Dendritic cells (DCs) comprise a diverse system of antigen-presenting cells that are responsible for maintaining self-tolerance, and initiating innate or adaptive immune responses to pathogens, rejection of organ transplants, and graft-versus-host disease. DCs exist in low frequency in living organisms and therefore they are difficult to isolate. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and tumor necrosis factor-α (TNF-α) cooperate in the generation of human myeloid DCs from CD34+ hematopoietic cells.1 The use of these cytokines for ex vivo expansion has made available large numbers of myeloid DCs for studies of normal and pathologic immune responses and for vaccination trials.
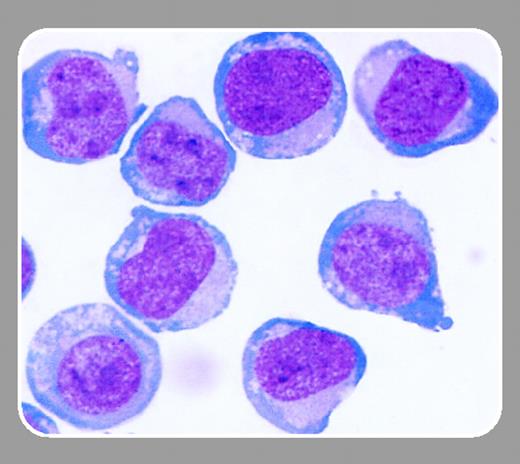
Chen and colleagues (page 2547) report that thrombopoietin cooperates with FLT-3 in the generation of plasmacytoid DC precursors from human CD34+ hematopoietic cells. Plasmacytoid DC precursors (also called plasmacytoid monocytes, plasmacytoid T cells, type 2 DC precursors, or type 1 interferon-producing cells [IPCs]) are negative for lineage markers and CD11c, are positive for HLA-DR and CD123 (interleukin-3 [IL-3] receptor α-chain), and are CD4 bright. They have plasmacytoid morphology, an oval shape, a round nucleus, and a basophilic cytoplasm. Freshly isolated plasmacytoid DC precursors produce high levels of type I interferons upon stimulation with viruses, including the herpes simplex virus and the human immunodeficiency virus, or bacterial oligodeoxynucleotides.2 Thus, plasmacytoid DC precursors have a critical role in innate immune responses against pathogens.
Initial functional experiments with plasmacytoid DC precursors were facilitated by the discovery that their survival depends on IL-3.3 In addition, exposure to CD40 ligand or TNF-α induces expression of high levels of the costimulatory molecules CD40, CD80, and CD86, as well as CD83, a marker of mature dendritic cells. Mature plasmacytoid DCs are as potent as mature myeloid DCs at stimulating proliferation of alloantigen-specific naive CD4 T cells. However, following stimulation of Toll-like receptor 7 (TLR-7), plasmacytoid DCs produce interferon-α, whereas myeloid DCs produce IL-12. T cells activated by plasmacytoid DCs produce high levels of IL-4 and IL-10, whereas T cells activated by myeloid DCs produce interferon-γ and IL-2 upon rechallenge. Alloantigen presentation by plasmacytoid DCs promotes the induction of IL-10–producing CD8 T regulatory cells.4 Thus, there are theoretical considerations and initial data for a potential role of plasmacytoid dendritic cells in regulating immunity and tolerance after hematopoietic cell transplantation.5
Chen and collaborators show that plasmacytoid DC precursors obtained from CD34+ cells by ex vivo culture with thrombopoietin and FLT-3 produce interferon-α upon activation and with appropriate stimuli differentiate into mature DCs. The mechanism by which thrombopoietin promotes differentiation from CD34+ progenitors into the plasmacytoid DC lineage remains unknown. The discovery by Chen and colleagues will allow the generation of large numbers of IPCs and mature plasmacytoid DCs and facilitate studies of their functions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal