Abstract
Hephaestin (Hp) plays an important role in intestinal iron absorption and is predicted to be a ferroxidase based on significant sequence identity to the serum multicopper ferroxidase ceruloplasmin. Here, we demonstrate that Hp has both amine oxidase and ferroxidase activity in cultured cells and primary intestinal enterocytes with the use of both gel and solution assays. The specificity of the activity is shown by immunoblotting, immunoprecipitation, and immunodepletion experiments. Surprisingly, the truncated hephaestin expressed in sex-linked anemia (sla) mice still has measurable, but decreased, oxidase activity. Molecular modeling of the truncated hephaestin suggests retention of a minimum catalytic core required for enzymatic activity. We suggest that hephaestin, by way of its ferroxidase activity, facilitates iron export from intestinal enterocytes, most likely in cooperation with the basolateral iron transporter, Ireg1.
Introduction
Iron enters the body across the epithelium of the proximal small intestine. A brush border ferri-reductase, Dctyb, is likely to be involved in the uptake process,1 and this enzyme provides the substrate for the apical divalent metal transporter 1 (DMT1). DMT1 carries ferrous iron from the intestinal lumen into enterocytes.2-4 The mechanism(s) of iron transport within the enterocyte remains obscure, but iron moves to the basolateral side and is secreted across the basolateral membrane, likely by way of Ireg1 (also known as ferroportin or MTP1), a Fe (II) transporter.5-7 It is uncertain how iron moves from the basolateral surface of enterocytes through the intestinal mucosa to the plasma, but it is ultimately bound to transferrin as Fe (III) in the intestinal circulation.
Insights into the mechanism of iron export from enterocytes to the plasma have been obtained from the study of the sex-linked anemia (sla) mouse.8,9 Basolateral iron transport is impaired in these mice, leading to iron accumulation in the enterocytes and systemic iron deficiency.8-10 The sla mouse contains a mutation resulting in an in-frame deletion of 582 bases in the mRNA of the heph gene which produces a truncated protein missing 194 amino acids.9 The predicted full-length hephaestin (Hp) protein has significant sequence identity to ceruloplasmin (Cp), a serum multicopper ferroxidase. Modeling of hephaestin based on the backbone of ceruloplasmin11 showed a conserved structure12 with 6 distinct domains. Cp contains 6 integral copper atoms designated type 1, 2, and 3 with distinct spectroscopic properties. All of the type 1 (T1 or “blue” copper), type 2 (T2 or “normal” copper), and type 3 (T3 or binuclear copper lacking an electron paramagnetic resonance [EPR] signal) sites for the 6 copper atoms in Cp are also present in Hp. Three of the copper atoms form a trinuclear metallic unit at the interface of domains 1 and 6. The other 3 copper atoms form the mononuclear type 1 centers and, as in Cp, are located in domains 2, 4, and 6. In the ceruloplasmin structure,13 additional atypical labile metal ion binding sites were identified close to 2 of the 3 mononuclear sites (Cu 42 and 62) in domains 4 and 6, respectively. It was postulated that these sites might be involved in the ferroxidase activity of ceruloplasmin. Hp is also predicted to contain these atypical sites that may serve a similar ferroxidase function. On the basis of the structural similarity with ceruloplasmin and its role in iron export, we hypothesized that hephaestin is a ferroxidase.8,12
Hp is predicted to contain a transmembrane domain at the C-terminus8 like Fet3p,14 a cell surface ferroxidase in Saccharomyces cerevisiae. Although Cp is soluble in plasma, a glycophosphatidyl inositol–linked form has been found in astrocytes.15 Copper-dependent ferroxidase activities of both Cp16-18 and Fet3p14,19 are crucial for plasma membrane iron transport. Fet3p interacts directly with the yeast high-affinity iron importer Ftr1p, and Ftr1p cannot reach the plasma membrane without its Fet3p partner.20 Cp circulates in the plasma and likely plays a role in facilitating iron efflux from a variety of cells, but notably the macrophages of the reticuloendothelial system. Studies of individuals and mice with congenital absence of Cp support a role for this protein in the removal of iron from Kupffer cells for redistribution to the body.21,22 Previously, it has been shown that hephaestin is a copper-dependent oxidase protein that can rescue the low-growth phenotype of S cerevisiae Δfet3 strain in iron-poor media.23 Moreover, we have shown that hephaestin is present in a supranuclear compartment and on the basolateral surface of intestinal enterocytes.24 We suggested that the predicted ferroxidase activity at the basolateral location may facilitate intestinal iron export through the basolateral membrane by ways of the iron permease Ireg1.24,25
Here, we show that Hp, in addition to diamines, oxidizes iron and that Hp represents the predominant oxidase activity in isolated mouse enterocytes. We demonstrate that the truncated version of Hp in the sla mouse retains reduced oxidase activity. Structural modeling provides a possible explanation for the residual activity. We hypothesize that the Hp protein, through its oxidase activity, works in concert with the predominantly basolaterally located transporter Ireg124 to facilitate iron transport across the basolateral membrane of intestinal enterocytes.
Materials and methods
Dietary studies and tissue preparation
Three separate mouse diets were used. For each diet, C57BL/6J male mice were separated at weaning into 3 groups of 5 to 11 mice each. The mice were fed the following commercial diets obtained from Dyets (Bethlehem, PA): (1) AIN-93M control diet (catalog no. 110900, ∼50 ppm iron); (2) iron-deficient diet (catalog no. 115111, 12 ppm iron AIN-93M); or (3) iron overload diet (catalog no. 115122; 2% carbonyl iron-supplemented AIN-93M) for 6 months. The mice were allowed unlimited access to the diets and distilled water and were housed in cages designed to minimize coprophagy and environmental iron contamination (stainless steel grid bases used instead of bedding material and silicon stoppers in water bottles). The sla mice were fed the control AIN-93M diet for 6 months. All mouse protocols were in accordance with the National Institutes of Health guidelines and approved by the Office of Lab Animal Care at the University of California, Berkeley. At the end of the dietary treatment, mice were killed without fasting. For all of our studies, we examined duodenal enterocytes rather than whole gut which contains multiple tissue types, including epithelial cells, lamina propria, muscle, blood vessels, and other tissues. Each duodenum was first rinsed with ice-cold phosphate-buffered saline (PBS) and then filled with PBS containing 1.5 mM EDTA (ethylenediaminetetraacetic acid) for 10 minutes to release enterocytes. As previously described, we assessed enterocyte purity by morphologic criteria.26 Enterocytes have a distinct columnar shape compared with other cell types in the intestine, and examination of the freshly isolated cells under the microscope usually shows that the purity is more than 95%. The enterocytes were then washed twice with ice-cold PBS and snap-frozen in liquid nitrogen for subsequent protein and enzyme analysis.
Cell growth and lysis procedures
Nondifferentiated HT29 cells were grown in McCoy 5A medium (Invitrogen, Carlsbad, CA) until 70% confluent. The medium was supplemented with 10% fetal bovine serum (FBS) and a 1% penicillin-streptomycin cocktail (catalog no. 15140-122; Invitrogen). Cultured cells and C57BL/6J and sla enterocytes were washed twice with ice-cold PBS and lysed in PBS containing 1.5% Triton X-100 and supplemented with a cocktail of protease inhibitors (1206893; Roche Molecular Biochemicals, Mannheim, Germany) by passage through a 27-gauge needle. The extracts were centrifuged at 10 000g for 20 minutes at 4° C, and the supernatants were collected. Protein concentrations were determined with the use of a protein assay kit (Bio-Rad Laboratories, Hercules, CA).
Antiserum to hephaestin
An affinity-purified antipeptide polyclonal antiserum to Hp was used in these studies as previously described.9 The antiserum was raised against the C-terminal 15 amino acids (QHRQRKLRRNRRSIL) (peptide 1) and is termed Hp1a.
Western blot analysis
Intestinal enterocyte and HT29 cell protein extracts were separated by polyacrylamide gel electrophoresis under nondenaturing, nonreducing conditions using a 4% to 20% Tris (tris(hydroxymethyl)aminomethane)–glycine running gel and transferred to polyvinylidene diflouride (PVDF) membranes. (It is important to note that native gel electrophoresis does not resolve protein species as sharply as denaturing, reducing sodium dodecyl sulfate [SDS] gel electrophoresis and, therefore, can lead to nonuniform, broad, and often U-shaped bands.27 ) Nonspecific binding was blocked by incubating the membrane with PBS containing 0.1% Tween 20 (PBS-T), 5% bovine serum albumin (BSA), and 5% fat-free dry milk for 1 hour at room temperature on a rotating platform. The membrane was then washed in PBS-T and incubated overnight with a 1:6000 dilution of affinity-purified anti-Hp antibody in PBS-T containing 2% BSA. The membrane was then washed in PBS-T and incubated with a 1:60 000 dilution of a peroxidase-conjugated antirabbit immunoglobulin antiserum (Santa Cruz Biotechnology, Santa Cruz, CA) in PBS-T containing 2% BSA for 1 hour. After washing the membrane 3 times with PBS-T, immunoreactive proteins were visualized by using enhanced chemiluminescence (ECL) with the ECL detection system (Amersham Pharmacia Biotech, Piscataway, NJ).
pPD oxidase activity assay
The oxidase activity of Hp was determined by using lysates of mouse enterocytes and HT29 cells prepared as described earlier. Cell homogenates were centrifuged at 10 000g for 10 minutes to remove unlysed cells and nuclei. The clear lysate was applied to a native nonreducing, nondenaturing 4% to 20% Tris-glycine polyacrylamide gel electrophoresis (PAGE) gel (Invitrogen). The gels were then incubated with 0.1% P-phenylene diamine (pPD) in 0.1 M acetate buffer, pH 5.45, for 2 hours and air-dried in the dark. Purified human ceruloplasmin (Vital Products, Boynton Beach, FL) was used as a positive control. For the in-tube assay, cell extracts were incubated with 0.01% pPD substrate in acetate buffer, pH 5.45, for 30 minutes at 37° C in the dark. Color development was monitored as absorbance at 570 nm.
Ferroxidase activity assay
The ferroxidase-specific assay differs from the pPD gel assay only in the final assay step. The gels (prepared as described in “pPD oxidase activity assay”) are placed for 2 hours at 37° C in a fresh solution of 0.007 84% Fe (NH4)2(SO4)2 6H2O in 100 mM sodium acetate, pH 5.0. Gels were then washed and rehydrated with 15 mM ferrozine solution in the dark. Color development was then monitored continuously and quantified by scanning densitometry. Ceruloplasmin activity was detected with this in-gel assay and served as a positive control.
Immunoprecipitation and immunodepletion of Hp
Enterocyte and HT29 cell extracts (500-800 μg protein), prepared as described earlier, were precleared by incubation with Protein G Sepharose (Amersham Pharmacia Biotech) for 1 hour with rotation at 4° C. The supernatants were then incubated with affinity-purified anti-Hp1a (1:500 dilution) and Protein G Sepharose (50 μL) together and rotated overnight at 4° C. After incubation, the agarose beads were washed 3 times with 1-mL volumes of lysis buffer, and the immunoprecipitates were eluted with 40 μL peptide 1 (10 mg/mL).
Molecular modeling
Results
Enterocytes contain a ferroxidase activity that comigrates with hephaestin
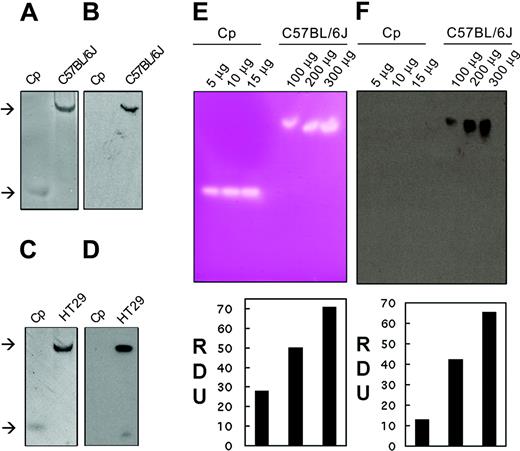
Nondenaturing, nonreducing (native) gel electrophoresis was used to investigate the oxidase activity of intestinal enterocytes by using extracts isolated from C57BL/6J mice. We initially used the amine substrate pPD which becomes purple and insoluble when oxidized, enabling visualization of the localized reaction. As can be seen in Figure 1A, a single reaction product is present in the enterocyte extract similar to that seen for purified ceruloplasmin. Similarly, extracts from a human colon cell line with intestinal cell properties (HT29) (Figure 1C) contain an oxidase activity which migrates at a similar rate to that in the enterocyte extract. The relative positions of oxidase activity attributable to ceruloplasmin and Hp are consistent with the mobilities of standards. Our results are comparable to previous studies of cell extracts using similar assays for Fet3p28,29 and Cp.30 That the oxidase activity in enterocyte and HT29 cell extracts was due to Hp was confirmed by Western blotting using a Hp-specific antiserum (Figure 1B,D). The antibody is raised against the C-terminal tail of hephaestin (a region not present in Cp), and no cross-reactivity of the antiserum was seen, even with excess purified Cp. We conclude that mouse intestinal enterocytes and the HT29 cell line contain a pPD oxidase activity attributable to hephaestin. No other oxidase activity was detected in these cells under the experimental conditions used, a result consistent with the presence of heph and the absence of Cp expression in the intestine.
Hp is an intestinal pPD oxidase and ferroxidase. (A) In-gel pPD oxidase activity was measured after separating 30 μg mouse enterocyte protein extract (C57/BL/6J), isolated under nondenaturing conditions, by native gel electrophoresis. Gels were immediately immersed in pPD solution and photographed as color developed. (B) Immunoblot of a duplicate sample of the enterocyte extract (C57/BL/6J) using Hp1a antiserum to the C-terminus of Hp. (C) In-gel pPD oxidase activity of 30 μg HT29 cell protein extract (HT29). (D) Immunoblot of a duplicate sample of HT29 cell extract (HT29) using Hp1a antiserum. Human ceruloplasmin (Cp) (10 μg) was used as a positive control. The upper and lower arrows indicate the mobilities of Hp and Cp, respectively. (E) In-gel ferroxidase activity was measured by separating serial dilutions of mouse enterocyte protein extracts (C57/BL/6J) under nondenaturing conditions by nondenaturing, nonreducing electrophoresis. Gels were immediately immersed in ferrous ammonium sulfate solution, developed with ferrozine, and scanned as color developed. Ferroxidase signals were analyzed by densitometric measurement expressed as relative densitometric units (RDUs). (F) Immunoblot and densitometric analysis expressed as RDU of replicate samples of mouse intestinal extracts (C57/BL/6J) using Hp1a antiserum. Purified human Cp was used as control.
Hp is an intestinal pPD oxidase and ferroxidase. (A) In-gel pPD oxidase activity was measured after separating 30 μg mouse enterocyte protein extract (C57/BL/6J), isolated under nondenaturing conditions, by native gel electrophoresis. Gels were immediately immersed in pPD solution and photographed as color developed. (B) Immunoblot of a duplicate sample of the enterocyte extract (C57/BL/6J) using Hp1a antiserum to the C-terminus of Hp. (C) In-gel pPD oxidase activity of 30 μg HT29 cell protein extract (HT29). (D) Immunoblot of a duplicate sample of HT29 cell extract (HT29) using Hp1a antiserum. Human ceruloplasmin (Cp) (10 μg) was used as a positive control. The upper and lower arrows indicate the mobilities of Hp and Cp, respectively. (E) In-gel ferroxidase activity was measured by separating serial dilutions of mouse enterocyte protein extracts (C57/BL/6J) under nondenaturing conditions by nondenaturing, nonreducing electrophoresis. Gels were immediately immersed in ferrous ammonium sulfate solution, developed with ferrozine, and scanned as color developed. Ferroxidase signals were analyzed by densitometric measurement expressed as relative densitometric units (RDUs). (F) Immunoblot and densitometric analysis expressed as RDU of replicate samples of mouse intestinal extracts (C57/BL/6J) using Hp1a antiserum. Purified human Cp was used as control.
We subsequently assessed whether hephaestin can oxidize Fe (II) to Fe (III) by using an assay based on ferrozine. This Fe (II) specific chelator is usually colorless but turns red-purple when Fe (II) is bound. As can be seen in Figure 1E, in gel incubation of ceruloplasmin with Fe (II) and ferrozine results in a discrete clear area in the red-purple background. The clear area is indicative of the presence of Fe (III) from the ferroxidase activity of Cp. Similarly, increased amounts of intestinal enterocyte protein extract result in increased observable ferroxidase activity indicative of a dose response (Figure 1E) confirmed by densitometric measurements of the ferroxidase signal (shown below the gel). Oxidase activity could be detected with as little as 80 μg cellular extract. Western blotting with an antiserum specific to Hp reveals that the ferroxidase activity comigrates with Hp (Figure 1F). No cross-immunoreactivity was detected with Cp (Figure 1F). The ferroxidase activity seen in whole cell intestinal extracts is comparable to that seen for purified microgram quantities of ceruloplasmin. We do not know the concentration of hephaestin in the intestinal extract, although Northern blot analysis of Hp indicates a low to average abundance message in enterocytes.9 Even if we make the very conservative assumption that Hp constitutes 1% of the extract (eg, 0.8 μg Hp in 80 μg total protein), the observed activity is at least as high as that of ceruloplasmin and likely higher.
Immunoprecipitated hephaestin has a ferroxidase activity
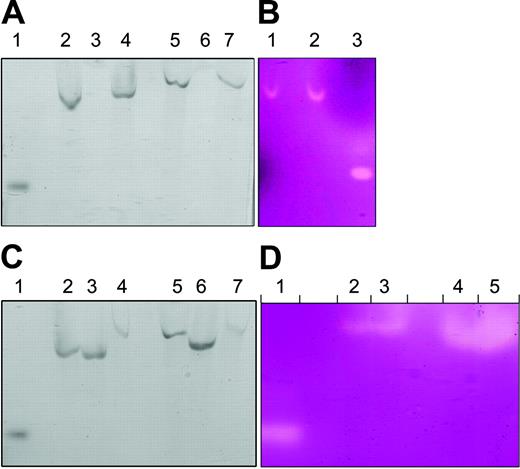
We immunoprecipitated Hp from intestinal enterocyte and HT29 cell protein extracts by using affinity-purified antipeptide Hp-specific antiserum (Hp1a to the C-terminus tail of Hp). We eluted Hp with an excess of the peptide to which the antiserum was raised (QHRQRKLRRNRRSIL). The eluted protein was subjected to native gel electrophoresis and showed in-gel pPD oxidase activity (Figure 2A, lanes 4,7). Cell extracts from intestinal enterocytes (Figure 2A, lane 3) and HT29 cells (Figure 2A, lane 6) showed no signal when immunoprecipitated with preimmune serum. Similarly, immunoprecipitated Hp from intestinal enterocytes showed ferroxidase-specific activity with the use of the ferrozine assay, further supporting the hypothesis that Hp is the enterocyte ferroxidase (Figure 2B, lane 2), whereas extracts immunoprecipitated with Hp1a antiserum in the presence of peptide 1 had a 3.5-fold reduction in signal as determined by densitometric analysis (Figure 2B, lane 1). Immunodepletion with Hp1a antiserum of extracts from intestinal enterocytes (Figure 2C, lane 4) and HT29 cells (Figure 2C, lane 7) showed reduced pPD oxidase activity. Intestinal enterocyte (Figure 2C, lane 3) or HT29 cell (Figure 2C, lane 6) extracts immunodepleted with the preimmune serum retained considerable pPD signal that was comparable to that of nontreated enterocyte (Figure 2C, lane 2) or HT29 cell (Figure 2C, lane 5) extracts, respectively. Similarly, enterocyte extracts immunodepleted of Hp following immunoprecipitation had 4.5-fold reduced ferroxidase activity as determined by densitometric analysis (Figure 2D, lanes 2-3) compared with nontreated extracts (Figure 2D, lanes 4-5). These results, collectively, demonstrate that the pPD and ferroxidase activity observed in intestinal enterocytes and HT29 cells is mediated by hephaestin.
Immunoprecipitated Hp has a pPD oxidase and ferroxidase activity. (A) Enterocyte (lanes 2-4) and HT29 (lanes 5-7) extracts were precleared with protein G Sepharose, and supernatants were collected. Supernatants were incubated with preimmune serum or Hp1a antiserum with protein G Sepharose for 16 hours. Sepharose beads were separated by centrifugation and washed with reaction buffer, and bound Hp was eluted with peptide 1. Eluates were tested with the in-gel pPD oxidase assay. Lane 1 is purified human ceruloplasmin control, lanes 2 and 5 are precleared supernatant controls. Lanes 3 and 6 are eluates from preimmune immunoprecipitates, whereas 4 and 7 are eluates from Hp1a antiserum immunoprecipitates. (B) Enterocyte extracts incubated with protein G Sepharose in the presence of Hp1a antiserum and peptide 1 (lane 1) or Hp1a antiserum only (lane 2). Sepharose beads were separated by centrifugation and washed with reaction buffer, and bound Hp was eluted with peptide 1 and tested with the ferroxidase assay. Purified human ceruloplasmin was used as control (lane 3). (C) Supernatants of enterocyte extracts preincubated with protein G Sepharose in the presence of buffer (lane 2), preimmune serum (lane 3), or Hp1a antiserum (lane 4) were tested with the in-gel pPD oxidase assay. Supernatants of HT29 cell extracts incubated with protein G Sepharose in the presence of buffer (lane 5), preimmune serum (lane 6), or Hp1a antiserum (lane 7) were tested with the in-gel pPD oxidase assay. Purified human ceruloplasmin (lane 1) was used as control. (D) Supernatants of mouse enterocyte extracts preincubated with protein G Sepharose in the presence of Hp1a antiserum (lanes 2-3) or buffer only (lanes 4-5) were tested with the ferroxidase assay. Purified human ceruloplasmin (10 μg) (l ane 1) was used as a control.
Immunoprecipitated Hp has a pPD oxidase and ferroxidase activity. (A) Enterocyte (lanes 2-4) and HT29 (lanes 5-7) extracts were precleared with protein G Sepharose, and supernatants were collected. Supernatants were incubated with preimmune serum or Hp1a antiserum with protein G Sepharose for 16 hours. Sepharose beads were separated by centrifugation and washed with reaction buffer, and bound Hp was eluted with peptide 1. Eluates were tested with the in-gel pPD oxidase assay. Lane 1 is purified human ceruloplasmin control, lanes 2 and 5 are precleared supernatant controls. Lanes 3 and 6 are eluates from preimmune immunoprecipitates, whereas 4 and 7 are eluates from Hp1a antiserum immunoprecipitates. (B) Enterocyte extracts incubated with protein G Sepharose in the presence of Hp1a antiserum and peptide 1 (lane 1) or Hp1a antiserum only (lane 2). Sepharose beads were separated by centrifugation and washed with reaction buffer, and bound Hp was eluted with peptide 1 and tested with the ferroxidase assay. Purified human ceruloplasmin was used as control (lane 3). (C) Supernatants of enterocyte extracts preincubated with protein G Sepharose in the presence of buffer (lane 2), preimmune serum (lane 3), or Hp1a antiserum (lane 4) were tested with the in-gel pPD oxidase assay. Supernatants of HT29 cell extracts incubated with protein G Sepharose in the presence of buffer (lane 5), preimmune serum (lane 6), or Hp1a antiserum (lane 7) were tested with the in-gel pPD oxidase assay. Purified human ceruloplasmin (lane 1) was used as control. (D) Supernatants of mouse enterocyte extracts preincubated with protein G Sepharose in the presence of Hp1a antiserum (lanes 2-3) or buffer only (lanes 4-5) were tested with the ferroxidase assay. Purified human ceruloplasmin (10 μg) (l ane 1) was used as a control.
Hephaestin in sla has ferroxidase activity
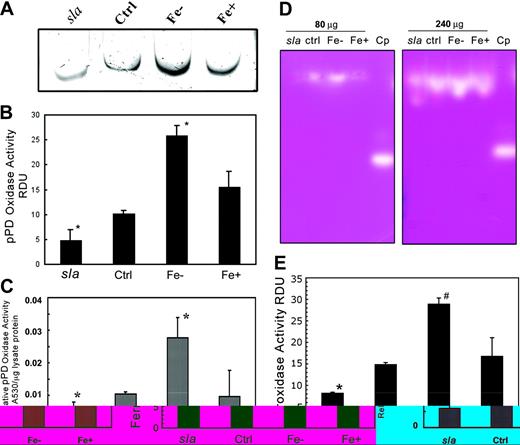
We have previously shown that sla mice produce reduced amounts of a truncated hephaestin protein.9 As depicted in Figure 3A, Hp in these mice still had detectable ferroxidase activity (lane 1). The ferroxidase activity in sla mice migrates faster than that of wild-type mice, consistent with the smaller size of the truncated protein in sla mice. As observed in Figure 3B, ferroxidase activity is detectable with 80 μg protein of a control enterocyte extract but not with a similar amount of an sla enterocyte extract. This finding is consistent with decreased levels and subsequently decreased activity of Hp in sla mice. We estimated the specific activity of the mutant protein by densitometric measurements of ferroxidase activity determined by the in-gel assay and protein levels as determined by Western blot and found no evidence of decreased specific activity of the mutant protein (data not shown).
Hp from sla retains a reduced ferroxidase activity. (A) Mouse enterocyte protein extract ([A] 200 μg or [B] 80 μg) was prepared from a normal mouse (C57/BL/6J) or an sla mouse was resolved by nondenaturing, nonreducing gel electrophoresis. Gels were incubated with ferrous ammonium sulfate, developed with ferrozine, and photographed as color developed. Purified human ceruloplasmin (Cp) was used as a control. The upper and lower arrows indicate the mobilities of Hp and Cp, respectively.
Hp from sla retains a reduced ferroxidase activity. (A) Mouse enterocyte protein extract ([A] 200 μg or [B] 80 μg) was prepared from a normal mouse (C57/BL/6J) or an sla mouse was resolved by nondenaturing, nonreducing gel electrophoresis. Gels were incubated with ferrous ammonium sulfate, developed with ferrozine, and photographed as color developed. Purified human ceruloplasmin (Cp) was used as a control. The upper and lower arrows indicate the mobilities of Hp and Cp, respectively.
pPD oxidase activity is increased in iron deficiency
We previously showed that Hp protein levels increase in iron deficiency.9 As seen in Figure 4A, the in-gel Hp pPD oxidase activity, confirmed by densitometric measurements of the pPD signal (Figure 4B), increases under iron-deficient conditions. Hp activity in sla enterocytes (Figure 4A) is lower than that of control enterocytes in accordance with previous results (Figure 3A). Similarly, in-tube assays (Figure 4C) show that iron deficiency leads to an approximate 2-fold increase in pPD oxidase activity compared with controls. Oxidase activity was similar in control and iron-overloaded enterocyte protein extracts. Similar results were obtained with the ferroxidase assay (Figure 4D) and confirmed by densitometric analysis of the ferrozine signal (Figure 4E). This increased activity under iron-deficient conditions may facilitate the enhanced movement of iron from the enterocytes to the body.
pPD and ferroxidase activities of mouse Hp are increased in iron deficiency. (A) In-gel pPD oxidase assay of extracts from sla, C57/BL/6J (Ctrl), iron-deficient (Fe–), or iron-replete (Fe+) mouse enterocyte protein extracts. (B) Relative pPD levels of sla, Ctrl, Fe–, and Fe+ extracts were quantified using National Institutes of Health (NIH) Image (NIH, Bethesda, MD), and densitometry values are expressed as relative densitometric units (RDUs). Data are means ± SD from 5 independent experiments. *Lanes are significantly different from each other and the control at P < .05. (C) In-tube pPD oxidase activity of replicate samples was measured by adding protein extracts to pPD oxidase assay solution for 30 minutes at 37° C and measuring the A530. Each bar represents the mean ± SD (n = 5). *Significant difference at P < .05. (D) Ferroxidase activity assay of 80 μg and 240 μg sla, C57/BL/6J (Ctrl), iron-deficient (Fe–), and iron-overload (Fe+) mouse enterocyte protein extracts. (E) Relative ferroxidase levels of sla, Ctrl, Fe–, and Fe+ extracts were quantified by using NIH Image and densitometry values expressed as relative densitometric units (RDUs). Data are means ± SD from 5 independent experiments. Lanes marked with different symbols are significantly different from each other and the control at P < .05.
pPD and ferroxidase activities of mouse Hp are increased in iron deficiency. (A) In-gel pPD oxidase assay of extracts from sla, C57/BL/6J (Ctrl), iron-deficient (Fe–), or iron-replete (Fe+) mouse enterocyte protein extracts. (B) Relative pPD levels of sla, Ctrl, Fe–, and Fe+ extracts were quantified using National Institutes of Health (NIH) Image (NIH, Bethesda, MD), and densitometry values are expressed as relative densitometric units (RDUs). Data are means ± SD from 5 independent experiments. *Lanes are significantly different from each other and the control at P < .05. (C) In-tube pPD oxidase activity of replicate samples was measured by adding protein extracts to pPD oxidase assay solution for 30 minutes at 37° C and measuring the A530. Each bar represents the mean ± SD (n = 5). *Significant difference at P < .05. (D) Ferroxidase activity assay of 80 μg and 240 μg sla, C57/BL/6J (Ctrl), iron-deficient (Fe–), and iron-overload (Fe+) mouse enterocyte protein extracts. (E) Relative ferroxidase levels of sla, Ctrl, Fe–, and Fe+ extracts were quantified by using NIH Image and densitometry values expressed as relative densitometric units (RDUs). Data are means ± SD from 5 independent experiments. Lanes marked with different symbols are significantly different from each other and the control at P < .05.
Molecular modeling of sla Hp
We modeled the predicted Hp protein in sla in an effort to understand the retention of partial oxidase activity (Figure 5A). The deletion corresponds to residues 507 to 694 of mHp and results in the removal of a large portion of domains 3 and 4, a region that contains a T1 mononuclear Cu site, from the murine protein.12 In our analysis, we noted the predicted wild-type hephaestin may contain an additional active T1 site which is not functional in Cp (Figure 5B). Although Cp has 3 T1 Cu atoms in domains 2, 4, and 6, the domain 2 T1 Cu is thought not to participate in the oxidation process.34 This domain also does not have an affiliated substrate binding site13 ; therefore, Cp can function adequately as a ferroxidase despite this “defective” site. Interestingly, examination of the hephaestin model reveals the presence of a conserved axial methionine (mHp: M357) in domain 2, which would be able to coordinate the Cu atom and makes this site equivalent to the 4-coordinate mononuclear sites in domains 4 and 6 (Figure 5B-C). We suggest this T1 site in domain 2 may be functional in Hp (Figure 6B) in contrast to Cp (Figure 6A). The minimum catalytic unit that is necessary for the 4-electron reduction to water coupled to substrate oxidation in multicopper oxidases is the T2/T3 multimetallic domain and a single T1 Cu.35 The modeling, therefore, indicates that the T2/T3 cluster (domains 1 and 6) and the T1 Cu sites in domains 2 and 6 of hephaestin are preserved in the mutant sla protein. As outlined in Figure 5A, we suggest that the T1 Cu sites in domains 2 and 6 (Cu2-H304, V303, D302, I301, E300, H-bond.... [2.85 [angstrom] (2.85 × 10–10 m), H1050-Cu6) in sla Hp are sufficient to oxidize the substrates and shuttle the electrons to the T2/T3 oxygen binding and reducing site.
Molecular modeling of sla Hp reveals retention of core catalytic domains. (A) Ribbon diagram of mouse hephaestin. A top view along the pseudo–3-fold axis highlighting the sla deletion in black with the missing T1 Cu in domain 4. The figures were generated by using a modified version of Molscript31 and subsequently rendered in Raster3D version 2.0.32,33 (B) An alignment of Cp and Hp sequences showing the residues coordinating the mononuclear Cu in domain 2. The canonical blue Cu environment for mononuclear type 1 Cu is preserved in Hp and interestingly in zebrafish Cp but not in higher mammalian Cps. Accession numbers for the sequences are U49430, mouse Cp (mCp); L33869, rat Cp (rCp); M13699, human Cp (hCp); BC048037, zebrafish Cp (zCp); AF082567, mouse Hp; AF246120, rat Hp; and AJ296162, human Hp (hHp). *Indicates sequence identity in all proteins, whereas: indicates conservative substitutions. (C) The putative structural arrangement of Cu-binding sites in mHp. The additional methionine (mHp: 357) in domain 2 mononuclear type 1 site of Hp replaces the nonpolar leucine of hCp (L348) at the homologous position. The figure is based on the Cu centers in human Cp.11 The distances between the different Cu atoms are indicated. The brackets indicate the T1 Cu atoms in domains 2 and 4 that are unavailable for participation in the redox reactions in Cp and sla Hp, respectively.
Molecular modeling of sla Hp reveals retention of core catalytic domains. (A) Ribbon diagram of mouse hephaestin. A top view along the pseudo–3-fold axis highlighting the sla deletion in black with the missing T1 Cu in domain 4. The figures were generated by using a modified version of Molscript31 and subsequently rendered in Raster3D version 2.0.32,33 (B) An alignment of Cp and Hp sequences showing the residues coordinating the mononuclear Cu in domain 2. The canonical blue Cu environment for mononuclear type 1 Cu is preserved in Hp and interestingly in zebrafish Cp but not in higher mammalian Cps. Accession numbers for the sequences are U49430, mouse Cp (mCp); L33869, rat Cp (rCp); M13699, human Cp (hCp); BC048037, zebrafish Cp (zCp); AF082567, mouse Hp; AF246120, rat Hp; and AJ296162, human Hp (hHp). *Indicates sequence identity in all proteins, whereas: indicates conservative substitutions. (C) The putative structural arrangement of Cu-binding sites in mHp. The additional methionine (mHp: 357) in domain 2 mononuclear type 1 site of Hp replaces the nonpolar leucine of hCp (L348) at the homologous position. The figure is based on the Cu centers in human Cp.11 The distances between the different Cu atoms are indicated. The brackets indicate the T1 Cu atoms in domains 2 and 4 that are unavailable for participation in the redox reactions in Cp and sla Hp, respectively.
A potentially redox active domain 2 T1 Cu-binding site in Hp. (A) Human Cp and (B) the analogous putative site in murine Hp. The Met357 residue is well positioned to bind a Cu atom in the binding cavity. Residue numbers correspond to full-length amino acid sequences for human Cp (M13699) and mouse Hp (AF246120).
A potentially redox active domain 2 T1 Cu-binding site in Hp. (A) Human Cp and (B) the analogous putative site in murine Hp. The Met357 residue is well positioned to bind a Cu atom in the binding cavity. Residue numbers correspond to full-length amino acid sequences for human Cp (M13699) and mouse Hp (AF246120).
Discussion
We have established that intestinal enterocytes contain a ferroxidase activity. The Hp protein explains the observed oxidase activity in both intestinal enterocytes and a cell-culture model of intestinal enterocytes. Furthermore, Hp can mediate conversion of Fe (II) to Fe (III) and can, therefore, be considered a ferroxidase. Our finding of no other ferroxidase activity in these cells is consistent with earlier studies showing that Hp is expressed at high levels in the gut, whereas Cp is expressed in the liver and several other tissues but not the gut. Despite an in-frame, interstitial deletion removing 192 amino acids, Hp from mice with sex-linked anemia still has appreciable, if reduced, oxidase activity, suggesting that sla mice do not represent a true null phenotype for Hp.
Hp is a recently identified member of the blue multicopper oxidase family which is found in organisms as diverse as bacteria and humans. Our results now suggest that Hp should be considered a member of the ferroxidase subfamily of multicopper oxidases that are characterized by the presence of signature copper binding sites, including at least one blue T1 site and a multicopper T3 site. All of these proteins couple the reduction of O2 to H20 with simultaneous oxidation of substrate. These oxidases include laccases, which can oxidize a variety of organic substrates, and substrate-specific proteins such as the ascorbate oxidases.35 Remarkably, some members of this family, like Hp, oxidize iron as their primary substrate. Multicopper ferroxidases were initially defined by mammalian ceruloplasmin,36 but this family now includes S cerevisiae Fet3p19 and related fungal ferroxidases,37 an algal ferroxidase38,39 and one from Pseudomonas aeruginosa40 which all share the ability to enzymatically convert Fe (II) to Fe (III).
We and others have proposed that the interstitial deletion in Hp inactivates the protein and, therefore, explains the phenotypic changes.8,12 However, our results show that the truncated Hp from sla mice has residual in vitro oxidase activity. Homology modeling of hephaestin12 suggests that the in frame deletion in the sla gene removes a type I (T1) copper binding ligand (histidine 662) and cysteine residues that form a disulfide bridge. These T1 mononuclear sites are characteristically redox active Cu centers involved in long-range electron transfer pathways, shuttling electrons to the T2/3 Cu center that is the site for O2 reduction. A single T1 blue copper site and the T2/T3 multicopper domain are adequate for catalytic activity.35 The Fet3p molecule is a good example of a known ferroxidase with a single T1 Cu site in addition to the T2/T3 Cu cluster.41 The T1 site in domain 2 of Cp lacks the fourth axial Met ligand which is mutated to a nonpolar leucine residue.11 A very high reduction potential of approximately 1000 mV has been attributed to this T1 site in domain 2,34 which likely results in this T1 site being constitutively reduced and unable to participate in redox reactions. Interestingly, we show that this axial Met ligand is present in Hp, suggesting this is an active T1 site unlike in Cp. Cp, therefore, has only 2 redox active T1 sites in domains 4 and 6, whereas Hp has 3 potential T1 sites. On this basis, one might expect Hp to have higher intrinsic ferroxidase activity than Cp, consistent with our experimental results from the in-gel oxidase assays. The truncated Hp in sla mice, therefore, retains the same number of active T1 sites as intact Cp as well as all other requisite copper binding sites. The decreased activity seen in sla may, therefore, result from the decreased Hp protein levels, perhaps secondary to protein instability or misfolding, rather than the loss of any critical structural/enzymatic features.
The presence of detectable oxidase activity in the sla mouse suggests the possibility that other factors may contribute to the decreased iron efflux in the sla mouse. Indeed, we have recently noted that the Hp protein is mislocalized in sla enterocytes.24 In wild-type mice, Hp is present both in a supranuclear compartment and the basolateral membrane, whereas we were not able to detect Hp on the basolateral membrane in sla. However, we cannot rule out a small amount of less active protein at the basolateral membrane. The mislocalization in combination with the reduced activity may be responsible for the defective iron efflux in the sla mouse.
We have suggested that Hp ferroxidase activity may be necessary for the effective release of iron from enterocytes and that it acts in concert with Ireg1 to transport iron across the basolateral membrane.8 The role of hephaestin in the supranuclear compartment is not known, but it is important to note that several groups have found that DMT1 localizes to a supranuclear compartment with apotransferrin so there may be an intersection between apical and basolateral transport in this uncharacterized compartment.42,43 Sla mice are iron deficient at birth and weaning and have a severe hypochromic microcytic anemia.44 The severity of the anemia decreases with age,45 although the animals remain iron deficient.9,46 Despite this defect, sla mice absorb sufficient iron for normal development and reproduction. Previously, we suggested that either Hp is not absolutely required for intestinal iron transport25 or that additional ferroxidases such as ceruloplasmin could compensate. Our finding that Hp retains some oxidase activity in sla mice raises the possibility that this activity is sufficient for the residual iron transport in these animals.
Prepublished online as Blood First Edition Paper, January 29, 2004; DOI 10.1182/blood-2003-09-3139.
Supported by grants from the National Institutes of Health (5-R01-DK57800 and 5-R01-DK56376) and Human Frontier Science Program (HFSP) (RGY0328).
H.C. and Z.K.A. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 3. Hp from sla retains a reduced ferroxidase activity. (A) Mouse enterocyte protein extract ([A] 200 μg or [B] 80 μg) was prepared from a normal mouse (C57/BL/6J) or an sla mouse was resolved by nondenaturing, nonreducing gel electrophoresis. Gels were incubated with ferrous ammonium sulfate, developed with ferrozine, and photographed as color developed. Purified human ceruloplasmin (Cp) was used as a control. The upper and lower arrows indicate the mobilities of Hp and Cp, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/10/10.1182_blood-2003-09-3139/6/m_zh80100461380003.jpeg?Expires=1767778424&Signature=jVRN3X2jgQ2t9dsbkfpOvi3lUlweHKlz7KNrkMn1NSTd-snqPQ99DPFE97IIuR0SJ2kd6~wnFc2Y3Fd4rN3nn7qSLXfhhw0W5Kfs51Kut9nJcwUtr-O7DKY4uc0MTbdYuNBaJGfVlu7su7TN2sX~KhOWfG8lbh3ghg8xbCJsFimUnCBy07ekPYO~XYTpOAbWWhWeVZ5Zs4ZW2ecmejxFn6vlyUidvEYsmg3UAY-gN3n-g5ln7sHrdlp9rCsfByqAm~dsvqfzEzZb8ubV2I7Oyuu61ijkrCpBBqSXkHfOlGcVNKEQmkoag6FgyNqmtj~hH2JEwlPEhJ6LfYaw7tc9kg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal