Abstract
Thrombopoietin stimulates extracellular signal-related kinase 1/2 (ERK1/2) phosphorylation in megakaryocytes, and the classic mitogen-activated protein (MAP) kinase (Raf/mitogen-induced extracellular kinase [MEK]/ERK) pathway has been implicated directly and indirectly to play a critical role in megakaryocytopoiesis. However, the involvement of specific Raf family members in megakaryocytopoiesis is unknown. raf-1-/- mice were therefore used to directly determine the role of Raf-1 in megakaryocytopoiesis. Surprisingly, raf-1-/- mice have a modestly higher platelet count than their raf-1+/+ littermates. Nonetheless, the absence of Raf-1 does not alter thrombopoietin-induced expansion of primary megakaryocyte-lineage cells, the development of apoptotic megakaryocytes in the presence or absence of thrombopoietin, or the development of megakaryocyte DNA ploidy distribution. Moreover, raf-1-/- megakaryocytes do not have a compensatory increase in A-Raf or B-Raf expression, and thrombopoietin-induced ERK1/2 phosphorylation is similar in raf-1-/- and raf-1+/+ megakaryocytes. These unexpected findings demonstrate that Raf-1 is dispensable for megakaryocytopoiesis, and for thrombopoietin-induced ERK1/2 activation in primary megakaryocyte-lineage cells.
Introduction
Megakaryocytopoiesis is a complex and poorly understood process involving the maturation of small lineage-committed CD41+ precursor cells into large polyploid megakaryocytes that release platelets into the circulation, and thrombopoietin (TPO) is the most important known cytokine regulator of this developmental process.1-3 Although TPO engagement of its receptor, Mpl, activates many intracellular signaling cascades,4,5 the role of specific pathways in megakaryocytopoiesis remains unclear.
TPO induces extracellular signal-related kinase 1/2 (ERK1/2) phosphorylation,4,5 and several reports implicate ERK and the classic mitogen-activated protein (MAP) kinase (Raf/mitogen-induced extracellular kinase [MEK]/ERK) cascade in megakaryocytopoiesis. For example, the MEK inhibitor PD98059 can increase megakaryocyte precursor number and delay megakaryocytic maturation of human umbilical cord CD34+ cells cultured in TPO,6 and it can inhibit DNA polyploidization of murine bone marrow CD41+ cells cultured in TPO.7 Furthermore, constitutively active MEK or ERK mutants can induce megakaryocyte differentiation of CMK cells8 and K562 human erythroleukemia cells,9 and the latter can be blocked by PD98059.9 Moreover, in the human megakaryocytic cell line UT-7, Raf-1 and B-Raf are required for TPO-induced sustained ERK1/2 activation,10 which itself is required for TPO-induced megakaryocytic maturation by this cell line.11
As an entry point to the classic MAP kinase cascade, Raf family members play a key role in controlling signaling through this pathway.12-14 Given the well-known role of Raf-1 in classic MAP kinase signaling, and the many reports supporting the importance of MEK/ERK activation on different aspects of megakaryocytopoiesis, we used raf-1-/- mice to directly investigate the role of Raf-1 in this physiologic process. Surprisingly, we found Raf-1 to be dispensable for megakaryocytopoiesis and for TPO-induced ERK1/2, implying that Raf-1 has no unique role in these processes.
Study design
Mice and genotyping
raf-1+/- mice15 were backcrossed to an MF1 background, and MF1 raf-1-/- mice were obtained by crossing MF1 raf-1+/- males and females. Genotyping was performed as described.15 Tissue samples were obtained at 18 to 21 days of age, and littermates were used as wild-type controls. All procedures were performed in accordance with University of California, San Francisco, animal care guidelines.
Blood counts
Peripheral blood was obtained by retro-orbital puncture using EDTA (ethylenediaminetetraacetic acid) anticoagulation. Complete blood counts were determined using a HEMAVET HV850 (CDC Technologies, Oxford, CT) analyzer.
Hematopoietic cell culture
Liquid cultures of low-density bone marrow mononuclear cells (BMMNCs) and fetal liver cells were grown in serum-free media16 supplemented with 50 ng/mL human TPO (hTPO; Genentech, South San Francisco, CA). Highly purified bone marrow (BM) megakaryocytes were obtained by bovine serum albumin density gradient.7 Colony-forming assays were performed at a plating density of 5 × 104 BMMNCs/35-mm plate in methylcellulose media (Methocult 3234; Stem Cell Technologies, Vancouver, BC) supplemented with 50 ng/mL murine stem cell factor (SCF; Peprotech, Rocky Hill, NJ), 10 ng/mL murine interleukin-3 (IL-3; Peprotech), 50 ng/mL murine IL-6 (Peprotech), 4 U/mL human erythropoietin (hEPO; Ortho Biotech, Raritan, NJ), and 50 ng/mL hTPO. The colonies were identified by microscopy after 4 to 10 days in culture.
Cell analysis
Cell surface CD41 expression and DNA ploidy analysis were performed essentially as described.16,17 Terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) analysis was performed using a fluorescein In Situ Cell Death Detection Kit (Roche, Indianapolis, IN) according to the manufacturer's instructions. For TUNEL assays, BM-derived mature megakaryocytes were identified by their unique high side scatter distribution on flow cytometry.16 Fetal liver–derived mature megakaryocytes were identified by CD41 expression (biotinylated anti-CD41 antibody and phycoerythrin-streptavidin; Pharmingen, San Diego, CA) and high side scatter using flow cytometry.
Immunoblotting
Purified BM-derived megakaryocytes were starved in phosphate-buffered saline for one hour, and then stimulated by 250 ng/mL hTPO for 0 to 60 minutes. Protein lysates were generated and Western blots performed essentially as described,5 using anti–A-Raf (1:1000), anti–B-Raf (1:1000), and anti-ERK (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA); anti–Raf-1 (1:1000; Transduction Laboratories, Lexington, KY); and anti–phospho-p44/42 MAPK (1:1000; Cell Signaling, Beverly, MA).
Results and discussion
MF1 background allows for survival of raf-1-/- mice to 25 days after birth
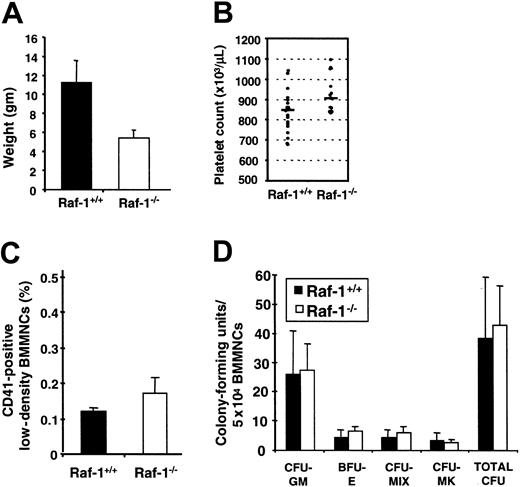
C57BL6 raf-1-/- mice die by embryonic day 9.5 (E9.5) due to increased apoptosis of many tissues, including the neovasculature.15,18 Crossing the raf-1-/- genotype onto the outbred MF1 background allowed raf-1-/- mice to survive after birth. Fetal genotyping at E15.5 to E16.5 from MF1 raf-1+/- × raf-1+/- matings demonstrated a normal mendelian distribution, but only 35% to 40% of the E15.5 to E16.5 raf-1-/- fetuses resulted in live births. The initial 2 raf-1-/- mice died at 26 and 30 days after birth. All subsequent raf-1-/- mice were therefore analyzed at 18 to 21 days after birth. The raf-1-/- mice are small, weighing one third to one half that of their raf-1+/+ littermates (Figure 1A). The early embryonic lethal phenotype previously reported for raf-1-/- mice15,18 is therefore a function of both the Raf-1 deficiency and the genetic background.
Size and hematopoietic characteristics of raf-1-/- mice. (A) The weight (gm) for raf-1+/+ (n = 18) and raf-1-/- (n = 16) littermates at 18 to 21 days after birth is presented as the mean ± SD. The size difference is readily appreciated beginning 2 to 3 days after birth. (B) Circulating platelet counts for raf-1+/+ (n = 21) and raf-1-/- (n = 15) littermates at 18 days after birth are presented, with the mean value indicated by the bar. The mean (± SD) values (× 103) are 832 ± 102 (raf-1+/+) and 929 ± 94 (raf-1-/-); P = .006. (C) The mean percentage (± SD) of CD41+ cells in freshly harvested bone marrow from raf-1+/+ (0.12%) and raf-1-/- (0.17%) littermates are presented; n = 3 for each genotype. Student t test analysis indicates that the 2 groups are not statistically different; P = .12. (D) The relative representation of colony-forming progenitor cells per 50 000 low-density bone marrow mononuclear cells from raf-1+/+ (n = 9) and raf-1-/- (n = 6) littermates at 18 days after birth is presented. Colonies were generated in methylcellulose media supplemented with SCF, IL-6, IL-3, EPO, and TPO. CFU-GM indicates colony-forming unit granulocyte-macrophage; BFU-E, blast-forming unit erythrocyte; CFU-Mix, 3 lineages or less; and CFU-MK, CFU megakaryocyte. Error bars indicate means ± SD.
Size and hematopoietic characteristics of raf-1-/- mice. (A) The weight (gm) for raf-1+/+ (n = 18) and raf-1-/- (n = 16) littermates at 18 to 21 days after birth is presented as the mean ± SD. The size difference is readily appreciated beginning 2 to 3 days after birth. (B) Circulating platelet counts for raf-1+/+ (n = 21) and raf-1-/- (n = 15) littermates at 18 days after birth are presented, with the mean value indicated by the bar. The mean (± SD) values (× 103) are 832 ± 102 (raf-1+/+) and 929 ± 94 (raf-1-/-); P = .006. (C) The mean percentage (± SD) of CD41+ cells in freshly harvested bone marrow from raf-1+/+ (0.12%) and raf-1-/- (0.17%) littermates are presented; n = 3 for each genotype. Student t test analysis indicates that the 2 groups are not statistically different; P = .12. (D) The relative representation of colony-forming progenitor cells per 50 000 low-density bone marrow mononuclear cells from raf-1+/+ (n = 9) and raf-1-/- (n = 6) littermates at 18 days after birth is presented. Colonies were generated in methylcellulose media supplemented with SCF, IL-6, IL-3, EPO, and TPO. CFU-GM indicates colony-forming unit granulocyte-macrophage; BFU-E, blast-forming unit erythrocyte; CFU-Mix, 3 lineages or less; and CFU-MK, CFU megakaryocyte. Error bars indicate means ± SD.
raf-1-/- mice are not thrombocytopenic
Contrary to our expectations, the raf-1-/- mice were not thrombocytopenic. In fact, they had a modest but statistically significantly higher mean platelet count than their raf-1+/+ littermates (Figure 1B). This difference does not, however, reflect an expanded megakaryocyte lineage in raf-1-/- mice as defined by the percentage of CD41-expressing cells in freshly harvested bone marrow (Figure 1C). Furthermore, the absence of Raf-1 did not alter the number of colony-forming progenitor cells per 50 000 low-density BMMNCs, or the relative contribution of each colony type (Figure 2D). Colony size also was not affected. Therefore, while raf-1-/- mice are small and somewhat ill-appearing, their myeloid marrow compartment is not proportionately reduced in size or grossly altered in relative progenitor makeup, including the megakaryocyte progenitor cell compartment.
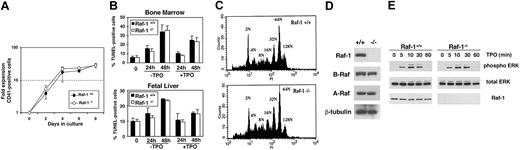
Raf-1 and in vitro megakaryocytopoiesis. (A) The fold expansion of raf-1-/- and raf-1+/+ CD41+ low-density bone marrow mononuclear cells grown in serum-free liquid culture supplemented with 50 ng/mL TPO is presented in semilog format. Data represent the mean ± SEM from 3 independent experiments. (B) Megakaryocytes generated by 4 days of culture in TPO-containing media were washed and resuspended in media with (+TPO) or without (-TPO) TPO, and TUNEL assays were performed following an additional 0, 24, or 48 hours of incubation. The percentage of TUNEL-positive bone marrow–derived megakaryocytes following culture for the indicated times in media with or without TPO is presented (left). Data are the mean ± SEM of 5 independent experiments. The same experiments performed on megakaryocytes derived from E13.5 to E15.5 fetal liver cells (n = 3) produced similar results (right). (C) DNA ploidy analysis of raf-1+/+ and raf-1-/- megakaryocytes stained with fluorescein isothiocyanate–conjugated antimouse CD41 antibody and propidium iodide. Data are from CD41+ megakaryocytes and are representative of 3 independent experiments that each produced the same results. DNA ploidy values ranging from 2N to 128N are indicated above their respective peaks. (D) Western blots demonstrate Raf-1, A-Raf, and B-Raf protein expression in purified mature megakaryocytes derived from raf-1+/+ (+/+) or raf-1-/- (-/-) bone marrow. Total protein (20 μg) was loaded in each lane, separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and probed with antibodies against Raf-1, B-Raf and A-Raf, and β-tubulin, the latter serving as an independent measure of protein loading. (E) A representative Western blot demonstrates similar kinetics and extent of TPO-induced ERK phosphorylation in purified raf-1-/- and raf-1+/+ BM-derived megakaryocytes. The proteins were separated by 10% SDS-PAGE, and blots were probed as indicated using anti–phospho ERK, anti–total ERK, or anti–Raf-1 antibody.
Raf-1 and in vitro megakaryocytopoiesis. (A) The fold expansion of raf-1-/- and raf-1+/+ CD41+ low-density bone marrow mononuclear cells grown in serum-free liquid culture supplemented with 50 ng/mL TPO is presented in semilog format. Data represent the mean ± SEM from 3 independent experiments. (B) Megakaryocytes generated by 4 days of culture in TPO-containing media were washed and resuspended in media with (+TPO) or without (-TPO) TPO, and TUNEL assays were performed following an additional 0, 24, or 48 hours of incubation. The percentage of TUNEL-positive bone marrow–derived megakaryocytes following culture for the indicated times in media with or without TPO is presented (left). Data are the mean ± SEM of 5 independent experiments. The same experiments performed on megakaryocytes derived from E13.5 to E15.5 fetal liver cells (n = 3) produced similar results (right). (C) DNA ploidy analysis of raf-1+/+ and raf-1-/- megakaryocytes stained with fluorescein isothiocyanate–conjugated antimouse CD41 antibody and propidium iodide. Data are from CD41+ megakaryocytes and are representative of 3 independent experiments that each produced the same results. DNA ploidy values ranging from 2N to 128N are indicated above their respective peaks. (D) Western blots demonstrate Raf-1, A-Raf, and B-Raf protein expression in purified mature megakaryocytes derived from raf-1+/+ (+/+) or raf-1-/- (-/-) bone marrow. Total protein (20 μg) was loaded in each lane, separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and probed with antibodies against Raf-1, B-Raf and A-Raf, and β-tubulin, the latter serving as an independent measure of protein loading. (E) A representative Western blot demonstrates similar kinetics and extent of TPO-induced ERK phosphorylation in purified raf-1-/- and raf-1+/+ BM-derived megakaryocytes. The proteins were separated by 10% SDS-PAGE, and blots were probed as indicated using anti–phospho ERK, anti–total ERK, or anti–Raf-1 antibody.
Raf-1 and megakaryocytopoiesis
While various kinase active forms of exogenously introduced Raf-1 can affect proliferation, differentiation, and apoptosis,14,18 and Raf-1 can abrogate cytokine dependency of hematopoietic cell lines,19 little is known about the role of endogenous Raf-1 in specific physiologic processes. To begin to understand the role of Raf-1 in megakaryocytopoiesis, low-density BMMNCs from raf-1-/- and raf-1+/+ littermates were cultured in TPO-supplemented serum-free liquid media. The expansion of CD41+ cells was not altered by the absence of Raf-1 (Figure 2A). Mesenchymal tissue from C57BL6 raf-1-/- mice and mouse embryonic fibroblasts (MEFs) from C57BL6 and MF1 raf-1-/- mice have increased levels of apoptosis relative to their raf-1+/+ counterparts.15 In contrast, the absence of Raf-1 had no detectable effect on the development of apoptotic megakaryocytes derived from bone marrow or fetal liver (Figure 2B). Late megakaryocytopoiesis involves DNA replication without cell division, generating a population of mature megakaryocytes with variable chromosome copy number, or DNA ploidy. Although MEK inhibitor studies implicate the classic MAP kinase pathway in this late step of megakaryocytopoiesis,7 there was no difference in the DNA ploidy distribution in bone marrow–derived megakaryocytes from raf-1-/- and raf-1+/+ mice (Figure 2C). Taken together, these in vitro findings fail to identify an essential, cell-autonomous role for Raf-1 in megakaryocyte lineage expansion, apoptosis, or differentiation. Moreover, the lack of a significant megakaryocytopoiesis abnormality in raf-1-/- mice is not due to compensatory expression of other Raf family members, A-Raf or B-Raf (Figure 2D).
As outlined in “Introduction,” TPO stimulates ERK phosphorylation, and Raf-1 is well known to activate MEK/ERK signaling. However, Raf-1 is dispensable for TPO-induced ERK1/2 phosphorylation in marrow-derived megakaryocytes (Figure 2E), a finding similar to that recently reported for raf-1-/- fibroblasts.15 The growing appreciation for MEK-independent roles of Raf-115,20,21 underscores the need for genetic approaches to more fully understand the MEK-dependent and MEK-independent roles of Raf family members in specific biologic processes such as megakaryocytopoiesis. Attention now turns to other likely MEK activators in megakaryocytopoiesis. As B-Raf has been identified as an important physiologic MEK activator in some cell types, including hematopoietic cells,22-24 genetic approaches to clarify its role in megakaryocytopoiesis are currently under way.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-06-1803.
Supported by the National Institutes of Health (NIH) Transfusion Medicine Specialized Centers of Research grant P50 HL54476 and NIH grant RO1 HL65198.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Jing Kang for excellent mouse colony management, Martin McMahon (UCSF) and Koji Eto (Scripps Research Institute) for many helpful discussions, and members of the Leavitt lab for ongoing support and discussions. We are grateful for the generous support of Genentech for recombinant human TPO and Johnson & Johnson for recombinant human EPO.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal