Abstract
We report the outcomes of reduced-intensity allogeneic stem cell transplantation using BEAM-alemtuzumab conditioning (carmustine, etoposide, cytosine arabinoside, melphalan, and alemtuzumab 10 mg/d on days –5 to –1) in 6 United Kingdom transplant centers. Sixty-five patients with lymphoproliferative diseases underwent sibling (n = 57) or matched unrelated donor (n = 8) transplantation. Sustained donor engraftment occurred in 60 (97%) of 62 patients. Of the 56 patients undergoing chimerism studies, 35 (63%) had full donor chimerism. Overall, 73% were in complete remission (CR) after transplantation. At a median follow-up of 1.4 years (range, 0.1-5.6 years), 37 remain alive and in CR. Acute graft-versus-host disease (GVHD) occurred in 11 (17%) of 64, grades I-II only. Estimated 1-year transplantation-related mortality (TRM) was 8% for patients undergoing first transplantation but was significantly worse for those who had previously undergone autologous transplantation. Six patients relapsed (estimated 2-year relapse risk, 20%). Histologic diagnosis (mantle cell lymphoma and high-grade non-Hodgkin lymphoma) and age at transplantation (> 46 years) were significantly associated with higher relapse risk and worse event-free survival. Relapse did not occur in any patient who developed acute or chronic GVHD. This study demonstrates that reduced-intensity allogeneic stem cell transplantation for lymphoproliferative diseases using a BEAM-alemtuzumab preparative regimen is associated with sustained donor engraftment, a high response rate, minimal toxicity, and a low incidence of GVHD.
Introduction
Allogeneic hemopoietic stem cell transplantation is a curative therapy for patients with lymphoproliferative disorders both as a result of the intensity of the conditioning regimen and the application of graft-versus-lymphoma effect. However, use of conventional conditioning regimens has been associated with a 39% to 47% risk of transplant-related mortality (TRM) in low-grade lymphoma1-3 and 24% to 61% in advanced Hodgkin lymphoma (HL).4-7 Therefore, despite encouragingly low relapse rates reported in some studies,1,2 the high TRM has prevented the widespread adoption of allogeneic transplantation in lymphoproliferative disorders.
Over the past few years a number of investigators have reported on the use of reduced-intensity conditioning (RIC) regimens in lymphoma with encouraging results.8,9 These regimens have been designed to reduce TRM and provide a platform of durable donor stem cell engraftment to exploit a graft-versus-lymphoma (GVL) effect. A number of such regimens have been reported, including fludarabine plus cyclophosphamide9 and fludarabine plus melphalan.5,10 These regimens are designed to be primarily immunosuppressive to facilitate donor engraftment but have the potential drawback, compared with conventional myeloablative conditioning regimens, of allowing disease progression prior to the development of any GVL effect. Furthermore, graft-versus-host disease (GVHD) remains a significant clinical problem following reduced-intensity conditioning and is a major cause of TRM in several series of such transplantations.11-13
To circumvent this effect, the anti-CD52 antibodies Campath-1G and alemtuzumab (Campath-1H; Schering, West Sussex, United Kingdom) have been used to reduce the risk of developing acute GVHD following both conventional and RIC allogeneic transplantation regimens.9,14 We have previously reported on a pilot study combining BEAM (carmustine, etoposide, cytosine arabinoside, melphalan) with pretransplantation campath-1G antibodies as a reduced-intensity conditioning regimen for patients with lymphoproliferative diseases and high risk of relapse.15 BEAM is a widely used and effective conditioning regimen for autologous transplantation in lymphoma with low toxicity. Administration of anti-CD52 antibodies prior to stem cell infusion depletes host T cells and consequently reduces the risk of graft rejection. The rationale for this RIC regimen in allogeneic transplantation for lymphoma is, therefore, to exploit the well-established antilymphoma efficacy of BEAM while reducing the risks of rejection and GVHD. Here, we report the results of this approach in 65 consecutive patients with lymphoproliferative disease undergoing transplantation in 6 United Kingdom centers.
Patients and methods
Patients
Between January 1997 and May 2002, data were collected on 65 consecutive patients undergoing RIC allogeneic stem cell transplantation with BEAM-alemtuzumab conditioning for lymphoproliferative diseases at 6 United Kingdom centers. The local ethics committee for each participating center approved the study. Informed consent was provided according to the Declaration of Helsinki. Patients were considered for this treatment if they were ineligible for conventional allogeneic transplantation, including relapse after previous autologous transplantation and previous mediastinal irradiation, or were unsuitable for autologous transplantation, including extensive bone marrow disease or failure to mobilize peripheral blood stem cells (PBSCs). Patient and transplant characteristics are given in Table 1. Pretransplantation histology was low-grade non-Hodgkin lymphoma (NHL; n = 28), mantle cell lymphoma (MCL; n = 5), high-grade NHL (n = 8), chronic lymphocytic leukemia/prolymphocytic leukemia (CLL/PLL; n = 13), peripheral T-cell lymphoma (PTCL; n = 6), and HL (n = 5). Patients with lymphoblastic lymphoma did not receive transplants with this regimen. The source of stem cells was PBSCs (n = 56), bone marrow (n = 8), or both (n = 1) from HLA-identical (n = 52) or 1 antigen–mismatched (n = 5) sibling donor or from matched unrelated donors (n = 8). The median CD34+ cell dose was 4.3 × 106/kg (range 3.5 × 105-41.4 × 106/kg). The median age was 45.6 years (range, 18.8-60.4 years), and the median number of chemotherapy regimens administered prior to transplantation was 2 (range, 1-6). A total of 7 patients (11%) had undergone a prior high-dose procedure with autologous stem cell support, 6 with BEAM and 1 with total body irradiation–based conditioning. Disease status at transplantation was classified as good risk (patients in first and second complete remission [CR]), poor risk (primary refractory disease, refractory relapse, and greater than first partial remission [PR]), and intermediate risk (all others). At the time of transplantation, 13 patients had good-risk disease, 17 had intermediate-risk disease, and 27 (47% of classifiable cases) had poor-risk disease.
Patient and transplantation details
Characteristics . | . |
|---|---|
| Sex, n, M/F | 46/19 |
| Median age at transplantation, y (range) | 45.6 (18.9-60.4) |
| Histology at diagnosis, n | |
| Low-grade NHL* | 28 |
| Mantle cell lymphoma | 5 |
| High-grade NHL | 8 |
| CLL/PLL | 13 |
| PTCL | 5 |
| Hodgkin lymphoma | 5 |
| Risk group at transplantation, n† | |
| Good risk | 13 |
| Intermediate risk | 17 |
| Poor risk | 27 |
| Unknown | 8 |
| Prior chemotherapy regimes, n (range) | 2 (1-6) |
| Transplantation characteristics, n | |
| HLA-identical related donor | 52 |
| 1 Ag mismatch related donor | 5 |
| Matched unrelated donor | 8 |
| PBSCs | 56 |
| BM | 8 |
| PBSCs and BM | 1 |
| CMV serostatus, n | |
| Recipient negative/donor negative | 26 |
| Recipient negative/donor positive | 11 |
| Recipient positive | 28 |
Characteristics . | . |
|---|---|
| Sex, n, M/F | 46/19 |
| Median age at transplantation, y (range) | 45.6 (18.9-60.4) |
| Histology at diagnosis, n | |
| Low-grade NHL* | 28 |
| Mantle cell lymphoma | 5 |
| High-grade NHL | 8 |
| CLL/PLL | 13 |
| PTCL | 5 |
| Hodgkin lymphoma | 5 |
| Risk group at transplantation, n† | |
| Good risk | 13 |
| Intermediate risk | 17 |
| Poor risk | 27 |
| Unknown | 8 |
| Prior chemotherapy regimes, n (range) | 2 (1-6) |
| Transplantation characteristics, n | |
| HLA-identical related donor | 52 |
| 1 Ag mismatch related donor | 5 |
| Matched unrelated donor | 8 |
| PBSCs | 56 |
| BM | 8 |
| PBSCs and BM | 1 |
| CMV serostatus, n | |
| Recipient negative/donor negative | 26 |
| Recipient negative/donor positive | 11 |
| Recipient positive | 28 |
CLL/PLL indicates chronic lymphocytic leukemia/prolymphocytic leukemia; PTCL, peripheral T-cell lymphoma; CR1, first complete remission; CR2, second complete remission; PR1, first partial remission; Ag, antigen; PBSCs, peripheral blood stem cells; BM, bone marrow; and CMV, cytomegalovirus.
Low-grade and transformed low-grade NHL.
Good risk indicates CR1, CR2; intermediate risk, CR > 2, PR1; and poor risk, PR > 1, refractory relapse, or primary progressive disease.
Transplantation regimen
BEAM-alemtuzumab conditioning was used in all patients: carmustine (300 mg/m2) on day –6, cytosine arabinoside (200 mg/m2) on days –5 to –2, etoposide (200 mg/m2) on days –5 to –2, and melphalan (140 mg/m2) on day –1. Fludarabine (30 mg/m2) on days –9 to –7 was added in 5 cases (3 matched unrelated donors [MUDs] and 2 sibling RIC allografts). Campath antibodies (10 mg/d) were administered intravenously on days –5 to –1 (50 mg total) except in one center, where a total dose of 100 mg was used (n = 15 patients). Campath-1G antibodies were used in the early part of the study, but this preparation was discontinued from May 2000 and was replaced by alemtuzumab. PBSCs were mobilized with granulocyte colony-stimulating factor (filgrastim) at a dose of 10 μg/kg for 4 to 5 days with the aim of collecting more than 4 × 106/kg CD34+ cells. GVHD prophylaxis was with cyclosporin and methotrexate (10 mg/m2 days +1, +3, +6). Cyclosporin was tapered from day 50 and withdrawn at 3 months in the absence of GVHD.
Minimal residual disease
Minimal residual disease (MRD) was monitored by using polymerase chain reaction (PCR) techniques for those patients in whom a molecular marker of disease was identified prior to transplantation. These markers included rearrangement of the immunoglobulin heavy-chain (IgH) T-cell receptor and the t(14;18) major breakpoint region/IgH translocation.
Chimerism studies
Chimerism was studied locally by using variable numbers of tandem repeats analysis of peripheral blood at day 30, day 60, day 90, and every 3 months thereafter.
Donor lymphocyte infusions
Donor lymphocyte infusions (DLIs) were permitted for the treatment of persisting disease, relapse/progression, or minimal residual disease. Patients not evolving to 100% donor chimerism after withdrawal of immunosuppression were also considered eligible for DLI if mixed chimerism was confirmed by repeat testing 4 weeks later and if there was no evidence of GVHD. The initial lymphocyte dose was between 5 × 106 and 1 × 107 CD3+ cells/kg, with dose escalations of 1 × 107 CD3+ cells/kg if there were no response (persisting clinical disease, MRD, or mixed chimerism as appropriate) after 6 to 8 weeks.
Definitions
Histologic diagnosis was based on local review. NHL cases were classified according to the International Working Formulation. Transformed low-grade lymphomas were classified with low-grade lymphomas. CR, unconfirmed complete remission (CRu), PR, stable disease, and progressive disease were defined according to International Workshop criteria for NHL16 and HL.17 Complete molecular remission was defined for those patients with a known molecular marker as absence of same-sized PCR product at further analysis after transplantation. Acute GVHD was graded according to the standard criteria.18 The incidence of chronic GVHD was calculated in patients followed for at least 90 days and was classified as none, limited, or extensive. Relapse was defined as disease progression in those patients in CR or CRu with transplantation-related deaths (all deaths following RIC not attributable to disease) being censored. Event-free survival was measured from the time of transplantation to relapse (patients in CR and CRu), progressive disease (patients in PR, nonresponders), or death from any cause.
Statistical analysis
Relapse, TRM, and survival estimates were calculated by the method of Kaplan and Meier. Patient, disease, and transplantation-related variables were studied for associations with outcomes by univariate analysis by using the log-rank test and by multivariate analysis with the use of Cox proportional hazards regression. For the purpose of analysis, 2 diagnostic groups were defined: low-grade lymphoproliferative diseases (low-grade NHL, CLL/PLL, PTCL, and HL) and aggressive NHL (MCL and high-grade NHL). In all statistical calculations, P values less than .05 were considered significant. Statistical analyses were performed with the use of SPSS software (SPSS, Chicago, IL).
Results
Engraftment and chimerism
Three patients died within 30 days of transplantation, prior to engraftment (1 of TRM, 2 of progressive disease) and were, therefore, excluded from engraftment analysis. Primary graft failure occurred in 3 patients (1 sibling bone marrow, 2 sibling PBSCs). Two of these patients engrafted following additional stem cell infusions and 1 patient died. The median time to neutrophil engraftment (neutrophils > 0.5 × 106/L) and platelet engraftment (platelets > 20 × 109/L) was 15 days (range, 8-29 days and 6-176 days, respectively). Chimerism results were available in 56 cases: 35 (62.5%) were full donor chimeras (> 98% donor DNA) and 19 were mixed chimeras. Of the 2 patients engrafting following second stem cell infusion, 1 attained full donor chimerism and the other achieved mixed chimerism. Two further patients were found to have unsuspected autologous reconstitution, although chimerism studies were not documented prior to day 90 in either of these patients. Late graft failure occurred in 1 patient at 5 months after transplantation, necessitating autologous stem cell transplantation.
Transplantation-related mortality
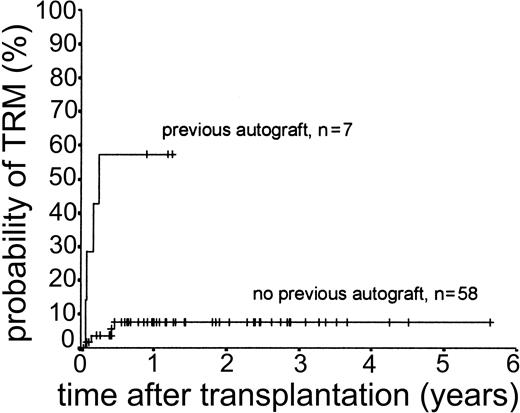
Eight patients (12.3%) died of transplantation-related causes between 20 days and 5.7 months after transplantation. All 8 had undergone matched sibling allogeneic transplantation. The causes of these deaths were infection (n = 4), multiorgan failure (n = 2), primary graft failure (n = 1), and iatrogenic bleeding (n = 1). Four of these 8 deaths occurred in patients who had previously undergone autologous transplantation (3 with BEAM conditioning, 1 with TBI-based regimen). There were no deaths attributable to GVHD or to late onset (> day 100 after transplantation) opportunistic infection. The 2-year actuarial TRM for all patients was 13.3%. Results of univariate analysis for factors associated with TRM are shown in Table 2. There was a statistically significant difference between patients previously treated with autologous transplantation (1-year actuarial TRM, 57.1%) and those undergoing first transplantation (1-year actuarial TRM, 7.6%; log-rank chi-square, P < .001) (Figure 1). Age at transplantation, risk group at transplantation, and diagnostic group did not have an effect on TRM.
Univariate analysis of factors influencing 2-year actuarial TRM
. | TRM, % . | P . |
|---|---|---|
| Age | .14 | |
| 46 y or younger | 6.5 | |
| Older than 46 y | 20.3 | |
| Donor | .29 | |
| Related | 15.1 | |
| Unrelated | 0.0 | |
| Prior autograft | < .001 | |
| Yes | 57.1* | |
| No | 7.6* | |
| Risk group | .63 | |
| Low | 7.7 | |
| Intermediate | 12.2 | |
| High | 9.6 | |
| GVHD | .22 | |
| Yes | 5.0 | |
| No | 17.4 | |
| Diagnostic group | .59 | |
| Low-grade disease† | 15.8 | |
| MCL/high-grade NHL | 7.7 |
. | TRM, % . | P . |
|---|---|---|
| Age | .14 | |
| 46 y or younger | 6.5 | |
| Older than 46 y | 20.3 | |
| Donor | .29 | |
| Related | 15.1 | |
| Unrelated | 0.0 | |
| Prior autograft | < .001 | |
| Yes | 57.1* | |
| No | 7.6* | |
| Risk group | .63 | |
| Low | 7.7 | |
| Intermediate | 12.2 | |
| High | 9.6 | |
| GVHD | .22 | |
| Yes | 5.0 | |
| No | 17.4 | |
| Diagnostic group | .59 | |
| Low-grade disease† | 15.8 | |
| MCL/high-grade NHL | 7.7 |
TRM at 1 year.
Low-grade lymphoproliferative diseases and HL.
Kaplan-Meier plot of TRM probability stratified by previous autologous transplantation.
Kaplan-Meier plot of TRM probability stratified by previous autologous transplantation.
Disease response
Fifty-six patients were evaluable for response at 3 months (5 early TRM, 3 deaths from progressive disease, 1 too early for assessment). Thirty-seven patients were in CR, 3 were in CRu, 11 were in PR, 2 had stable disease, 2 had progressive disease, and 1 was not assessed. Beyond 3 months, 1 patient with CRu and 2 patients in PR later achieved CR with no further treatment, and 1 patient in PR received DLI and achieved CR giving a final CR rate of 73.2%. Risk group at transplantation did not have an effect on CR/CRu rate, which was 84.6% in the good-risk group, 58.8% in the intermediate-risk group, and 59.2% in the poor-risk group (good and intermediate risk compared with poor risk, chi-square = 0.57). Neither acute GVHD nor diagnostic group had an effect on final CR/CRu. Seventeen patients had markers for MRD: after transplantation, 7 were in complete molecular remission, 5 had MRD, and 5 had positive PCR with clinical evidence of disease.
Response to DLI
Seventeen patients received DLI, for stable persistent disease after transplantation (n = 2) relapse/progressive disease (n = 8), MRD (n = 3), or lone mixed chimerism (n = 4). In total, 10 patients with mixed chimerism received DLI. One patient receiving DLI for persistent disease achieved CR; the second failed to respond. Of the 8 patients who received DLI for relapse or progressive disease, 6 were treated with antitumor chemotherapy prior to DLI. Only 1 patient responded and remains alive in CR; the remaining 7 died as a result of disease progression, including the 2 patients who received DLI alone. Of the 3 patients receiving DLI for MRD, all are alive and in complete molecular remission. Of the 4 patients receiving DLI for lone mixed chimerism, 3 are alive and in CR (1 full donor chimera, 1 mixed chimera, 1 not assessed) and 1 later relapsed and died.
GVHD
Sixty-four patients were evaluable for acute GVHD. Eleven patients (17.2%) developed acute GVHD following transplantation, all at grades I to II. Of the 16 patients who received DLI, 6 (37.5%) subsequently developed acute GVHD (grades I-III). Nine (17.0%) of 53 patients alive at day 100 after transplantation developed chronic GVHD, which was of limited extent in all cases. In 2 of these patients, chronic GVHD developed following DLI therapy. Subgroup analysis by dose of alemtuzumab antibodies (50 mg compared with 100 mg total) confirmed no significant differences in risk of acute GVHD (Fisher exact test, P = .45) nor acute or chronic GVHD (chi-square, P = .40)
Relapse
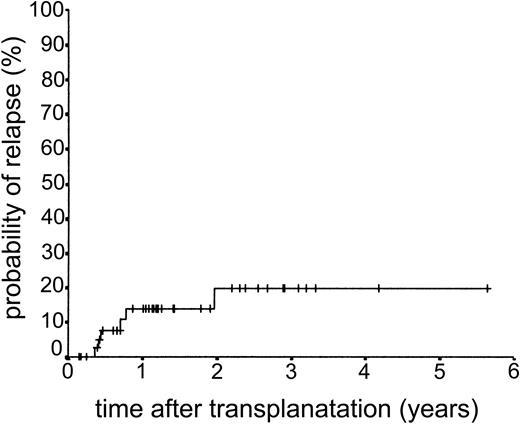
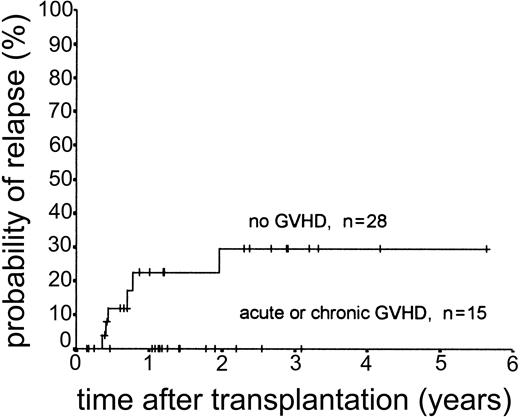
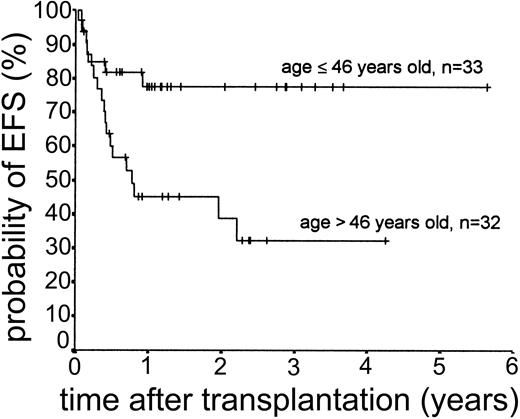
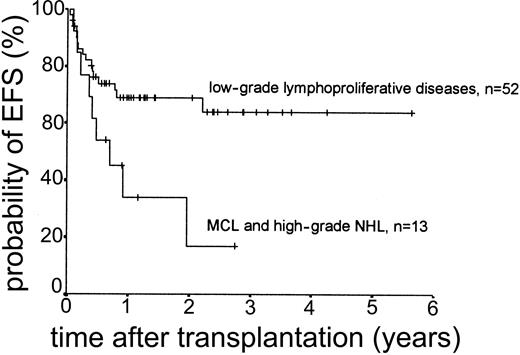
Relapse occurred in 6 of 43 cases. The 2-year actuarial risk of relapse for all patients was 20.0% (Figure 2). Results of univariate analysis for factors associated with relapse risk are shown in Table 3. Neither donor type (sibling or unrelated donor) nor risk group had a significant effect on relapse risk (2-year actuarial relapse risk, 11.1% for good risk, 37.0% for intermediate risk, and 7.1% for poor risk; log-rank chi square, P = .30). Attainment of full donor chimerism did not have an effect on relapse risk (Fisher exact test, P = .30). There was a significant difference in relapse risk for patients younger than 46 years at transplantation (2-year actuarial relapse risk, 4.8%) and patients older than 46 years (2-year actuarial relapse risk, 37.3%; log-rank chi square, P = .047). There was a trend toward lower relapse risk for patients who developed either acute or chronic GVHD (where no relapses occurred) compared with patients with no GVHD (2-year actuarial relapse risk, 29.4%; log-rank chi square, P = .059) (Figure 3). The relapse risk was significantly higher in the aggressive NHL group (2-year actuarial relapse risk, 67.9%) compared with the low-grade lymphoproliferative diseases group (2-year actuarial relapse risk, 10.0%; log-rank chi square, P = .014) (Figure 4). By multivariate analysis, only diagnostic group (aggressive NHL) had a significant effect on relapse risk (Table 4).
Univariate analysis of factors influencing 2-year actuarial relapse risk, overall survival (OS), and event-free survival (EFS)
. | Relapse, % . | P . | OS, % . | P . | EFS, % . | P . |
|---|---|---|---|---|---|---|
| Age | .047 | .066 | .005 | |||
| 46 y and younger | 4.8 | 84.1 | 77.6 | |||
| Older than 46 y | 37.8 | 53.4 | 38.7 | |||
| Donor | .72 | .39 | .53 | |||
| Related | 19.5 | 65.9 | 57.0 | |||
| Unrelated | 16.7 | 85.7 | 68.6 | |||
| Prior autograft | .53 | .013 | .01 | |||
| Yes | 11.1* | 42.8† | 42.9‡ | |||
| No | 0.0* | 74.8† | 63.9‡ | |||
| Risk group | .30 | .79 | .42 | |||
| Low | 11.1 | 76.9 | 75.2 | |||
| Intermediate | 37.0 | 59.2 | 51.8 | |||
| High | 7.1 | 65.6 | 47.9 | |||
| GVHD | .059 | .26 | .35 | |||
| Yes | 0.0 | 71.5 | 68.0 | |||
| No | 29.4 | 67.8 | 55.6 | |||
| Diagnostic group | .014 | .066 | .018 | |||
| Low-grade disease§ | 10.0 | 74.3 | 69.0 | |||
| MCL/high-grade NHL | 67.9 | 45.4 | 16.8 |
. | Relapse, % . | P . | OS, % . | P . | EFS, % . | P . |
|---|---|---|---|---|---|---|
| Age | .047 | .066 | .005 | |||
| 46 y and younger | 4.8 | 84.1 | 77.6 | |||
| Older than 46 y | 37.8 | 53.4 | 38.7 | |||
| Donor | .72 | .39 | .53 | |||
| Related | 19.5 | 65.9 | 57.0 | |||
| Unrelated | 16.7 | 85.7 | 68.6 | |||
| Prior autograft | .53 | .013 | .01 | |||
| Yes | 11.1* | 42.8† | 42.9‡ | |||
| No | 0.0* | 74.8† | 63.9‡ | |||
| Risk group | .30 | .79 | .42 | |||
| Low | 11.1 | 76.9 | 75.2 | |||
| Intermediate | 37.0 | 59.2 | 51.8 | |||
| High | 7.1 | 65.6 | 47.9 | |||
| GVHD | .059 | .26 | .35 | |||
| Yes | 0.0 | 71.5 | 68.0 | |||
| No | 29.4 | 67.8 | 55.6 | |||
| Diagnostic group | .014 | .066 | .018 | |||
| Low-grade disease§ | 10.0 | 74.3 | 69.0 | |||
| MCL/high-grade NHL | 67.9 | 45.4 | 16.8 |
Populations presented: relapse, n = 43; OS, n = 65; and EFS, n = 65.
1-year actuarial relapse risk.
1-year actuarial OS.
1-year actuarial EFS.
Low-grade lymphoproliferative diseases and HL.
Kaplan-Meier plot of probability of relapse stratified by occurrence of GVHD.
Kaplan-Meier plot of probability of relapse according to diagnostic group.
Results of multivariate analysis
Outcome . | Variable . | Relative risk . | Confidence interval . | P . |
|---|---|---|---|---|
| Relapse | Age > 46 y | 6.0 | 0.7-52.0 | .10 |
| Relapse | MCL/high-grade NHL | 5.4 | 1.1-27.2 | .04 |
| EFS | Age > 46 y | 3.3 | 1.4-7.9 | .01 |
| EFS | MCL/high-grade NHL | 2.7 | 1.2-6.1 | .02 |
Outcome . | Variable . | Relative risk . | Confidence interval . | P . |
|---|---|---|---|---|
| Relapse | Age > 46 y | 6.0 | 0.7-52.0 | .10 |
| Relapse | MCL/high-grade NHL | 5.4 | 1.1-27.2 | .04 |
| EFS | Age > 46 y | 3.3 | 1.4-7.9 | .01 |
| EFS | MCL/high-grade NHL | 2.7 | 1.2-6.1 | .02 |
Only variables that were statistically significant in univariate analysis are shown.
Of the 6 patients who relapsed, 3 have subsequently died of disease (1 not treated, 2 received DLI ± additional therapy), 2 are alive in CR (1 following second allogeneic transplantation; 1 following DLI, surgery, and radiotherapy), and the third has not yet received treatment.
Overall survival and EFS
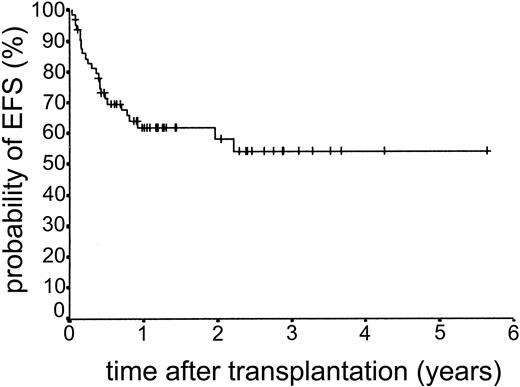
The median duration of follow-up after transplantation was 1.4 years (range, 0.1-5.6 years). Forty-nine patients remain alive. The estimated overall survival at 2 and 3 years was 68.0% and 63.1%, respectively (Figure 5). The overall survival (OS) according to diagnosis is shown in Figure 6. The 2- and 3-year actuarial event-free survival (EFS) results were 58.1% and 54.2%, respectively (Figure 7). The results of univariate analyses for effect on OS and EFS are shown in Table 3. Prior autologous transplantation had a significant effect on OS (42.8% OS at 1 year compared with 74.8% without prior autologous transplantation; log-rank chi square, P = .013) but had no significant effect on EFS. There was a trend toward improved OS for patients younger than 46 years at transplantation, and this difference was significant for EFS (77.6% EFS at 2 years for age < 46 years compared with 38.7% EFS for age > 46 years, log-rank chi square, P = .005) (Figure 8). Diagnostic group had a significant effect on EFS, which was significantly worse in aggressive NHL (16.8% EFS at 2 years) compared with low-grade lymphoproliferative disease group (69.0% EFS at 2 years; log-rank chi square, P = .018) (Figure 9). There was no difference between patients with low-grade NHL (n = 23) and those with transformed low-grade NHL (n = 5) for OS or EFS (log-rank chi square P = .48 and P = .60, respectively). Risk group, donor type (related or unrelated), and occurrence of GVHD did not have an effect on OS or EFS. By multivariate analysis, both being 46 years old or younger and low-grade lymphoproliferative disease were associated with significantly better EFS (Table 4).
Discussion
Most RIC regimens have used fludarabine to provide pretransplantation immunosuppression and to facilitate hemopoietic engraftment.8,9,12,13,19 Preliminary experience in a single center had previously demonstrated that the addition of pretransplantation Campath-1G antibodies to the BEAM regimen permitted the engraftment of stem cells from sibling donors with a low risk of acute and chronic GVHD.15 We have now used this regimen for 65 patients in a multicenter study, with fludarabine as additional immunosuppression in 5 patients, and overall 97% of the patients achieved donor engraftment.
An important finding is that BEAM-alemtuzumab conditioning was well tolerated, with a low risk of TRM (7.6%) in patients undergoing first transplantation. Indeed the TRM is comparable to that which has been reported for BEAM conditioning prior to autologous transplantation in lymphoma.20 Four of the 8 transplant-related deaths occurred in patients who had previously undergone autologous transplantation, and we now use a less-toxic RIC regimen, comprising fludarabine, melphalan, and alemtuzumab, in this setting.
The incidence of acute GVHD was low, and only grades I to II disease was observed. This finding is consistent with previous reports of RIC regimens that incorporate alemtuzumab antibodies.8,9 In the early part of this study Campath-1G antibodies, the rat monoclonal antibody, were used. The mechanism by which Campath antibodies decrease the risk of GVHD may be complex. In our pilot study we found that the serum concentration of Campath-1G antibodies at the time of stem cell infusion was sufficient to kill donor T cells.15 Furthermore, a recent study has shown that Campath antibodies administered in vivo deplete host dendritic cells,21 and this depletion may have contributed to the low incidence of GVHD observed. From May 2000, when Campath-1G was no longer available, the humanized version alemtuzumab was used at the same dose and schedule. Alemtuzumab has a longer half-life than Campath-1G22 and is, therefore, at least as likely to deplete donor T cells effectively. The total dose of Campath antibodies used for most of the patients in this study (50 mg) was lower than that used by Kottaridis et al8 (100 mg) in their alemtuzumab-containing regimen. Despite this difference in dose, the risk of GVHD was similar. However, we used methotrexate for additional GVHD prophylaxis, and our schedule of antibody administration was different. The lower dose of alemtuzumab may be desirable if this results in a shorter period of posttransplantation immunosuppression. Although immune reconstitution was not formally studied here, it is notable that we did not observe any death from infection beyond day 100 in this study.
In terms of response, more than 70% of the patients were in complete remission following BEAM-alemtuzumab transplantation. Interestingly, we found that risk group at transplantation had no effect on the response to transplantation. This finding contrasts to the findings of Perez-Simon et al,23 in which no patient categorized as high risk attained CR/CRu/PR at 3 months following RIC with fludarabine, melphalan, and alemtuzumab conditioning, although delayed DLI successfully induced responses in this group. This difference may reflect the enhanced antilymphoma efficacy of BEAM in this setting compared with fludarabine and melphalan. This difference may also account for the good response of patients with transformed low-grade NHL. Analysis of this group demonstrated that there was no significant difference in CR rate or overall survival for patients with transformed low-grade NHL compared with low-grade NHL. However, all of the patients with transformed low-grade NHL had chemosensitive disease at the time of transplantation.
The major cause of treatment failure in this study was disease relapse/progression. The risk of relapse was related to the histologic grade of lymphoma. Patients with low-grade lymphoproliferative diseases, including Hodgkin lymphoma, had a relapse risk of only 10% at 2 years. Others have reported the low risk of relapse and excellent survival for patients with low-grade NHL undergoing RIC transplantation.8,9,24 Our results confirm this result and also show comparable results for patients with CLL/PLL and peripheral T-cell lymphoma. The results for high-grade NHL and MCL were less encouraging because of the higher risk of early relapse for this group (68% at 2 years). Treatment of relapse in aggressive lymphoma, including the use of DLI, was generally unsatisfactory with only 1 patient, who underwent a second RIC from an alternative sibling donor at relapse, responding. Patients with mantle cell lymphoma have a high risk of relapse following autologous transplantation,26 and overall our data are in agreement with the European Group for Blood and Marrow Transplantation (EBMT) analysis, which shows a high risk of relapse in high-grade NHL and mantle cell lymphoma following RIC allogeneic transplantation.25
The second major factor apparently influencing relapse was the absence of acute or chronic GVHD. Although this absence did not reach statistical significance in this study, relapse did not occur in any patient who developed GVHD compared with a 29% relapse risk at 2 years in patients who did not develop GVHD. These observations are in keeping with the existence of a GVL effect and suggest that this effect could be further exploited to improve the outcome for patients with a high risk of relapse.
We found that delayed DLI was able to induce disease response, as with other studies of RIC allogeneic transplantation.11,23-25 This response was most effective for patients with persistent disease after transplantation or with MRD. However, this response was at the expense of a higher incidence and severity of acute GVHD following delayed DLI than following initial transplantation.
The major factors following multivariate analysis affecting event-free survival were age at transplantation and the diagnostic subgroup. The beneficial effect of age on survival in this study was due to a combination of higher, but not statistically significant, risk of TRM and relapse in older patients. For patients younger than 46 years the outcome was excellent with EFS and OS at 2 years of 78% and 84%, respectively. The superior outcome for patients with low-grade disease has been previously seen in other studies of RIC transplantation for lymphoma.25 In our study, patients with low-grade NHL, CLL, PTCL, and HL had a comparable outcome with EFS of 69% and OS of 74% at 2 years for the whole group (Table 3; Figure 9). This finding contrasts with the poorer outcome for patients with MCL and high-grade NHL primarily because of the higher risk of relapse. Given our observations concerning the influence of GVHD on relapse, it is possible that the results for aggressive NHL might be improved by a reduction in posttransplantation immunosuppression. Indeed, we have now altered our protocol such that methotrexate is not used in this group to elicit an earlier and more significant GVL effect.
In summary, these results demonstrate that it is possible to undertake allogeneic transplantation for lymphoma by using a regimen that combines the benefit of significant antilymphoma conditioning therapy with Campath antibodies to reduce GVHD and transplant-related mortality from GVHD. BEAM would appear to be a logical conditioning regimen for lymphoma, given its efficacy and favorable toxicity profile in autologous transplantation. Despite the use of Campath antibodies and the minimal risk of GVHD, the relapse risk, particularly for patients with low-grade disease, was low. Given the acceptable toxicity of the procedure, it would appear justified to consider this as an alternative therapy strategy for selected patients with low-grade NHL and Hodgkin lymphoma considered to be at high risk of treatment failure following autologous transplantation.
Prepublished online as Blood First Edition Paper, September 11, 2003; DOI 10.1182/blood-2003-05-1406.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal