to DBX and antigen specificity was confirmed by Western blot (data not shown). The ability to detect DBY antibody in women who have not undergone allogeneic transplantation demonstrates that alternative methods of antigen exposure, perhaps during pregnancy or following blood transfusion, can also elicit significant immune responses to mHA. The lower titers of anti–H-Y in these individuals may reflect the lower levels and transient nature of antigen exposure as well as the possibility that such exposure may have occurred many years before specific antibody was measured. A recent report by James et al38 also documents the presence of H-Y–specific T cells in a single multiparous woman using HLA-A2 SMCY tetramers.6
A single HLA class II–restricted DBY T-cell epitope has been identified thus far.12 This epitope is restricted to HLA DQB5, an allele that is present in 30% of our HSCT cohort. DQB5 expression did not correlate with serologic response (data not shown). Nevertheless, the high degree of antibody reactivity directed at specific regions of DBY disparate with DBX suggests that these regions are highly immunogenic and other HLA class II T-cell epitopes may be present. Although none have been identified thus far, HLA class I T-cell epitopes may be present in this protein as well. Once additional T-cell epitopes have been identified, further studies in larger patient cohorts will be needed to better define the relationship between B- and T-cell responses in vivo.
The presence of DBY antibodies in healthy stem cell donors and in patients after allogeneic HSCT may have important clinical implications. Further studies will be necessary to determine whether transplantation of hematopoietic stem cells from donors with pre-existing DBY antibodies is associated with increased risk of subsequent GVHD, or if the presence of DBY antibodies in female patients with male donors is associated with increased rate of graft rejection. In preliminary studies, we have also detected antibodies to UTY and other H-Y antigens. Antibody responses to multiple H-Y antigens are thus likely to occur frequently after exposure and may also play a role in the immune response to male organ grafts in female recipients. If the presence of antibodies to DBY or other H-Y antigens increases the risk of subsequent GVHD or graft rejection, screening for such individuals prior to transplantation may help in the selection of appropriate donors. Following transplantation, IgG antibodies to DBY were not detected until 4 to 6 months after stem cell infusion. It is therefore unlikely that these antibody responses are associated with acute GVHD, but this is the time at which many patients develop chronic GVHD. Although chronic GVHD is presumed to be mediated primarily by donor T cells, the possible role of other immune cells has not been investigated. Chronic GVHD is associated with many features of scleroderma, a well-recognized autoimmune disease. As with autoimmune diseases,39 antibodies to H-Y antigens may also contribute to the pathogenesis of chronic GVHD after HSCT.
Antibodies reactive with DBY peptides preferentially target regions of DBY with amino acid disparity compared with DBX. We tested serum from 60 male HSCT patients with female donors by ELISA against 93 overlapping peptides corresponding to the entire amino acid sequence of DBY, and 82% of the anti-DBY responses measured by ELISA were directed against DBY peptides that differed in comparison with DBX. In contrast, only 18% of the anti-DBY responses were directed against peptides that were identical in DBY and DBX. These nondiscriminating antibodies may be present in vivo as 2 of 60 male HSCT patients with female donors tested positive for both recombinant DBY and DBX. Alternatively, antibodies that appear to be reactive with nondisparate peptides in vitro may reflect specificity for different epitopes in the native protein that may include conformational changes dependent on other disparate residues. Nonetheless, most antibodies target DBY peptides that are disparate in comparison with DBX, and this ability to discriminate was confirmed by directly comparing reactivity to 10 frequently recognized DBY peptides and their corresponding DBX homologs.
DNA sequences of major histocompatibility alleles are now clearly defined, and molecular methods are routinely used to provide HLA typing of patients and prospective donors. Nevertheless, initial characterization of these antigens was greatly facilitated by the generation of specific antibody responses to these antigens in vivo in healthy multiparous women.40 Minor histocompatibility antigens are critical targets of GVHD, but the identification of mHAs has relied exclusively on the analysis of T-cell responses and relatively few well-defined mHAs have been identified. The present studies demonstrate that H-Y mHAs are capable of generating strong B-cell responses in vivo, and the serologic response to these antigens may facilitate the rapid identification of new H-Y antigens and multiple immunogenic B-cell epitopes. In contrast to H-Y antigens, autosomal mHAs most often reflect single amino acid polymorphisms. Such antigens may be less immunogenic, but our analysis of DBY peptides demonstrates that single amino acid disparities are immunogenic in vivo, and the antibody response to these peptides distinguishes between DBY and DBX. Thus, antibody responses to these antigens in vivo may also facilitate the identification of new autosomal mHAs and lead to better matching of recipients and potential stem cell donors.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-03-0984.
Supported by National Institutes of Health (NIH) grants AI29530, HL70149, HL69132 and by the Ted and Eileen Pasquarello Tissue Bank and Research Fund; D.M. supported by Clinical Investigator Training Program: Harvard/MIT Health Sciences and Technology–Beth Israel Deaconess Medical Center, in collaboration with Pfizer Inc and K08 HL69132; R.J.S. is a Scholar in Clinical Research of the Leukemia and Lymphoma Society.
Presented in abstract form at the 44th annual meeting of the American Society of Hematology, Philadelphia, PA, December 10, 2002 (Abstract no. 802).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal