Abstract
Hepatitis-associated aplastic anemia (HAA) is a syndrome of bone marrow failure following an acute attack of seronegative hepatitis. Clinical features and liver histology suggest a central role for an immune-mediated mechanism. To characterize the immune response, we investigated the T-cell repertoire (T-cell receptor [TCR] Vβ chain subfamily) of intrahepatic lymphocytes in HAA patients by TCR spectratyping. In 6 of 7 HAA liver samples, a broad skewing pattern in the 21 Vβ subfamilies tested was observed. In total, 62% ± 18% of HAA spectratypes showed a skewed pattern, similar to 68% ± 18% skewed spectratype patterns in 3 of 4 patients with confirmed viral hepatitis. Additionally, the T-cell repertoire had similarly low levels of complexity. In the peripheral blood lymphocytes (PBLs) of a separate group of HAA patients prior to treatment, 60% ± 15% skewed spectratypes were detected, compared with only 18% ± 8% skewed spectratypes in healthy controls. After successful immunosuppressive treatment, an apparent reversion to a normal T-cell repertoire with a corresponding significant increase in T-cell repertoire complexity was observed in the HAA samples. In conclusion, our data suggest an antigen-driven T-cell expansion in HAA and achievement of a normal T-cell repertoire during recovery from HAA. (Blood. 2004;103:4588-4593)
Introduction
Hepatitis-associated aplastic anemia (HAA), the development of hematopoietic failure with bone marrow hypocellularity within 6 months of an episode of hepatitis, is not uncommon, with hepatitis preceding the onset of bone marrow failure in 2% to 5% of aplastic anemia (AA) cases in Europe and the United States.1 Aplastic anemia is also frequent following orthotopic liver transplantation for non-A, non-B, non-C hepatitis in young patients: 23% to 8% of non-A, non-B, non-C hepatitis patients receiving transplants developed aplastic anemia, compared with fewer than 1% of all liver transplant patients.2,3 The hepatitis/aplastic anemia syndrome shows a stereotypical pattern; most often affecting young males, the hepatitis generally follows a benign course, but the onset of aplastic anemia 2 to 3 months later can be explosive and is usually fatal if untreated.4 The presumed infectious cause of the hepatitis is unknown, but most cases are seronegative for known hepatitis viruses, including hepatitis A, B, C, and G (GB virus C[GBV-C]).5-7 We previously reported 10 cases of HAA seen at the National Institutes of Health (NIH) that had evidence of lymphocyte activation; 70% responded to immunosuppression with antithymocyte globulin and cyclosporine.8 Apart from case reports, the presence of lymphocyte activation,9,10 and the clinical response to either immunosuppression or bone marrow transplantation,11 little is known of the immunopathogenesis of this syndrome.
The time interval between the occurrence of hepatitis and the onset of bone marrow failure suggests that the initial target organ of the immune response is the liver. For both hepatitis B and hepatitis C infection, large numbers of lymphocytes infiltrate the liver at the time of maximal abnormal liver function,12,13 and T cells are the predominant inflammatory cells infiltrating the liver parenchyma.14,15 Analysis of the T-cell repertoire in these cases has demonstrated clonal expansions, and conserved features of antigen specificity in many of these expansions are linked to the immunopathogenesis of viral hepatitis.16-18 Corresponding information concerning the T-cell immune response in HAA in liver or blood is unreported.
T-cell receptor (TCR) analysis by spectratyping is a powerful tool to assess the clonal composition of the T-cell repertoire in both infectious and immune-mediated diseases.19-21 The technique depends on the tissue-specific expression of certain TCRs (on T cells) and on the diversity, somatic rearrangements, and insertions at the nucleotide level that create TCR diversity. The majority of mature T cells express an αβ TCR, of which the β chain has 3 different regions that correspond to the 3 complementary determined regions (CDRs) of immunoglobulin. The first 2 CDRs are germ line-encoded, but the third CDR is the product of somatic rearrangements. During T-cell development, the multiple TCRβ genes for the variable (V), diversity (D), and joining (J) segments rearrange with concomitant nucleotide excision and addition at the VD and DJ junctions, creating the third hypervariable CDR. Depending on the combination of segments and transferase activity, CDR3 sequences of varying lengths are produced, and it is these different CDR3 lengths that the spectratype analysis detects.22
We have assessed the T-cell repertoire in the liver and peripheral blood lymphocytes in HAA patients and compared the pattern of Vβ subfamily expansion in HAA patients and individuals with hepatitis B and/or C infection by spectratyping. Our results show that many different T-lymphocyte clones accumulate in the liver of HAA patients, with a similar broad skewing pattern of the T-cell repertoire observed in the liver of patients with HAA as well as known viral hepatitis disease. In addition, we determined the T-cell repertoire in peripheral blood lymphocytes before and after immunosuppressive therapies and showed an apparent reversion to a more normal T-cell repertoire in HAA patients after successful treatment.
Patients, materials, and methods
Patients and control population
HAA was defined as severe bone marrow aplasia within 6 months of an episode of documented seronegative (non-A, non-B, non-C) hepatitis. Severity was defined as pancytopenia with at least 2 of the following abnormalities: absolute neutrophil count below 0.5 × 109/L (below 500/μL), platelet count below 20 × 109/L (below 20 000/μL), and reticulocyte count below 60 × 109/L (below 60 000/μL), in association with bone marrow cellularity less than 30%. Hepatitis was defined as an increase in serum transaminases to at least 3 times the upper limit of normal (normal alanine transaminase, 6 to 41 IU/L; normal aspartate transaminase, 9 to 34 IU/L).8 Anonymized liver samples were provided by the University of Minnesota Liver Tissue and Distribution System (LTPADS) NIH contract no. N01-DK-9-2310) after Institutional Review Board (IRB) approval. Additional liver samples were obtained from Cleveland, OH; Columbus, OH; Denver, CO; and Miami, FL. All tissues were obtained after informed consent and following the human experimentation guidelines of the US Department of Health and Human Services and NIH. Liver samples were obtained at the time of liver transplantation, and diagnoses included 7 HAAs, 4 cases of confirmed hepatitis B and/or hepatitis C infection, and 4 cases of biliary atresia as controls. All patients with HAA were negative for hepatitis A virus (HAV), hepatitis B virus (HBV), and hepatitis C virus (HCV) infection. All liver samples were immediately frozen and stored at -80°C or in the gaseous phase of liquid nitrogen until RNA was extracted.
Peripheral blood samples or follow-up samples were not available from the HAA liver patients. However, peripheral blood was obtained from 3 additional HAA patients at the time of presentation and after immunosuppressive treatment. All patients were enrolled on National Heart, Lung and Blood Institute (NHLBI) IRB-approved protocols, and information on one of these patients, had been previously reported.8 In addition, lymphocytes were obtained from 10 healthy donors (younger than 40 years old) who served as healthy controls and from 3 additional healthy volunteers to assess the sensitivity of spectratype analysis. Lymphocytes were isolated from heparinized peripheral blood by Ficoll-Hypaque density centrifugation (ICN Pharmacentical, Costa Mesa, CA) and used immediately or stored at -80°C until RNA was extracted.
Cell sorting by flow cytometry (FACS) for Vβ spectratype sensitivity analysis
To confirm that we could detect specific Vβ spectratypes in tissue RNA samples, we first examined the sensitivity of our spectratype assay. Three representative Vβ subfamilies, Vβ2, Vβ14, and Vβ22, were randomly selected. Purified peripheral blood lymphocytes (PBLs) (107) were suspended in 50 μL fluorescence-activated cell sorter (FACS) buffer (phosphate-buffered saline plus 0.4% bovine serum albumin [BSA]) and incubated on ice with phycoerythrin-conjugated antibody specific for either TCR Vβ2, Vβ14, or Vβ22 (7 μL, 17 μL, or 7 μL, respectively) (Biodesign International, Saco, ME) for 20 to 30 minutes. After incubation, the cells were washed once and resuspended in 500 μL FACS buffer for cell sorting of antibody-positive and antibody-negative cells on a Cytomation MoFlo cell sorter (Dako-Cytomation, Fort Collins, CO). The sorted Vβ2+, Vβ14+, and Vβ22+ T cells were mixed with their respective Vβ- T cells to produce fixed ratios of the specific Vβ's in negative cell populations (0, 10, 102, 103, 104, and 105 Vβ+ cells in 106 total cells for each Vβ subfamily). Total RNA (approximately 1 μg) was extracted from cells (106), and standard spectratyping performed. The experiments were repeated 3 times.
Vβ subfamily specific and run-off polymerase chain reactions
RNA was extracted from ground frozen liver samples or PBLs (106) by direct suspension in 0.1 g/mL RNAStat 60 (TelTest, Friendswood, TX). RNA was precipitated by centrifugation according to the manufacturer's instructions and redissolved in approximately 20 μL RNase-free distilled water, and the concentration calculated by optical density measurement. First-strand cDNA was synthesized with the use of 1 μg total RNA, reverse transcriptase, and oligo-deoxythymidine (oligo-dT) at 42°C for 50 minutes and in a final volume of 50 μL following manufacturer's instruction (Invitrogen Life Technologies, Gaithersburg, MD).
CDR3 length analysis was conducted by using a modification of the polymerase chain reaction (PCR) followed by the run-off reaction method previously described.20 First, PCR amplification of cDNA was performed with the use of 22 specific Vβ primers (21 Vβ primers in the liver samples [Vβ18 was not included for technical reasons]; 0.5μL cDNA per reaction) for the TCR Vβ-gene families (excluding Vβ10 and Vβ19, which are pseudogenes) and the common Cβ primer (cgg-gct-gct-cct-tga-ggg-gtctgc-g)20 end-labeled with a fluorescence tag (6-FAM). Amplifications were performed with ExTaq buffer (Takara, Tokyo, Japan), 0.2 mM mixed deoxynucleoside triphosphate (dNTP), 0.5 mM each primer, and 0.5 U ExTaq polymerase (Takara) in a 20 μL vol, on a DNA engine machine (PTC-200; MJ Research, Waltham, MA). The amplification profile was denaturation at 94°C for 1 minute, annealing of primers at 60°C for 1 minute, and extension at 72°C for 4 minutes for 40 cycles with a final extension step at 72°C for 10 minutes. Amplified products were electrophoresed on 1.5% agarose gels and detected by ethidium bromide staining. For the run-off reaction, a second round of amplification (3 cycles) was performed with a single 6-hexachlorofluorescein (HEX)-labeled fluorescent constant primer internal to the Cβ primer (0.1 mM) in a volume of 10 μL. A standard size marker was added (500 ROX; Applied Biosystems, Foster City, CA), and first-round and run-off products were analyzed on a 310 DNA sequencer by means of 310 GeneScan Software (Applied Biosystems).
Analysis of spectratype
Owing to the recombination events that occurs during TCR generation, the length of the amplicon varies, and in a normal population of T cells, CDR3 length analysis produces approximately 5 to 10 identifiable peaks spaced by 3 nucleotides, with fluorescence intensity following a quasi-Gaussian distribution.20,22 Spectratypes were analyzed in 3 ways. First, the spectratype pattern was visually assessed. A normal spectratype profile was defined as showing an approximate Gaussian bell-shaped distribution, with discrete peaks spaced by 3 nucleotides. If discrete peaks were observed but did not have the Gaussian profile, the spectratype was classified as skewed; if discrete peaks were not present, it was scored as absent. To obtain an indication of the magnitude of skewing, each spectratype was assessed as either normal, skewed, or absent by 3 different observers in a blinded fashion. Second, spectratypes were scored mathematically, as previously described.17,23 Evidence of oligoclonal expansion or skewing was assessed by calculating the relative fluorescence intensity (RI) of each peak (RI [%] = 100 × clonal peak area ÷ total peak area). A skewed profile was determined if either (1) a single peak was observed and the RI of the dominant peak was greater than 35% of total peak area; (2) 2 dominant peaks were present and each peak's RI was greater than 25% of total peak area; or (3) there were multipeaks with the dominant peaks differing from a Gaussian pattern and the RI of the peaks was greater than 25% of total peak area. Finally, overall complexity within a Vβ subfamily was determined by counting the number of discrete peaks per Vβ subfamily, with each subfamily graded on a score of 0 to 5.24 Spectratypes containing more than 5 peaks were given a score of 5, and a score of 0 was assigned if no spectratype signal was obtained; spectratypes with 1, 2, 3, or 4 peaks were given a score of 1, 2, 3, or 4, respectively. The overall spectratype complexity score per sample was calculated as the sum of the scores for each subfamily, with a maximum complexity score for any one patient of 110 (22 Vβ × 5).
Statistical analysis
The Student t test was used to assess the differences in Vβ skewing or complexity scores in the different groups of patients. The paired Wilcoxon test (normalizing transformation by log) was used to determine the significance before and after immunosuppressive treatment. All statistical analysis was performed by using GraphPad InStat software (GraphPad Software, San Diego, CA).
Results
Sensitivity of T-cell repertoire spectratyping
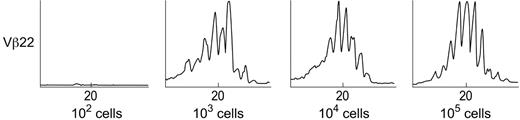
A classic Gaussian spectratype was reproducibly obtained with 102 Vβ2 or Vβ14 lymphocytes per 106 cells, and with 103 Vβ22 lymphocytes per 106 cells (Figure 1). With lower proportions of cells, no amplicon was detected by ethidium bromide staining, and no products of the correct size were seen by GeneScan analysis. Thus, the sensitivity of our spectratype analysis for each Vβ was between 100 and 1000 cells in a 1 μg total RNA sample.
Determination of the sensitivity of the TCR Vβ spectratyping technique. Detection of spectratyping sensitivity was performed on the basis of flow cytometry, and the sorted positive cells were mixed with negative cells to produce fixed proportions of the specific Vβ's in negative cell populations (0, 10, 102,103,104, and 105 Vβ+ cells in 106 cell population for each Vβ subfamily). Total RNA (aapproximately 1 μg) was extracted from cells (106), and standard spectratyping performed. The experiments were repeated 3 times. The lowest cell number detectable in 106 mixed population is 100 to 1000 cells for random selection of Vβ2, Vβ14, and Vβ22.
Determination of the sensitivity of the TCR Vβ spectratyping technique. Detection of spectratyping sensitivity was performed on the basis of flow cytometry, and the sorted positive cells were mixed with negative cells to produce fixed proportions of the specific Vβ's in negative cell populations (0, 10, 102,103,104, and 105 Vβ+ cells in 106 cell population for each Vβ subfamily). Total RNA (aapproximately 1 μg) was extracted from cells (106), and standard spectratyping performed. The experiments were repeated 3 times. The lowest cell number detectable in 106 mixed population is 100 to 1000 cells for random selection of Vβ2, Vβ14, and Vβ22.
T-cell repertoire of intrahepatic lymphocytes in HAA patients
Liver histology was not available on 2 of the HAA livers, but in the other 5 samples hepatic necrosis with a lymphocytic or mononuclear infiltrate was universally reported. CD3 staining was not performed on any of the livers, although one liver was stained for CD43. Despite this, in one of the HAA patients we were unable to detect any Vβ signal with any primers, despite the presence of RNA as indicated by reverse transcriptase-PCR (RT-PCR) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Histology of this liver was reported as having a mononuclear infiltrate with neutrophils and occasional eosinophils, and from our sensitivity data, we would conclude that the T-cell infiltrate was below our detection limit. Similarly we did not detect any Vβ amplicons in any of the 4 biliary atresia samples. In 6 of 7 HAA livers, 15 to 21 of the 21 Vβ subfamilies analyzed had detectable PCR products by ethidium bromide staining and production of a spectratype profile on GeneScan analysis, indicating the presence of a T-lymphocyte infiltrate in the livers.
A typical spectratype pattern in HAA is shown in Figure 2: many of the Vβ's have a highly skewed pattern. In 70% of the spectratypes, there was 100% concordance among all 3 observers. As a measure of the skewing in the different patient groups, the number of skewed Vβ spectratypes as a percentage of all Vβ spectratypes detected were calculated. There were 59.5% ± 16.7% (mean ± standard deviation [SD]) skewed Vβ's in the 21 tested Vβ subfamilies, with 4.8% ± 11.7% giving no detectable spectratype in HAA livers (Table 1). Similar results were obtained if the relative fluorescence intensity (RI) index was used to determine skewed spectratypes (Table 1). As a measure of the polyclonality of the T-cell repertoire, the complexity of each Vβ spectratype was assessed and summed to give a total complexity score.24 The complexity score of the spectratypes varied between 56 and 80, with a mean of 68 (Table 1).
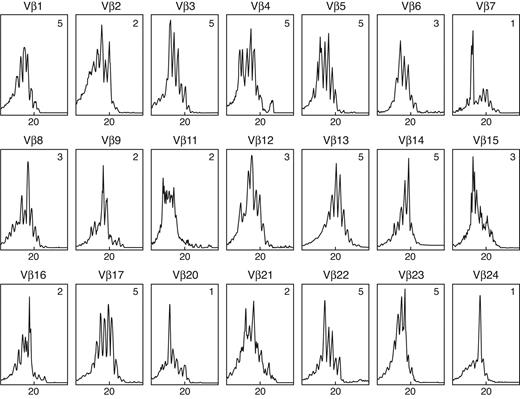
Representative TCR Vβ CDR3 size spectratype obtained from the liver of a patient with HAA showing many highly skewed spectratypes. Vβ13 and Vβ23 show a normal or Gaussian bell-shaped distribution; Vβ7 and Vβ24 demonstrate a skewed spectratype profile with a single dominant peak; Vβ2 and Vβ9 illustrate a skewed spectratype profile with double peaks; Vβ16 shows multiple peaks but with non-Gaussian distribution and skewed spectratype. The complexity score for each spectratype is given in the upper right corner. The overall complexity score was calculated as 70. The abscissa shows CDR3 length (amino acids), and the ordinate shows fluorescence intensity.
Representative TCR Vβ CDR3 size spectratype obtained from the liver of a patient with HAA showing many highly skewed spectratypes. Vβ13 and Vβ23 show a normal or Gaussian bell-shaped distribution; Vβ7 and Vβ24 demonstrate a skewed spectratype profile with a single dominant peak; Vβ2 and Vβ9 illustrate a skewed spectratype profile with double peaks; Vβ16 shows multiple peaks but with non-Gaussian distribution and skewed spectratype. The complexity score for each spectratype is given in the upper right corner. The overall complexity score was calculated as 70. The abscissa shows CDR3 length (amino acids), and the ordinate shows fluorescence intensity.
Comparison of the Vβ spectratype analysis by different methods in liver-infiltrating lymphocytes in HAA and viral hepatitis patients
. | . | Observation type . | . | Skewed by R1 measurement, % . | Total complexity score . | |
|---|---|---|---|---|---|---|
| Patients . | N . | Absent, % . | Skewed, % . | . | . | |
| HAA | 6 | 4.8 ± 11.7* | 59.5 ± 16.7 | 61.9 ± 17.8 | 68.3 ± 7.7 | |
| Viral hepatitis | 3 | 11.1 ± 12.5 | 66.7 ± 0 | 68.3 ± 18.4 | 69.7 ± 6.5 | |
. | . | Observation type . | . | Skewed by R1 measurement, % . | Total complexity score . | |
|---|---|---|---|---|---|---|
| Patients . | N . | Absent, % . | Skewed, % . | . | . | |
| HAA | 6 | 4.8 ± 11.7* | 59.5 ± 16.7 | 61.9 ± 17.8 | 68.3 ± 7.7 | |
| Viral hepatitis | 3 | 11.1 ± 12.5 | 66.7 ± 0 | 68.3 ± 18.4 | 69.7 ± 6.5 | |
Mean ± SD.
Individual Vβ spectratypes were also analyzed to determine if there were certain TCR families skewed in all HAA patients. Although Vβ1, Vβ7, Vβ11, Vβ12, Vβ15, Vβ16, Vβ20, and Vβ24 were skewed in more than 4 of 6 of the patients, when we further analyzed the length of the oligoclonal expansions, no peaks of the same size were found in more than half of the patients. Thus, although oligoclonal expansions affected many Vβ families, there did not appear to be any shared Vβ subfamily expansions for all cases, against a common Vβ CDR3 sequence for all HAAs.
The spectratype profiles obtained in the livers of patients with HAA were compared with profiles obtained from 4 patients with viral hepatitis of known etiology (hepatitis B and/or hepatitis C) (Figure 3). In one liver sample of a viral hepatitis patient, no signal could be observed for any of the PCR products, despite the presence of GAPDH product, suggesting a very low number of infiltrating lymphocytes. In the other 3 samples, there were 67% skewed Vβ's of the 21 Vβ subfamilies tested, with no signal in 11% ± 13% of reactions. No significant difference between HAA and hepatitis patients was shown statistically in the number of skewed Vβ spectratypes (P > .05); similarly, the complexity score was not significantly different between these patients.
Marked T-cell skewing in liver-infiltrating lymphocytes of HAA and hepatitis patients. Skewing was observed in HAA patients (n = 7) and hepatitis patients (n = 4). ▪ indicates the skewing Vβ profile by observation and RI; ▨ indicates the normal Vβ profile; and □ indicates no detectable signal. Overall, there were 60% ± 17% skewed spectratypes, with 5% ± 12% absent spectratypes in the HAA patients, compared with 67% ± 0% skewed Vβ's, with no signal in 11% ± 13% of reactions, in the viral hepatitis patients. Thus, no significant difference was shown in the number of skewed Vβ spectratypes in each group (P > .5).
Marked T-cell skewing in liver-infiltrating lymphocytes of HAA and hepatitis patients. Skewing was observed in HAA patients (n = 7) and hepatitis patients (n = 4). ▪ indicates the skewing Vβ profile by observation and RI; ▨ indicates the normal Vβ profile; and □ indicates no detectable signal. Overall, there were 60% ± 17% skewed spectratypes, with 5% ± 12% absent spectratypes in the HAA patients, compared with 67% ± 0% skewed Vβ's, with no signal in 11% ± 13% of reactions, in the viral hepatitis patients. Thus, no significant difference was shown in the number of skewed Vβ spectratypes in each group (P > .5).
T-cell repertoire of peripheral blood lymphocytes (PBLs) in HAA patients before and after immunosuppressive treatment
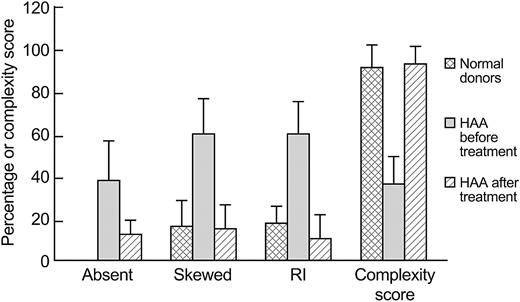
Spectratype analysis was performed on PBLs from 3 HAA patients and compared with the PBL spectratype analysis of healthy volunteers. The 10 healthy controls showed predominantly normal or Gaussian spectratype profiles, with only 17% ± 12% having a skewed Vβ pattern; the complexity score was 92 (± 11). In contrast, the HAA patients showed a highly skewed Vβ pattern (60% ± 17%) (P < .001), similar to the Vβ skewing seen in the liver-infiltrating lymphocytes. However, for many of the spectratypes, no signal was observed, and the complexity score was markedly reduced to 37 ± 13 (P < .001) (Figure 4).
Comparison of the T-cell repertoire by different methods in 10 healthy donors and 3 HAA patients before and after immunosupression therapy. Data (mean ± SD) illustrate a return toward a normal T-cell repertoire after successful treatment. The percentage of absent and skewed spectratypes in peripheral blood lymphocytes were first evaluated by observation, then the percentage of skewings of Vβ spectratypes were evaluated by RI, and the complexity scores were calculated.
Comparison of the T-cell repertoire by different methods in 10 healthy donors and 3 HAA patients before and after immunosupression therapy. Data (mean ± SD) illustrate a return toward a normal T-cell repertoire after successful treatment. The percentage of absent and skewed spectratypes in peripheral blood lymphocytes were first evaluated by observation, then the percentage of skewings of Vβ spectratypes were evaluated by RI, and the complexity scores were calculated.
All 3 patients were treated by immunosuppressive therapy, with good hematologic response. Follow-up samples were available on 2 patients 1 year after treatment, and in the third at 2.5 years after treatment. After immunosuppressive therapy, many skewed TCR Vβ's reversed to a normal Gaussian distribution (Figure 5), and many of the Vβ's that were previously not detected now gave a spectratype signal. The number of both skewed Vβ's and absent Vβ's before and after treatment decreased. Similarly, there was a significant increase in mean complexity scores (P < .01), with a mean score after treatment of 94 (± 8), not different from the complexity scores of the healthy donors (mean score, 92 ± 11) (Figure 4).
Reversion of the T-cell repertoire to normal in 3 HAA patients after immunosuppressive therapy. Samples for case no. 1 and no. 2 were collected at 1 year after immunosuppressive treatment and for case no. 3 at 2.5 years (antithymocyte globulin [ATG], cyclosporine [CsA], and mycophenolate mofetil [MMF]). Shown are the CDR3 length (40 amino acids) in the x-axis versus the fluorescence intensity in the y-axis. The spectratype profiles of the 21 Vβ subfamilies were analyzed in 3 ways: observation, measuring the relative fluorescence intensity, and complexity scoring. Absent indicates no signal detected with the use of the current technique; skewed spectratype (observation), the percentage of non-Gaussian spectratypes as determined by eye; skewed (RI), the percentage of skewed spectratypes detected by means of a calculation based on the relative fluorescence intensity of each peak; total complexity score, the total number of discrete peaks in each Vβ subfamily, as a measure of the polyclonality of the T-cell repertoire.
Reversion of the T-cell repertoire to normal in 3 HAA patients after immunosuppressive therapy. Samples for case no. 1 and no. 2 were collected at 1 year after immunosuppressive treatment and for case no. 3 at 2.5 years (antithymocyte globulin [ATG], cyclosporine [CsA], and mycophenolate mofetil [MMF]). Shown are the CDR3 length (40 amino acids) in the x-axis versus the fluorescence intensity in the y-axis. The spectratype profiles of the 21 Vβ subfamilies were analyzed in 3 ways: observation, measuring the relative fluorescence intensity, and complexity scoring. Absent indicates no signal detected with the use of the current technique; skewed spectratype (observation), the percentage of non-Gaussian spectratypes as determined by eye; skewed (RI), the percentage of skewed spectratypes detected by means of a calculation based on the relative fluorescence intensity of each peak; total complexity score, the total number of discrete peaks in each Vβ subfamily, as a measure of the polyclonality of the T-cell repertoire.
Discussion
Although the clinical characteristics and response to immunotherapy indicate that HAA is immune-mediated,8 there have been no studies of the T-cell repertoire in this syndrome. However a number of reports have studied the Vβ T-cell repertoire in hepatitis B infection, hepatitis C infection, and autoimmune hepatitis. Pham et al16 studied the Vβ composition of liver-infiltrating lymphocytes and showed that, in both hepatitis B and C infections, there was a preponderance of certain Vβ families; the overrepresented Vβ families differed in the 2 viral etiologies. Spectratype data have confirmed these observations. Sing et al25 compared the clonality of Vβ T-cell receptor-bearing population in the liver and peripheral blood of patients with hepatitis B and found clonotypic expansions in 4 to 9 TCR Vβ subfamilies, indicating a high restriction in the T-cell composition of liver-infiltrating lymphocytes. Similar experiments in hepatitis C and autoimmune hepatitis,17,18,26 showed many skewed spectratype patterns in lymphocytes in the liver.
Our results from the 4 cases of chronic hepatitis B and C for TCR-spectratyping analysis found a similarly highly skewed Vβ pattern consistent with these published results (and validating our use of frozen tissue). In addition, we demonstrated that we could detect a similar skewed spectratype pattern in the livers of patients with HAA. This was in contrast to livers from patients with biliary atresia that, as is the case in healthy livers, should not have an inflammatory or T-cell infiltrate. When we also compared the complexity of the T-cell repertoire in HAA and viral hepatitis, similar complexity scores were obtained in both diseases. Although we cannot conclude whether the same or different antigens are involved in this broad stimulation, our data are suggestive of a similar pathogenic mechanism, perhaps triggered by an antigen-specific immune response as in viral hepatitis and supporting the hypothesis, based on epidemiologic and clinical investigations, that an unknown pathogen may be involve in this disease.27
Some studies using peptides as antigens have suggested a limited T-cell response, with different clones having the same or related CDR3 sequences.28,29 Shared CDR3 sequences have been harder to identify in hepatitis infections, although putative shared motifs have been suggested.17,25,26 When we analyzed the specific CDR3 lengths in the TCR Vβ repertoire in these patients, we did not find any common peaks shared by 3 or more samples. However, our samples were not human leukocyte antigen (HLA) matched. The current negative finding nevertheless does not support the role of a superantigen or shared CDR3 sequences in the pathogenesis of HAA.
One of the major limitations of our study was that we were unable to obtain paired liver and peripheral blood samples from the same patients. However, when PBLs from different patients with HAA were examined, we observed a similar broadly skewed T-cell repertoire pattern (greater than 50% of Vβ families), as found in the liver samples, with a statistically significant increase in the complexity score of the spectratypes compared with controls, suggesting an antigen-driven TCR repertoire and limited usage of the TCR. In addition, evaluation of the TCR Vβ spectratypes before and after successful immunosuppressive therapy showed reversion to a normal spectratyping profile after treatment. This strongly suggests that pathogenic T cells had been eliminated or decreased so as not to be detectable, and a relationship between Vβ changes and an autoimmune pathophysiology.30
Our results in the HAA patients can be compared and contrasted with similar data in idiopathic aplastic anemia. Manz et al31 found a restrictive T-cell expansion in both bone marrow and PBLs of patients with severe AA, with 1 or 2 oligoclonal Vβ patterns per patient, and suggested that the T-cell repertoire expansion was random with respect to the Vβ chain. Data from a Japanese study32 showed that patients with cyclosporine-dependent AA had highly skewed Vβ spectratypes, with patients who responded to immunosuppressive therapy still showing skewing, but in fewer Vβ families (fewer than 20% had abnormal patterns). In an earlier study comparing TCR repertoire at initial presentation and after therapy in AA patients, we found much broader spectratype skewing (44% ± 33%), with oligoclonal expansions of Vβ15, Vβ21, and Vβ24 in more than 70% of AA patients with HLA-DR2.33 In contrast to the HAA patients reported here, after immunosuppressive therapy, no significant change was found in the degree of Vβ skewing, with patients treated with cyclophosphamide even showing more oligoclonality. Skewed T-cell repertoires34 were also seen in paroxysmal nocturnal hemoglobulinuria (PNH), a syndrome often associated with AA.35 In a study of CD4 and CD8 lymphocyte subpopulations in patients with AA and PNH, Risitano et al36 demonstrated that although the abnormal Vβ-distribution pattern was retained after immunosuppressive therapy, the degree of expansion of individual Vβ's was lower. For transformed CD4 and CD8 clones obtained from AA patients, Zeng et al37 reported that most CD4 clones displayed Vβ5 and CD8 clones displayed Vβ13, and that ATG and cyclosporine treatment led to marked decrease in clones bearing the dominant CDR3 Vβ5 sequence in HLA-DR2 patients.
Although HAA is usually considered a subset of AA, HLA restriction patterns may differ (J.L. et al, manuscript in preparation). We have observed no increased association of HLA-DR2 in HAA patients. Differences in T-cell repertoire have been observed in other immune-mediated diseases, such as multiple sclerosis,38,39 rheumatoid arthritis,40,41 and autoimmune hepatitis,42 where a limited number of T cells using a restricted diversity of the Vβ subfamilies to proliferate dominantly is revealed in different patients,21 but the TCR repertoire pattern is different among these diseases owing to the different antigenic triggers.39,43,44
In summary, we show that in HAA there is an infiltration of both clonal and many nonclonal T cells, giving rise to a markedly skewed T-cell repertoire, as seen in both viral and autoimmune hepatitis. This highly skewed T-cell repertoire is also evident in the blood at the time of presentation of bone marrow failure. The expanded T-cell clones are replaced by a normal Gaussian distribution of T-cell repertoire after immunosuppressive treatment, possibly associated with an antigen clearance and/or loss of T cells due to therapy. Further study on the disease-specific T-cell clones (CD8+ or CD4+) and their roles in the immunopathology of HAA is ongoing.
Prepublished online as Blood First Edition Paper, February 26, 2004; DOI 10.1182/blood-2003-11-3959.
Supported by the National Institutes of Health (NIH) Intramural Program.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Drs Alan Wayne (Miami, FL), John Dumot (Cleveland, OH), Ron Sokal (Denver, CO), Thomas Sferra (Columbus, OH), Peter Whitington (Chicago, IL), and Robert Squires (Pittsburgh, PA) for providing samples or referral of patients for these studies. We would like to thank Dr Nancy L. Geller, Office of Biostatistics Research, NHLBI, for advice in the biostatistical analysis of this study, and Dr Antonio Risitano, currently at the Division of Hematology, University of Naples “Federico II,” Italy, for helpful discussions.


![Figure 5. Reversion of the T-cell repertoire to normal in 3 HAA patients after immunosuppressive therapy. Samples for case no. 1 and no. 2 were collected at 1 year after immunosuppressive treatment and for case no. 3 at 2.5 years (antithymocyte globulin [ATG], cyclosporine [CsA], and mycophenolate mofetil [MMF]). Shown are the CDR3 length (40 amino acids) in the x-axis versus the fluorescence intensity in the y-axis. The spectratype profiles of the 21 Vβ subfamilies were analyzed in 3 ways: observation, measuring the relative fluorescence intensity, and complexity scoring. Absent indicates no signal detected with the use of the current technique; skewed spectratype (observation), the percentage of non-Gaussian spectratypes as determined by eye; skewed (RI), the percentage of skewed spectratypes detected by means of a calculation based on the relative fluorescence intensity of each peak; total complexity score, the total number of discrete peaks in each Vβ subfamily, as a measure of the polyclonality of the T-cell repertoire.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/12/10.1182_blood-2003-11-3959/5/m_zh80120462810005.jpeg?Expires=1769843982&Signature=QaPAnJNrQJ-0LHzyJhYjp7T4w~qC8cgksxNPMyl1H9w~BPEeaMB~5X0VpeKHAjnqz8QLys-AKPY7OYyLVRIb81~fPUeopvaCZD2zKahlKtQ9cifoWvGdXgrDK5PC3f1eRkYwfuYyMHKBQDM9AC6Y3YggmqW6b9DuIMoNJwXuebmBCidOv4VfwJD9huPHn3FusXh5L1kxQCebOMD8O0Ed6MRzPpk6tduvKNiLbBMyeMAG-iOsyqhBPamfBbf1Onubb33vJGQtJb5lhEPhq1DGN~19D6S5yeyk55RX1zelnDYL9K4wJkpf4CLjP~Q6FUwdGNu4sqgzipEB6Ihak-uk5Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal