Abstract
The adult hematopoietic stem cell (HSC) compartment contains a substantial population of lineage-biased (Lin-bi) HSCs. Lin-bi HSCs generate cells of all hematopoietic lineages, albeit with skewed ratios of lymphoid to myeloid cells. The biased ratios are stable through serial transplantation, demonstrating that lineage bias is an inherent function of the HSCs. To define the mechanisms that cause lineage bias, the developmental potential of myeloid-biased (My-bi) HSCs was characterized. In serial transplantation experiments, My-bi HSCs contributed significantly longer to repopulation than other types of HSCs. The long lifespan indicates that My-bi HSCs are important for the persistence of HSC function throughout life. My-bi HSCs produce normal levels of myeloid precursors but reduced levels of precursors for the T- and B- lymphocyte lineages. Gene array analysis suggested that the lymphoid progeny of My-bi HSCs express lowered levels of interleukin-7 (IL-7) receptor. Indeed, the progeny derived from My-bi HSCs failed to respond to IL-7 in vitro. Thus, My-bi HSCs are programmed for diminished lymphopoiesis through a mechanism that involves a blunted response of its progeny to the central lymphokine IL-7. The data demonstrate that epigenetic regulation on the level of the HSCs can directly affect the number, composition, and function of the mature progeny.
Introduction
Hematopoietic stem cells (HSCs) are capable of generating mature cells of all the hematopoietic lineages. Self-renewal capacity, the ability to proliferate without differentiation, is a similarly important hallmark of HSCs. The undifferentiated status of HSCs is characterized by promiscuous expression of genes, many of which were thought to be lineage specific.1-3 Commitment to differentiation increasingly limits the developmental potential of the original HSCs by regulating the level of expression of lineage-specific genes. Lineage-specific genes then maintain the differentiated state and allow the committed progeny to react to environmental stimuli. Such extrinsic signals promote the survival and expansion of differentiated progeny. The commitment process is accompanied by loss of the ability to self-renew.4,5 The mechanisms that control self-renewal and commitment are incompletely understood.
It has been assumed that all HSCs have similar developmental potential. For example, the usual diagrams of HSC differentiation cascade that can be found in any textbook imply that the commitment process generates a myeloid and a lymphoid progenitor from each HSC. This is thought to assure that these lineages can be produced in equal measure. However, there is evidence that the HSC compartment contains distinct and separable subsets of HSCs that can differ in phenotype and primitiveness. “Primativeness” describes the proliferative capacity of an HSC, where primitive HSCs show delayed onsets of repopulation and then sustain production of mature progeny for a long time. Less primitive HSCs contribute rapidly, albeit transiently, to peripheral hematopoiesis. These types of HSCs can be separated to some extent. For example, HSCs that lack c-kit are more primitive,6 whereas HSCs that express CD47 may be more mature than other types of HSCs. However, differences in primitiveness could be a reflection of the proliferative history of the HSC.8 According to the generation-age hypothesis,8 all HSCs may be born with the same level of primitiveness, but each proliferation results in loss of primitiveness. Thus, the subsets defined so far may reflect HSCs with different histories rather than cells with distinct functions. This raises the question of whether there is true heterogeneity in the HSC compartment.
To define the composition of the HSC compartment, we developed an efficient approach to isolate clonally derived HSCs. Repopulating HSCs were isolated after limiting-dilution cultures on the stromal cell line S17.9,10 The simplicity of the approach allowed the analysis of a large number of individual HSC clones. Among the HSC clones, we identified lineage-biased (Lin-bi) HSCs as a distinct class of HSCs.10 Lin-bi HSCs give rise to cells of all hematopoietic lineages, but they generate noticeably skewed ratios of myeloid and lymphoid cells in the periphery. HSCs with similar properties had been identified previously, though they had not been characterized in detail.10-13 The clonal analysis indicates that Lin-bi HSCs comprise approximately 30% of the HSC compartment, about equally divided into myeloid-biased (My-bi) and lymphoid-biased (Ly-bi) HSCs.10 A hallmark of Lin-bi HSCs is that the skewed ratios of myeloid to lymphoid cells are maintained through long-term, serial transplantation.10,13 Thus, lineage bias is a stable, “inherited” property of HSCs.
The ablation or mutation of genes important for hematopoietic lineage development can result in HSCs that fail to generate selectively myeloid or lymphoid cells.14-16 For example, ablation of the RAG genes causes a selective inability to generate mature lymphocytes, although precursors for the lymphocyte lineage are made at normal levels.14,15 Mutations in Ikaros preferentially reduce lymphocytes at an even earlier developmental state,17 although other HSC functions are also affected.16 However, there is no evidence that the normal HSC compartment routinely accumulates mutated HSCs, and Lin-bi HSCs are reproducibly found as part of the normal HSC compartment. Thus, the mechanisms that cause Lin-bi HSCs cannot be attributed to genetic defects. Rather, lineage bias must be caused by an epigenetically fixed developmental program of the HSCs that is executed on the level of the progeny. What then causes lineage bias of young HSCs? To address this question, we analyzed the developmental potential of My-bi HSCs. The data indicate that a change in the developmental program of the My-bi HSCs leads to lymphoid progeny with a blunted response to the central lymphopoietin interleukin-7 (IL-7). Thus, the normal HSC compartment contains primitive HSCs that are preprogrammed for inefficient lymphopoiesis.
Materials and methods
Isolation and classification of HSC clones
Bone marrow cells (BMCs) were cultured for 4 weeks in limiting dilution on the stromal cell line S17 as described.9,10 The clonal derivation of the cultures has been established.9 Individual cultures were injected into individual sublethally irradiated B6-W41W41 mice18 or lethally irradiated B6 mice (both CD45.2). Injected cells were derived from CD45.1, (CD45.1 × CD45.2) F1, or GFP-transgenic mice (all on the B6 background). When B6 hosts were used, twice-transplanted BMCs from a genetically distinguishable strain were coinjected to provide a stem cell–depleted19 source of radioprotecting cells. All mice were bled at the indicated time points, and the percentages of donor-type cells that were T lymphocytes (Thy-1), B lymphocytes (B220), and myeloid cells (Gr-1, Mac-1) were measured by immunofluorescence, as described.9 Mice were considered repopulated by clonally derived HSCs if the hosts showed 4% or more donor-type cells in the blood at 2 or more time points. HSCs were classified as balanced, My-bi, or Ly-bi after 5 months in the primary recipient on the basis of the ratio of lymphocytes to myeloid cells in blood, as described.10 For serial transplantations, 5 × 106 BMCs from the primary host were injected into lethally irradiated B6 mice. If the secondary hosts showed more than 20% donor type in the blood after 7 months, BMCs were transplanted into tertiary and (if possible) quaternary recipients. A clone was judged to have reached the end of its lifespan if it showed consistently declining levels of donor-type cells and it reached 20% or less donor-type cells. Experiments were approved by the Institutional Animal Care and Use Committee of the Sidney Kimmel Cancer Center.
Immunofluorescence
Spleen and BMCs were typed by immunofluorescence to determine the percentage of lymphoid and myeloid cells of donor type in these organs. Monoclonal antibodies (mAbs) specific for CD45.1 (clone A/20, a gift from Dr S. Kimoro, Sloan-Kettering Cancer Center, New York, NY), CD45.2 (clone ALI-4A3, gift from Dr G. Spangrude, University of Utah, Salt Lake City, UT), B220,20 Mac-1,21 Gr-1,22 Thy-1,23 and c-kit24 were purified from culture supernatants of the respective hybridomas. Other mAbs were purchased, including mAbs specific for the IL-7 receptor, c-fms (eBioscience, San Diego, CA), NK-1, IL-6 receptor, and interleukin receptor γ-chain (Becton Dickison/Pharmingen, San Diego, CA).
Limiting-dilution analysis for stroma-responsive precursors
Limiting-dilution cultures were performed as described.9,25,26 The frequencies of the initiating cells were calculated with maximum likelihood statistics using a program originally provided by D. Harrison (Jackson Laboratory, Bar Harbor, ME) as described.25,27,28 Lymphoid cultures (Whitlock-Witte conditions) on the stromal cell line AC3.GG29,30 were counted after 2 weeks. Myeloid cultures were maintained in Dexter conditions on the stromal cell line S179,25 and colonies were counted after 4 weeks. After counting, individual cultures were harvested, and the cells were stained for B220 (lymphoid cultures) or Gr-1 (myeloid cultures). At the same time, cells were costained with mAbs specific the appropriate donor-type antigen(s), as described,31 to determine the frequency of cultures that contained donor-type cells. Because the cultures were clonal, the genotype of the progeny reflects the genotype of the precursor.31 Information on the percentage of donor-type cultures was used to adjust the frequencies measured in limiting dilution. For example, if 50% of the cultures were of donor type and the frequency of precursors was 2 of 105, we report the frequency of donor-type precursors to be 1 per 105.
Colony-forming unit assays
BMCs from individual hosts were plated in methylcellulose (Stem Cell Technologies, Vancouver, BC, Canada), supplemented with optimal concentrations of WEHI-3 supernatant as a source of IL-3. All cultures were initiated in duplicate and were counted after 10 days. Individual colonies were picked and genotyped by immunofluorescence as outlined above. Colony size was measured by microscopy using a 21-mm reticle (AG Heinz, Lakeforest, CA).
Thymic colony-forming units
Thymic colony-forming unit (CFUt) assay is an in vivo limiting-dilution assay that measures the ability of T-cell precursors to home directly to the thymus and to proliferate in the thymic environment.32-34 Lethally irradiated B6 hosts were injected with 2 to 4 × 104 BMCs consisting of equal mixtures of BMCs from a My-bi HSC–repopulated host and control BMCs. At the same time, each recipient received 106 host-type BMCs as competitor. Eleven to 19 mice were injected for each experiment. All injected cells were genetically distinguishable (vide supra). After 4 weeks, the percentages of donor-type cells in thymi derived from the My-bi HSCs or the control BMCs were determined by immunofluorescence. Background staining was on the order of 0.06% of the cells. Frequencies of CFUt in control and My-bi–derived BM were calculated by maximum likelihood analysis based on the percentage of negative animals.
Cytokine cultures
Cytokine cultures were performance as described.35,36 Briefly, 106 BMCs or 5 × 105 enriched B220+ cells were plated in RPMI 1640 and 5% fetal calf serum. Cultures were supplemented with 10 to 100 ng IL-7. All cultures were set up in duplicate. Viable cells were enumerated after 4 to 7 days of culture, and surviving cells were phenotyped by immunofluorescence. Clonally and multiclonally repopulated mice used in the study had at least 70% donor-type cells.
cDNA microarray
Mouse cDNA microarrays were spotted using an Amersham Biosciences (Piscataway, NJ) Generation III spotter, onto Corning (Corning, NY) GAPS slides. Mouse cDNA chips was composed of 12 200 cDNAs. More than 90% of the clones on the arrays were IMAGE clones purchased from Research Genetics (Invitrogen, Carlsbad, CA). Each cDNA on the microarray was spotted in duplicate, and each RNA sample was hybridized onto 2 microarrays performed in parallel. Donor-type lymphoid cells were isolated from spleens of hosts repopulated by My-bi and balanced HSCs by depleting Mac-1–, Gr-1–expressing cells and all host-type cells (using CD45.2-specific mAb ALI-4A3) with avidin-conjugated magnetic beads. RNA was isolated using a kit from Qiagen (San Diego, CA). Cy-3–labeled cDNA probe preparation and hybridizations were performed according to the method of Luo et al.37 Arrays were scanned in a ScanArray 4000 (Perkin Elmer Life Sciences, Boston, MA). Quantification was performed using Imagene (Biodiscovery, Marina del Rey, CA). Intensities from each microarray were first log-transformed and then normalized.38 The first normalization step involved arrays within the same RNA sample, and the second normalization step involved the 2 samples within the same experiment. The ratio of each gene between both samples was calculated using the normalized data.
Results
Lineage contribution by Lin-bi HSCs
Lin-bi HSCs are defined by the ratio between lymphoid and myeloid cells in blood after clonal HSC transplantation.10 Mice that received balanced HSCs showed approximately 10% to 18% myeloid cells in blood; the remainder were B and T lymphocytes. The mean lymphoid- to-myeloid cell ratio in the blood of these mice was 6.0 ± 2.0, similar to that found in unmanipulated control animals.10 My-bi HSCs generated low ratios (less than 3 but greater than 0) of lymphoid to myeloid cells, and Ly-bi HSCs gave rise to high ratios (more than 10 but less than infinity) of lymphoid to myeloid cells in blood. However, all Lin-bi HSCs generated myeloid and lymphoid progeny at all times, as expected from pluripotent HSCs.
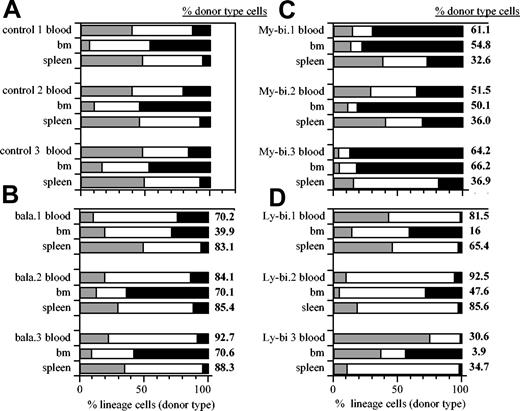
To determine whether lineage bias extends to organs other than blood, we measured the contribution of lymphoid and myeloid cells in BM and spleen in hosts repopulated by the different types of HSC clones (Figure 1). For comparison, data from unmanipulated control animals are shown (Figure 1A). Most HSC clones showed unique patterns of repopulation. Nevertheless, a consistent pattern emerged (Table 1). The ratios of lymphocytes to myeloid cells in control mice were not significantly different from those found in organs repopulated by balanced clones. However, these ratios were significantly skewed toward the myeloid lineage in organs repopulated by My-bi HSCs. Similarly, skewing toward the lymphoid lineage was seen in organs repopulated by Ly-bi HSCs (Figure 1D; Table 1). Thus, the lineage bias seen in blood was also reflected in BM and spleen. Interestingly, organ specificity was maintained. For example, in all cases, BM remains a predominantly myeloid organ with lymphocyte-to-myeloid ratios less than 3 (Table 1). Thus, the lineage composition of the peripheral hematopoietic organs is determined by a combination of organ specificity and the generative potential of the HSCs.
Lin-bi HSCs generate skewed lineage contribution in the hematopoietic organs. Shown are representative data of the percentage of donor-type T lymphocytes (▦), B lymphocytes (□), and myeloid cells (▪) in blood, BM, and spleen. Unmanipulated control mice (A). Host repopulated by individual balanced (B), My-bi (C), and Ly-bi (D) HSC clones. Data are from primary hosts 6 to 8 months after transplantation. Numbers at the right of each bar indicate the percentage of total donor-type cells in the corresponding organs.
Lin-bi HSCs generate skewed lineage contribution in the hematopoietic organs. Shown are representative data of the percentage of donor-type T lymphocytes (▦), B lymphocytes (□), and myeloid cells (▪) in blood, BM, and spleen. Unmanipulated control mice (A). Host repopulated by individual balanced (B), My-bi (C), and Ly-bi (D) HSC clones. Data are from primary hosts 6 to 8 months after transplantation. Numbers at the right of each bar indicate the percentage of total donor-type cells in the corresponding organs.
Ratios of lymphocytes to myeloid cells in clonally repopulated hosts
HSC type . | Lymphoid-to-myeloid ratio . | . | . | . | ||
|---|---|---|---|---|---|---|
| . | Blood (P) . | BM (P) . | Spleen (P) . | n . | ||
| Control | 6.5 ± 2.9 | 1.2 ± 0.3 | 13.6 ± 2.0 | 5 | ||
| Balanced | 5.5 ± 2.6 (.55) | 1.0 ± 0.8 (.63) | 14.8 ± 6.9 (.7) | 6 | ||
| My-bi | 1.1 ± 0.9 (.003) | 0.5 ± 0.5 (.02) | 5.4 ± 2.8 (.0004) | 9 | ||
| Ly-bi | 26 ± 21 (.035) | 2.1 ± 1.2 (.16) | 25.8 ± 12.2 (.06) | 6 | ||
HSC type . | Lymphoid-to-myeloid ratio . | . | . | . | ||
|---|---|---|---|---|---|---|
| . | Blood (P) . | BM (P) . | Spleen (P) . | n . | ||
| Control | 6.5 ± 2.9 | 1.2 ± 0.3 | 13.6 ± 2.0 | 5 | ||
| Balanced | 5.5 ± 2.6 (.55) | 1.0 ± 0.8 (.63) | 14.8 ± 6.9 (.7) | 6 | ||
| My-bi | 1.1 ± 0.9 (.003) | 0.5 ± 0.5 (.02) | 5.4 ± 2.8 (.0004) | 9 | ||
| Ly-bi | 26 ± 21 (.035) | 2.1 ± 1.2 (.16) | 25.8 ± 12.2 (.06) | 6 | ||
Donor-type ratios of lymphoid to myeloid cells were calculated for each animal and each organ. Shown are the mean ratios (±SD). Significance levels are indicated as P values derived from analysis of variance (ANOVA), comparing the clonally repopulated organs with unmanipulated controls. n indicates number of independent clones examined; and —,.
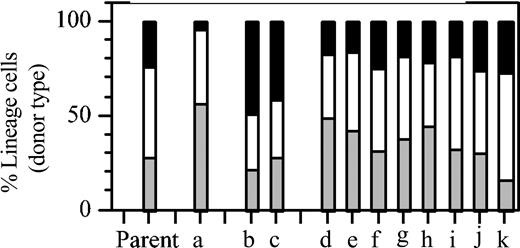
Although the observation of Lin-bi HSCs was clear, the possibility remained that the bias was somehow caused by the initial culture. To address this issue, HSC activity among clones derived entirely in vivo was examined. Limiting numbers of BMS from a host that had previously received a pauciclonal graft of 1 × 105 BMCs were transferred into secondary recipients. The percentages of T and B lymphocytes and of myeloid cells in blood of this “parent” mouse are shown in Figure 2. As shown previously,10 mixtures of HSCs reproducibly generate balanced repopulation patterns. When these mixtures are separated into their clonal components, Lin-bi HSCs can be revealed. In the secondary recipients, the patterns of donor-type repopulation in individual hosts showed the distinct kinetics that are indicative of clonal repopulation (data not shown). Of 11 recipients, 1 showed Ly-bi (Figure 2, a), 2 showed My-bi (Figure 2, b and c), and all others (Figure 2, d-k) showed balanced repopulation. Thus, Lin-bi HSCs are found in uncultured BM and are part of the normal HSC compartment.
Lin-bi HSCs are part of the normal HSC compartment. Eleven individual hosts (a-k) received a graft of 106 BMCs from a host that 8 months earlier had received a graft of 105 BMCs. This donor mouse is designated “Parent” and shows the balanced lineage composition reproducibly found after a multiclonal graft.10 Transplantation increases the number of radioprotecting cells but reduces the repopulation capacity of BMCs by approximately 8-fold.39 Therefore, each secondary host received on average 1.2 HSCs. Shown are the percentage of myeloid (▪) cells, B lymphocytes (□), and T lymphocytes (▦) in donor-type white blood cells in individual secondary hosts at the end of the experiment (parent: 8 months in primary host; a-k, 7 months in secondary hosts).
Lin-bi HSCs are part of the normal HSC compartment. Eleven individual hosts (a-k) received a graft of 106 BMCs from a host that 8 months earlier had received a graft of 105 BMCs. This donor mouse is designated “Parent” and shows the balanced lineage composition reproducibly found after a multiclonal graft.10 Transplantation increases the number of radioprotecting cells but reduces the repopulation capacity of BMCs by approximately 8-fold.39 Therefore, each secondary host received on average 1.2 HSCs. Shown are the percentage of myeloid (▪) cells, B lymphocytes (□), and T lymphocytes (▦) in donor-type white blood cells in individual secondary hosts at the end of the experiment (parent: 8 months in primary host; a-k, 7 months in secondary hosts).
Lifespan of Lin-bi HSCs
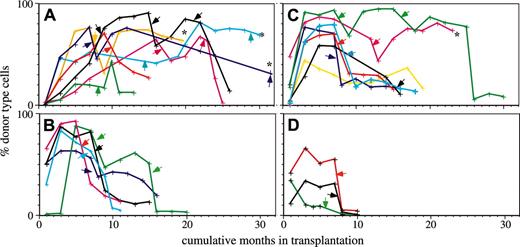
Lin-bi HSCs can self-renew to repopulate secondary hosts.10 However, it was unknown whether Lin-bi HSCs could contribute to mature cells as long as other types of HSCs could. To test this, we monitored the repopulation capacity of 7 My-bi, 7 balanced, and 5 Ly-bi HSC clones through serial transplantation. All groups showed considerable heterogeneity in the time that the HSCs contributed to the periphery. Nevertheless, there were clear differences (Figure 3). The group of My-bi HSCs stands out in that all My-bi HSC clones showed a delayed onset of repopulation. Whereas 4 of 5 Ly-bi and 5 of 7 balanced HSC clones already contributed to blood 1 month after transplantation, none (0 of 7) of the My-bi HSCs clones generated donor-type cells by this time. None of the Ly-bi HSC clones lasted for more than 18 months, but progeny of 5 of 7 My-bi HSC clones were still detected at this point. At 20 months after the initial transplantation, 2 balanced clones and 4 My-bi HSC clones continued to contribute to the periphery. An additional My-bi clone (Figure 3A, brown) is currently at 19 months, is still going strong with 62% donor-type cells, and is likely to continue beyond 20 months. Thus, most My-bi clones and a subset of balanced HSC clones have repopulation capacities that approach or even supersede the lifespan of the mouse.
My-bi HSCs contribute significantly longer to peripheral white blood cells than do other types of HSCs. Shown are the percentages of donor-type cells in blood measured at the time points indicated by crosses for each of the HSC clones. Clonally derived HSCs were classified as My-bi (A), Ly-bi (B), and balanced (C) according to the lymphoid-to-myeloid cell ratios in blood generated in the primary hosts. Early repopulation patterns of some of the clones have been reported previously.10 Primary hosts were rested at least 6 months, and BMCs from these mice were then transplanted into secondary hosts at the time points indicated by arrows. Whenever the secondary hosts showed good levels of repopulation at 7 months after transfer, BMCs were transplanted into tertiary and, if possible, into quaternary hosts at the time points indicated by the arrows. The time of injection into the first host is called month 0, and the cumulative number of months that an HSC clone contributed donor-type cells is shown on the x-axis. Multiple hosts were used in serial transplantation, and the mean levels of donor-type cells in blood are shown. As reported previously, 10 the extent of repopulation in multiple recipients of clonally derived HSCs is similar. Some HSC clones (indicated by asterisks) are still contributing to donor-type cells. Clones that exhausted and failed to self-renew in the primary host (and thus could not reconstitute secondary hosts) were classified as graft failures (D).
My-bi HSCs contribute significantly longer to peripheral white blood cells than do other types of HSCs. Shown are the percentages of donor-type cells in blood measured at the time points indicated by crosses for each of the HSC clones. Clonally derived HSCs were classified as My-bi (A), Ly-bi (B), and balanced (C) according to the lymphoid-to-myeloid cell ratios in blood generated in the primary hosts. Early repopulation patterns of some of the clones have been reported previously.10 Primary hosts were rested at least 6 months, and BMCs from these mice were then transplanted into secondary hosts at the time points indicated by arrows. Whenever the secondary hosts showed good levels of repopulation at 7 months after transfer, BMCs were transplanted into tertiary and, if possible, into quaternary hosts at the time points indicated by the arrows. The time of injection into the first host is called month 0, and the cumulative number of months that an HSC clone contributed donor-type cells is shown on the x-axis. Multiple hosts were used in serial transplantation, and the mean levels of donor-type cells in blood are shown. As reported previously, 10 the extent of repopulation in multiple recipients of clonally derived HSCs is similar. Some HSC clones (indicated by asterisks) are still contributing to donor-type cells. Clones that exhausted and failed to self-renew in the primary host (and thus could not reconstitute secondary hosts) were classified as graft failures (D).
To quantify the observations, a survival analysis was performed. For the sake of statistical analysis, the cessation of repopulation was called death. Relative risk (RR) ratios of the groups of different types of HSC clones were then calculated for each time point. Distribution of the RR was analyzed using a Cochran-Cox statistic. By its nature, this analysis excludes the marked differences seen in the early repopulation data; consequently, no significant difference was found in the first 10 months after transplantation. However, from months 10 to 30, a highly significant difference of My-bi compared with balanced and Ly-bi HSCs (P < .0001) was found. To quantify the differences seen in the early repopulation curves, a cross-sectional analysis was performed by which the repopulation levels of the groups of HSCs were compared using the Mann-Whitney test. This analysis confirmed significant differences between repopulation levels of the My-bi and balanced (P < .04) and the My-bi and Ly-bi (P = .007) HSCs at 1 month. The delayed onset of repopulation, together with the long lifespan, indicates that My-bi HSCs comprise the most primitive component of the HSC compartment.
Precursor content
My-bi HSCs are defined by their ability to generate mature progeny that is skewed toward the myeloid lineage.10 This prompted us to question whether My-bi HSCs generate too many myeloid or too few lymphoid progeny. To test this, BMCs from individual primary hosts that were repopulated by clonally derived My-bi, Ly-bi, or balanced HSCs were examined for precursor content.
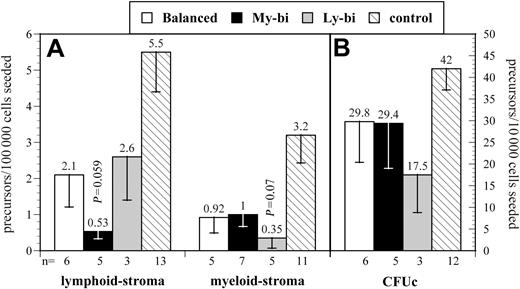
We focused on 2 types of precursor, immature precursors that are detected by their ability to repopulate stroma in limiting dilution and more mature precursors that respond to cytokines in colony-forming assays. Results varied noticeably among the HSC clones (Figure 4), consistent with the interpretation that each HSC clone has a distinct developmental program (Figure 1). All clonally derived HSCs had lower levels of precursors than unmanipulated controls. Therefore, precursor levels from Lin-bi HSCs were compared with those from balanced HSCs. Ly-bi and balanced HSCs gave rise to comparable levels of precursors in lymphoid condition. However, every My-bi HSC examined generated reduced levels (average reduction, 3-fold; P = .059) of this immature B-lineage precursor when compared with balanced HSCs. In contrast, the frequency of cells that respond to stroma in myeloid condition were comparable between My-bi and balanced HSCs but were reduced by approximately 60% in the progeny of Ly-bi HSCs. My-bi and balanced HSCs gave rise to similar levels of myeloid culture colony-forming unit (CFUc), but Ly-bi HSCs generated fewer CFUc. Although clonal variability precluded highly significant statistical values, the data are consistent with the interpretation that lymphoid bias is caused by a decrease in the production of myeloid cells, whereas myeloid bias is attributed to reduced production of lymphoid precursors.
Donor-type precursor levels in the progeny of different types of HSCs. Shown are the mean levels of donor-type precursors (± SE). The number of experiments (n), each using an individual animal repopulated by an independent balanced, My-bi, or Ly-bi HSC, is indicated below the x-axis. Data from unmanipulated animals (control) are shown for comparison. (A) Lymphoid and myeloid stroma. (B) CFUc. Note that panels A and B use different scales for the y-axis. Lymphoid stroma indicates the frequency of precursors that respond to stroma in Whitlock-Witte conditions to generate colonies of pre-B cells. The cell that repopulates the stroma to differentiate into pre-B cells and B cells is arguably the earliest B-lineage precursor.26 Myeloid stroma indicates the frequency of cells that respond to stroma in Dexter conditions to generate colonies of myeloid cells. This assay is identical to the long-term culture–initiating cell (LTC-IC) assay.9,40 However, the relationship between LTC-ICs and repopulating HSCs in previously transplanted BM has not been defined. Therefore, we chose to use a conservative interpretation of the data to indicate myeloid differentiation potential. CFUc indicates colony-forming units in response to IL-3. Individual colonies from all experiments were harvested and genotyped to determine the fraction of colonies derived from the transplanted HSC clone. The level of significance compared with precursors from balanced HSCs is indicated.
Donor-type precursor levels in the progeny of different types of HSCs. Shown are the mean levels of donor-type precursors (± SE). The number of experiments (n), each using an individual animal repopulated by an independent balanced, My-bi, or Ly-bi HSC, is indicated below the x-axis. Data from unmanipulated animals (control) are shown for comparison. (A) Lymphoid and myeloid stroma. (B) CFUc. Note that panels A and B use different scales for the y-axis. Lymphoid stroma indicates the frequency of precursors that respond to stroma in Whitlock-Witte conditions to generate colonies of pre-B cells. The cell that repopulates the stroma to differentiate into pre-B cells and B cells is arguably the earliest B-lineage precursor.26 Myeloid stroma indicates the frequency of cells that respond to stroma in Dexter conditions to generate colonies of myeloid cells. This assay is identical to the long-term culture–initiating cell (LTC-IC) assay.9,40 However, the relationship between LTC-ICs and repopulating HSCs in previously transplanted BM has not been defined. Therefore, we chose to use a conservative interpretation of the data to indicate myeloid differentiation potential. CFUc indicates colony-forming units in response to IL-3. Individual colonies from all experiments were harvested and genotyped to determine the fraction of colonies derived from the transplanted HSC clone. The level of significance compared with precursors from balanced HSCs is indicated.
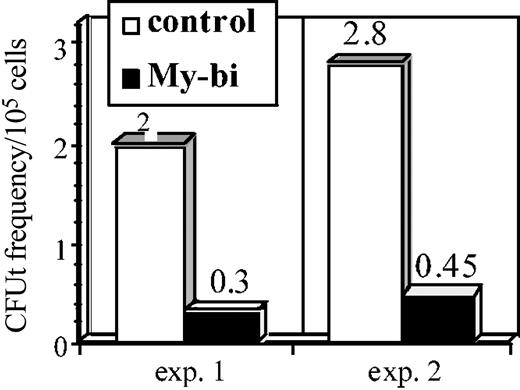
The data in Figure 4 indicate that My-bi HSCs show a reduction of primitive B-lymphoid precursors. To test whether the T-lymphocyte lineage is also impaired, we measured the frequency of CFUt, a primitive precursor that homes to the thymus, where it proliferates to generate mature T cells that replenish the periphery.32,33 The data in Figure 5 show a strong reduction in CFUt levels in the progeny of 2 independent My-bi HSCs compared with control cells. Collectively, the data show that My-bi HSCs generated reduced levels of B- and T-lymphoid precursors.
My-bi HSCs generate reduced levels of thymic precursors. Data are from an analysis of 2 independent My-bi HSC clones. BMCs derived from My-bi HSC–repopulated mice were mixed with equal numbers of control BMCs. Control BMCs served as an internal standard. The cell mixture was injected together with an excess of host-type BMCs. The number of mice that showed donor-type cells in thymus derived from the My-bi HSCs (▪) or the control BM (□) were enumerated by immunofluorescence. CFUt frequencies were calculated from the number of mice that had no thymic donor-type cells at 4 weeks after injection of mixtures of BMCs from My-bi HSC–repopulated and control mice. For details, see “Materials and methods.”
My-bi HSCs generate reduced levels of thymic precursors. Data are from an analysis of 2 independent My-bi HSC clones. BMCs derived from My-bi HSC–repopulated mice were mixed with equal numbers of control BMCs. Control BMCs served as an internal standard. The cell mixture was injected together with an excess of host-type BMCs. The number of mice that showed donor-type cells in thymus derived from the My-bi HSCs (▪) or the control BM (□) were enumerated by immunofluorescence. CFUt frequencies were calculated from the number of mice that had no thymic donor-type cells at 4 weeks after injection of mixtures of BMCs from My-bi HSC–repopulated and control mice. For details, see “Materials and methods.”
The data indicate that Lin-bi HSCs generate reduced numbers of precursors in the underrepresented lineage. This raised the question whether precursors that are made are capable of generating the standard numbers of mature cells. To test this, we measured the size of myeloid colonies from the CFU cultures (Figure 4) by microscopy. No significant differences were observed in the sizes of the colonies (P = .7) generated from My-bi and Ly-bi HSCs. Similarly, there was no significant difference (P = .58) in the size of the CFUt between control and My-bi–derived thymic contribution. Thus, though precursors are produced at a reduced level, the existing precursors generate normal levels of cells.
Mechanism of myeloid bias
The stability of My-bias through serial transplantation10 showed that My-bias is a stable property of an HSC. This HSC property results in a markedly changed progeny. Because the phenotype is on the level of the progeny, molecular analysis of the progeny may provide clues to the epigenetic program of My-bi HSCs. We isolated lymphocytes from hosts repopulated by a My-bi and a balanced HSC and compared the gene expression programs in an array analysis that focused on cytokines and their receptors. No differences (less than 2-fold) were found in the production of the cytokines IL-1, IL-2, IL-4, IL-5, IL-6, IL-7, IL-11, IL-12, IL-13; transforming growth factor-β2 (TGF-β2), interferon-β C-kit ligand (IFN-β KL), fetal kinase 2 ligand (FLKL), vascular endothelial growth factor (VEGF), Leukemia Inhibitory Factor (LIF), Oncostatin-M, macrophage inflammatory protein (MIP), or insulin-like growth factor-1 (IGF-1). Among the cytokine receptors tested, there was no difference in expression levels of IL-1 type 2 receptor (IL-1 2R), IL-3R, IL-4R, IL-5R, IL-6R (Gp130); IL-11R, IL-15R-α, IL-8R; granulocyte-macrophage colony-stimulating factor receptor-β (GM-CSFR-β), c-kit, FLK-2, tumor necrosis factor receptor-β (TNFR-β), platelet-derived growth factor receptor-α (PDGFR-α), CCR5, CCR7, IGF-1R, LIFR-α, and the common interleukin receptor γ-chain. However, IL-7R-α was 2.2-fold underrepresented in the lymphoid progeny of the My-bi HSCs.
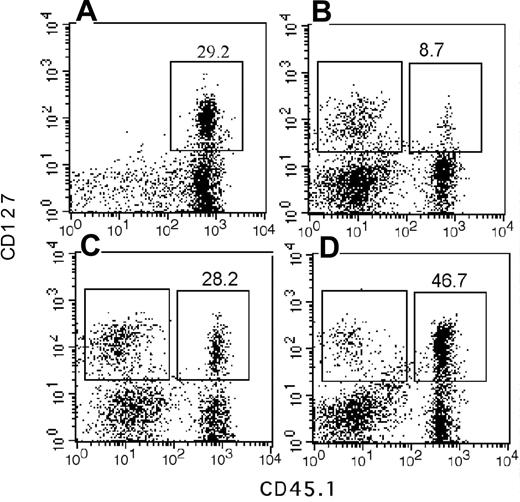
A blunted response to the central lymphokine IL-7 in the progeny could account for the reduced lymphopoietic potential of My-bi HSCs. Therefore, we sought independent confirmation of this finding using immunofluorescence. Unmanipulated BMCs contained on average 10.2% ± 4.2% IL-7R–expressing cells (n = 22 mice). We found that the number of cells that expressed IL-7R was lower by approximately 2-fold in BM repopulated by My-bi HSCs (6.1% ± 3.7%; n = 11) and was increased in BM from Ly-bi HSCs (16.9% ± 3.9%; n = 3). Similar results were found for spleen cells from these hosts (Figure 6).
Fewer IL-7R–positive cells in the progeny of My-bi HSCs. Shown are immunofluorescence plots of spleen cells stained for CD127 (IL-7R) and the donor-type marker CD45.1 from control mice (A) and chimeric mice that had received My-bi (B), balanced (C), or Ly-bi (D) HSCs 7 months earlier. Percentages of IL-7R–positive cells among the donor-type cells are indicated above the gates. Absolute numbers of donor-type cells were 34.7, 57.8, and 46.1 for mice shown in panels B, C, and D, respectively. Mean fluorescence levels for donor- and host-type cells of IL-7R–positive cells are shown in Table 2.
Fewer IL-7R–positive cells in the progeny of My-bi HSCs. Shown are immunofluorescence plots of spleen cells stained for CD127 (IL-7R) and the donor-type marker CD45.1 from control mice (A) and chimeric mice that had received My-bi (B), balanced (C), or Ly-bi (D) HSCs 7 months earlier. Percentages of IL-7R–positive cells among the donor-type cells are indicated above the gates. Absolute numbers of donor-type cells were 34.7, 57.8, and 46.1 for mice shown in panels B, C, and D, respectively. Mean fluorescence levels for donor- and host-type cells of IL-7R–positive cells are shown in Table 2.
My-bi HSCs generated fewer lymphoid cells than other HSCs (compare Figures 6 and 1), explaining to some extent the reduced number of cells that expressed IL-7R. However, array analysis used similar numbers of lymphoid cells, suggesting that there would be additional changes in IL-7R expression. Closer analysis of the immunofluorescence data showed that IL-7R staining was shifted toward lower levels when the progeny of My-bi HSCs was analyzed. Representative data are shown in Figure 6 and Table 2. Lowered levels of CD127 staining were found for 6 of 8 My-bi HSC clones analyzed. None of the balanced (n = 8) or Ly-bi (n = 4) HSCs tested generated progeny with lowered IL-7R expression. Consistent with the data from the array analysis, the common γ-chain (CD136) used by many interleukin receptors, including IL-7R, was expressed at normal levels on cells derived from all HSCs. Similarly, c-kit, c-fms, and the IL-6 receptors were expressed at normal levels on the progeny of My-bi HSCs (data not shown).
Mean fluorescence levels for IL-7R-positive cells
. | Mean fluorescence . | . | |
|---|---|---|---|
| Panel . | Donor . | Host . | |
| A | 101.0 | NA | |
| B | 56.4 | 101.0 | |
| C | 118.8 | 111.4 | |
| D | 98.2 | 128.5 | |
. | Mean fluorescence . | . | |
|---|---|---|---|
| Panel . | Donor . | Host . | |
| A | 101.0 | NA | |
| B | 56.4 | 101.0 | |
| C | 118.8 | 111.4 | |
| D | 98.2 | 128.5 | |
Cells were gated as indicated in Figure 6, and the Y-mean was calculated using CellQuest software (Becton Dickinson, Mountain View, CA). Note the normal levels of CD127 expression on the host-type cells in the chimera that received the My-bi clone. NA indicates not applicable.
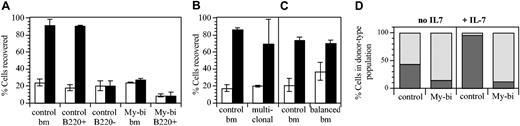
Measurements of density by immunofluorescence can be difficult to interpret, and not all My-bi HSCs generated progeny with lower IL-7R levels. Nevertheless, the observation that most My-bi HSCs generated progeny that appeared to selectively underexpress IL-7R was sufficiently intriguing to warrant further study. Therefore, we tested whether the progeny of My-bi HSCs responded to IL-7. BMCs were cultured with IL-7 as described.35,41 The number of viable cells of donor type was then evaluated. Representative data are shown in Figure 7. When BMCs from control mice were used, on average 3-fold more cells were recovered in the presence of IL-7 than without cytokine (P = .006). In agreement with published data,41 all IL-7–responsive cells were contained in the B220+ population (Figure 7A). The effect was specific for IL-7, because cells from mice in which the IL-7R was ablated by homozygous recombination (IL-7R knockout)42 failed to proliferate (data not shown). Interestingly, cells from 3 hosts repopulated by independent My-bi HSCs showed no response to IL-7. The progeny of one of the My-bi HSCs was analyzed in more detail (Figure 7). Adding IL-7 to the progeny of this My-bi HSC, as to the other My-bi HSCs tested, had no effect on the recovery of viable cells (Figure 7A). To determine whether the reduced response to IL-7 could have been caused by transplantation-related effects, we analyzed BMCs from a mouse that had received a polyclonal graft of 105 BMCs 8 months earlier. There was a slight, nonsignificant reduction in the previously transplanted cells (Figure 7B). Similar results were obtained when BMCs from a host repopulated by balanced HSCs were tested (Figure 7C), indicating that the failure to respond to IL-7 is not an artifact of transplantation but a unique hallmark of the progeny of My-bi HSCs.
My-bi HSCs give rise to progeny with a blunted response to IL-7. BMCs were cultured with (▪) or without (□) 10 ng IL-7 for 6 days. (A) The number of viable cells retrieved after culture is expressed as a percentage of the number of cells seeded. Data are representative of 7 independent experiments with control BM and 3 experiments with independent My-bi HSCs. The My-bi HSCs analyzed here had been injected into IL-7R knockout hosts. Thus, host-type cells could not contribute to the response. Cell numbers recovered from IL-7–supplemented cultures derived from a host repopulated by a My-bi clone are not different from those recovered from control BM cultured without IL-7 (P = .55). The number of cells recovered after IL-7 stimulation from control BMCs and previously transplanted (multiclonal) BMCs (B) or BMCs from a host that had received a balanced HSC clone (C) are not significantly different (P = .43 and P = .7644, respectively). The differences for both groups between cultures with (▪) and without (□) IL-7 are significantly different (P ≥ .03). All data are means ± SD of duplicate cultures. (D) Immunofluorescence analysis of the BMC cells from panel A after culture. The percentage of B220+ (▦) and Gr1+ plus Mac-1+ cells (▨) in the donor-type population after culture is shown. All cultures contained at least 70% donor-type cells.
My-bi HSCs give rise to progeny with a blunted response to IL-7. BMCs were cultured with (▪) or without (□) 10 ng IL-7 for 6 days. (A) The number of viable cells retrieved after culture is expressed as a percentage of the number of cells seeded. Data are representative of 7 independent experiments with control BM and 3 experiments with independent My-bi HSCs. The My-bi HSCs analyzed here had been injected into IL-7R knockout hosts. Thus, host-type cells could not contribute to the response. Cell numbers recovered from IL-7–supplemented cultures derived from a host repopulated by a My-bi clone are not different from those recovered from control BM cultured without IL-7 (P = .55). The number of cells recovered after IL-7 stimulation from control BMCs and previously transplanted (multiclonal) BMCs (B) or BMCs from a host that had received a balanced HSC clone (C) are not significantly different (P = .43 and P = .7644, respectively). The differences for both groups between cultures with (▪) and without (□) IL-7 are significantly different (P ≥ .03). All data are means ± SD of duplicate cultures. (D) Immunofluorescence analysis of the BMC cells from panel A after culture. The percentage of B220+ (▦) and Gr1+ plus Mac-1+ cells (▨) in the donor-type population after culture is shown. All cultures contained at least 70% donor-type cells.
In control cultures, more than 90% of the cells recovered after culture expressed B220+ (Figure 7C), demonstrating that IL-7 in these cultures acted on B-lineage cells. However, in the progeny of My-bi HSCs, the number of B220+ cells had not changed from the input levels, emphasizing the lack of response of lymphoid cells in these cultures. Because My-bi HSCs gave rise to lower levels of lymphoid cells, it was possible that the lack of response to IL-7 was caused by a paucity of responsive cells. However, B220+ cells enriched from the same host also failed to respond to IL-7 (Figure 7A). Extending the culture period to 7 days and adding 10-fold higher levels of cytokine did not overcome the lack of response (data not shown). Collectively, the data indicated that My-bi HSCs generated progeny characterized by a blunted response to IL-7.
Discussion
My-bi HSCs are primitive HSCs with a profoundly impaired ability to generate T and B lymphocytes. The blunted response to IL-7 of the progeny of My-bi HSCs provides a mechanism for the reduced lymphopoietic potential of My-bi HSCs. These findings have implications for defining the heterogeneity of HSCs and for the understanding of molecular mechanisms that control HSC commitment.
An unexpected feature of My-bi HSCs is the generation of lymphoid progeny with impaired responses to IL-7. Strikingly, all My-bi clones tested generated B-cell precursors that failed to mount a response to IL-7 in vitro. We have not formally tested whether other types of cells also show a blunted response to this cytokine. The lymphoid compartment generated by My-bi HSCs resembles closely that of IL-7r-/- mice35,43 both in the periphery and on the level of the precursors. This, together with the reduction of CFUt, suggests that the blunted IL-7 response extends to lymphoid progenitors and is likely to affect all lymphoid cells.
There is increasing evidence that chromosomal reorganization of lineage-specific genes precedes differentiation.44 HSCs do not express the IL-7R.45 However, the earliest lymphoid progenitors, the common lymphoid progenitors (CLPs), do express this receptor.4 Thus, it seems likely that the IL-7R locus is already primed in most HSCs. One interpretation of the behavior of My-bi HSCs is that these HSCs do not adequately open the IL-7R locus, resulting in depressed levels of expression of the receptor in the progeny. This model would predict that inefficient expression of the IL-7R, programmed on the level of the HSC, cannot be overcome in the mature progeny. Alternatively, My-bi HSCs could imprint for inefficient expression of molecule(s) necessary for signaling through the IL-7R. This would be consistent with the observation that even My-bi–derived progeny with normal levels of IL-7R failed to respond to IL-7. Regardless of the exact nature of the affected molecule(s), our data show that epigenetic events on the level of the HSC stably affect the number, composition, and function of the mature progeny.
The heterogeneity in lymphocyte generation seen for individual clones suggests that the IL-7 response may be impaired to a different degree in different My-bi HSCs. IL-7 provides a weak proliferative stimulus, and most cells require cytokines synergistic with IL-7 for proliferation. It is possible that the blunted IL-7 response of the progeny of My-bi HSCs can be partially overcome by other cytokines. Identifying such compensatory stimuli would open avenues to overcome myeloid bias in clinical settings. For example, accumulation of My-bi HSCs in the aged may contribute to the age-related decline in lymphopoiesis. Thus, intervention with co-stimulatory cytokines, rather than IL-7 itself,46 may be more successful in restoring the aged immune response. This is particularly pertinent because elevated levels of IL-7 can have adverse effects such as bone loss.47
The receptors analyzed here were far from exhaustive, and the data do not exclude that additional pathways are impaired in the progeny of My-bi HSCs. Nevertheless, the repopulation behaviors of My-bi HSCs and the normal levels of myeloid but reduced levels of lymphoid precursors are consistent with the view that a blunted response to IL-7 is a major reason for myeloid bias. We have not yet defined a mechanism for lymphoid bias. In many respects, Ly-bi HSCs appear to be the mirror image of My-bi HSCs. It is tempting to speculate that the progeny of Ly-bi HSCs will have a blunted response to myelopoietic signals.
The stability of the lineage contribution through serial transplantation indicates that there is no precursor progeny relationship between balanced, Ly-bi, and My-bi HSCs. Rather, Lin-bi HSCs are distinct subsets of the HSC compartment. Overall, the data suggest a scenario in which Ly-bi HSCs contribute rapidly and vigorously. In contrast, My-bi HSCs are primitive HSCs that contribute slowly, but for a long time, to peripheral hematopoiesis.
In humans, the ratio of lymphocytes and myeloid cells in blood changes noticeably during the first 20 years of life. Lymphocytes increase during the first year, peaking at appropriately 61% of blood cells. Thereafter, the contribution of myeloid cells in blood increases, and lymphocytes decline steadily until the age of 21 years, when a plateau is reached.48 At this point, blood in humans and other large animals (eg, dogs49 ) is strongly myeloid biased. The decrease in lymphocytes has been attributed to the decline of thymic function. However, thymic function also decreases in mice at approximately 4 to 5 weeks after birth. Yet, mice maintain a balanced ratio of white blood cells for much of their lifespans. Thus, it is tempting to speculate that the more rapidly and vigorously contributing HSCs (Ly-bi and most balanced HSCs) exhaust during the first few years in large animals. These HSCs may be needed to seed rapidly the expanding lymphoid organs early in life. Thereafter, hematopoiesis in large animals may be derived from My-bi and a subset of balanced HSCs, which persist in the HSCs compartment because of their long lifespans. This interpretation is further supported by data that IL-7 plays a lesser role in human than in mouse lymphopoiesis.50,51 In short-lived animals such as the mouse, the loss of Ly-bi HSCs and an accumulation of My-bi HSCs may be seen only in extremely old animals. This interpretation could be consistent with the report13 that myeloid-dominant HSCs accumulate in aged mice. However, whether the myeloid-dominant HSCs found in aged BM are identical with the My-bi HSCs found in young BM remains to be established.
Prepublished online as Blood First Edition Paper, February 19, 2004; DOI 10.1182/blood-2003-10-3448.
Supported by National Institutes of Health grant DK48150.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Becky Adkins (University of Miami) and Marilyn Thoman (Sidney Kimmel Cancer Center) for many inspiring discussions and for critical review of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal