Abstract
Stem cell leukemia (SCL) protein has been shown to be an essential transcription factor during hematopoietic development in the embryo. In adult hematopoiesis, however, the role for SCL has remained largely unknown, whereas it is expressed in bone marrow hematopoietic stem cells (HSCs). In this study, we performed HSC transplantation and an in vitro HSC differentiation assay using retrovirally transduced HSCs with wild-type (WT) and dominant-negative (DN) SCL. The transplantation experiments showed that SCL does not affect the long-term repopulating capacity of HSCs but that WT SCL and DN SCL increase the short-term contribution of the transduced HSCs in myeloid and lymphoid lineages, respectively. An in vitro single-cell assay using a fetal thymus organ culture system further demonstrated that WT SCL facilitates HSCs to differentiate into the myeloid lineage but that DN SCL facilitates HSCs to differentiate into the lymphoid lineage. We conclude that the up-regulation or down-regulation of SCL directs HSCs toward myeloid or lymphoid lineage, respectively, although SCL does not affect their long-term repopulating capacity. (Blood. 2004;103: 3336-3341)
Introduction
Hematopoietic stem cells (HSCs) that can give rise to all lineages of blood cells are enriched in surface marker-defined populations, such as those that are lineage marker (Lin)-negative, c-Kit-positive, Sca-1-positive, and CD34-low/negative (Lin-c-Kit+Sca-1+CD34low/- [34-KSL]1 ) in adult mouse bone marrow. Recently, common myeloid progenitors (CMPs)2 and common lymphoid progenitors (CLPs)3 were identified by surface marker profiles as clonogenic cells that can generate all lineages of myeloid and lymphoid cells, respectively, without any potential to differentiate into one another. Therefore, the first step in blood production from an HSC must be initiated by commitment to a CMP2 and a CLP.3 This process, like other differentiation processes, is governed by an expression profile of transcription factors. Although a number of transcription factors are known to be involved in commitment and differentiation processes during hematopoiesis,4 none have been identified that play roles in the progression from an HSC to a CLP and a CMP. It was reported that CLPs and CMPs differ in the expression pattern of transcription factors: stem cell leukemia (SCL), a basic helix-loop-helix (bHLH) motif-containing transcription factor,5 and GATA2, a zinc finger motif-containing transcription factor, are expressed in HSCs and CMPs but not in CLPs.2
SCL appears to play roles in hematopoietic cell differentiation into myeloid, erythroid, megakaryocyte, and mast cell lineages because it is preferentially expressed during early cell stages in these lineages.6-12 Studies in knockout mice have revealed that SCL is indispensable in the early stages of primitive embryonic hematopoiesis, when it functions in the specification of ventral mesoderm to blood cells and in the formation or maintenance of immature blood progenitors.13,14 Studies using embryonic stem cells in vitro have demonstrated that SCL is necessary for the development of bipotent progenitors of blood and endothelial cells in vertebrates.15,16 Furthermore, ectopic expression of SCL promotes the proliferation and inhibits the apoptosis of 32D, a late myeloid progenitor cell line; these effects are dependent on the bHLH domain.17 Essential roles for SCL in megakaryopoiesis and erythropoiesis in the adult mouse bone marrow were recently demonstrated by conditional targeting of the SCL gene.18,19
To clarify the roles of SCL in the commitment process of an HSC to a CLP and a CMP, we performed HSC transplantation and an in vitro HSC differentiation assay using retrovirally transduced HSCs with wild-type (WT) and bHLH domain-deleted—that is, dominant-negative (DN)—SCL.9,20 Our data show that the overexpression of WT SCL in HSCs strikingly promotes myelopoiesis. In sharp contrast, the overexpression of DN SCL (down-regulation of SCL) in HSCs results in a marked bias toward lymphopoiesis.
Materials and methods
Mice
C57BL/6 (B6-Ly5.2) mice were purchased from SLC (Tokyo, Japan). Mice congenic for Ly5 locus (B6-Ly5.1) were bred and maintained at the University of Tsukuba Animal Research Center (Tsukuba, Japan).
Purification of HSCs and flow cytometric analysis
We purified 34-KSL cells as HSCs (Figure 1A) from murine bone marrow cells and analyzed the surface phenotypes of cells from peripheral blood, bone marrow, spleen, and thymus as described.21 All antibodies were purchased from PharMingen (San Diego, CA), as follows: biotinylated and unmodified sets of rat immunoglobulin G2b (IgG2b) antilineage markers Gr-1, B220, CD4, CD8, Mac1, and Ter119; fluorescence-labeled antibodies phycoerythrin (PE)-Gr-1, PE-Mac1, PE-B220, allophycocyanin (APC)-B220, APC-Thy1.2, PE-Ly5.1, PE-Sca-1, PE-CD71, APC-c-Kit, and fluorescein isothiocyanate (FITC)-antimurine CD34; biotinylated antimurine α subunit of the receptor for interleukin-7 (IL-7Rα); APC-streptavidin and peridin chlorophyll protein (PerCP)-Cy5.5-streptavidin. All cytokines except for recombinant mouse IL-7 were from Kirin Brewery Research Laboratory (Takasaki, Japan). IL-7 was purchased from PEPROTECH EC (London, United Kingdom).
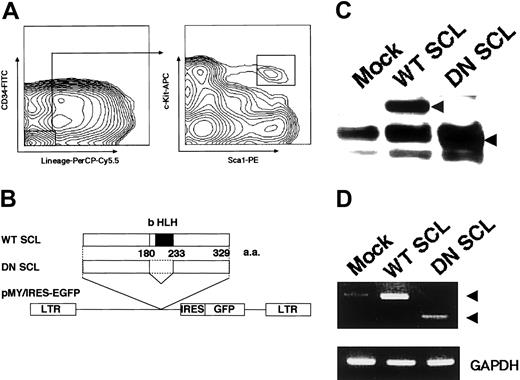
Construction of retroviral vector and target HSC for gene transduction. (A) Flow cytometric analysis of murine hematopoietic stem cells in adult bone marrow. Staining profile of lineage marker versus CD34 for lineage-depleted cells (left) and Sca-1 versus c-Kit in lineage-/CD34-/low-gated populations (right) in bone marrow is shown. (B) Schematic representation of the retroviral vector, pMY/WT SCL and pMY/DN SCL, encoding WT SCL and DN SCL linked by IRES to a cDNA encoding enhanced GFP (EGFP). (C) Expression of WT SCL and DN SCL proteins in retrovirally transduced 32D cells. Arrowheads indicate approximately 37 kDa (lane WT SCL) and 29 kDa (lane DN SCL). The other bands commonly seen in all the lanes, including the Mock control lane, represent nonspecific reaction of the antibody. (D) Expression of SCL mRNA in retrovirally transduced HSCs. Arrowheads indicate 308-base pair (bp) (lane WT SCL) and 170-bp (lane DN SCL) transcripts, the latter of which resulted from the deletion of a 138-bp sequence corresponding to the bHLH domain.
Construction of retroviral vector and target HSC for gene transduction. (A) Flow cytometric analysis of murine hematopoietic stem cells in adult bone marrow. Staining profile of lineage marker versus CD34 for lineage-depleted cells (left) and Sca-1 versus c-Kit in lineage-/CD34-/low-gated populations (right) in bone marrow is shown. (B) Schematic representation of the retroviral vector, pMY/WT SCL and pMY/DN SCL, encoding WT SCL and DN SCL linked by IRES to a cDNA encoding enhanced GFP (EGFP). (C) Expression of WT SCL and DN SCL proteins in retrovirally transduced 32D cells. Arrowheads indicate approximately 37 kDa (lane WT SCL) and 29 kDa (lane DN SCL). The other bands commonly seen in all the lanes, including the Mock control lane, represent nonspecific reaction of the antibody. (D) Expression of SCL mRNA in retrovirally transduced HSCs. Arrowheads indicate 308-base pair (bp) (lane WT SCL) and 170-bp (lane DN SCL) transcripts, the latter of which resulted from the deletion of a 138-bp sequence corresponding to the bHLH domain.
Plasmid construction
The cDNA fragment for SCL was obtained from a library made from an embryonic day-14 (E14) mouse embryo (a gift from Kirin Brewery Research Laboratory) by using a polymerase chain reaction (PCR) method based on the published sequence. DN SCL devoid of the DNA-binding domain (amino acid residues 180-233) was constructed with 2 PCR fragments. One of the PCR fragments was generated with primers 1 and 2 (ACAGAATTCTCTAAATATGCCCCAGGATGACGGAG and CACTACTTTGGTGTGAGGACCATCAG); the other fragment was generated with primers 3 and 4 (AAGTACATCAATTTCCTGGCCAAGTTACT and AAACTCGAGTCACCGGGGGCCAGCCCCATCA). A Flag sequence 5′-GACTACAAAGACGATGACGATAAATGA-3′) was fused in-frame to the 3′ end of the cDNAs. The obtained SCL and DN SCL fragments were cloned into pMY IRES-GFP,22 which allows the expression of green fluorescence protein (GFP) from an internal ribosomal entry site (IRES) (Figure 1B).
Retroviral gene transfer
Western blot analyses and reverse transcription-polymerase chain reaction
32D cells, which were infected with retrovirus and were selected by the expression of GFP, were cultured in RPMI 1640 medium containing 10% fetal calf serum (FCS) with 10 ng/mL mouse interleukin-e (IL-3). They were harvested in TNE buffer (10 mM Tris-HCl, pH 7.8, 150 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 1% NP-40) with protease inhibitor cocktail (Sigma, St Louis, MO) and were sonicated by Handy Sonic HR-20P (Tomy Seiko, Tokyo, Japan). Cell lysates were immunoprecipitated with anti-FLAG M2-Agarose Affinity Gel (Sigma), and Flag fusion proteins were eluted by competition with Flag peptide (Sigma) according to the manufacturer's instructions. The proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis using anti-Flag M2 monoclonal antibody (Sigma) and were detected by antimouse IgG antibody conjugated with horseradish peroxidase (DAKO, Glostrup, Denmark).
Reverse transcription-polymerase chain reaction (RT-PCR) analysis was performed as described previously.21 Primer pairs were: GAPDH, 5′-GCATTGTGGAAGGGCTCATG-3′ and 5′-TTGCTGTTGAAGTCGCAGGAG-3′; SCL, 5′-CTAGGCAGTGGGTTCTTTGG-3′ and 5′-TCCTCCTCCTGGTCATTGAG-3′.
Colony, noncompetitive and competitive reconstitution, and MLP assays
Colony and transplantation assays were performed as previously described.21 To perform a competitive long-term reconstitution (CLTR) assay and to evaluate whether SCL affects the long-term repopulating capacity of 34-KSL cells, competitor cells were prepared by culturing 34-KSL cells without retroviral transduction in the same conditions used for transducing 34-KSL cells. Competitor cells and GFP-sorted transduced cells (test cells) (1000 each) were then transplanted into irradiated recipient mice and were analyzed for the contribution of test cells against competitor cells (ie, ratio of GFP+ cells among donor [Ly5.1] cells) in the recipients' blood at various times. For noncompetitive assays, we transplanted 2000 transduced cells and 10 000 lineage-depleted fresh bone marrow cells into lethally irradiated recipient mice. A multilineage progenitor (MLP) assay was performed according to the original method previously described.23 In brief, to prepare hematopoietic cell-depleted fetus thymic lobes, thymi obtained from B6 fetuses 15 days after coitus were cultured on polycarbonate filters (pore size, 8 μm) (Nuclepore, Pleasanton, CA) floating on culture medium containing dGuo (1.35 mM) for 6 days. A single HSC placed in each well of a 96-well V-bottom plate was cultured together with a washed deoxyguanosine (dGuo)-treated lobe in the medium supplemented with recombinant murine IL-7 (rmIL-7) (100 U/mL). Plates were placed into a plastic bag (Ohmi Oder Air Service, Hikone, Japan) in which the air had been replaced by a gas mixture (70% O2, 25% N2, 5% CO2) and were incubated for 12 days.
Results
Effects of retrovirally transduced WT SCL and DN SCL on the properties of HSCs
First, we confirmed the expression of Flag-tagged WT SCL and DN SCL proteins in retrovirally transduced 32D cells (Figure 1C). The predicted molecular weight from an amino acid sequence of WT SCL is 34276.6 Da, and from an amino acid sequence of DN SCL it is 28764.46 Da. We next examined the expression of mRNA in retrovirally transduced HSCs (Figure 1D). In WT SCL-transduced HSCs, the level of SCL mRNA expression was higher than that of Mock GFP-transduced HSCs. mRNA for DN SCL was detected only in DN SCL-transduced HSCs.
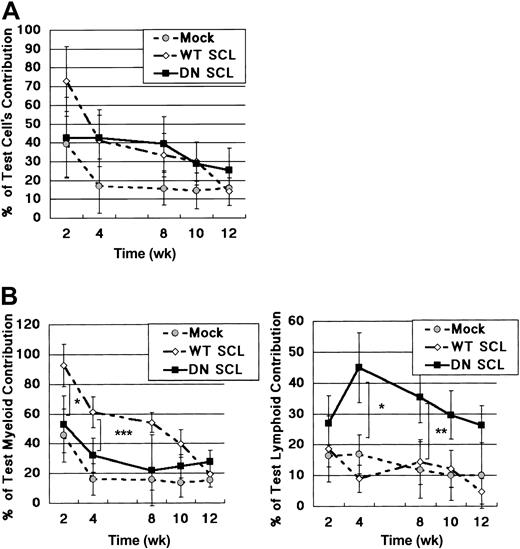
In a CLTR assay, the contribution of WT SCL- and DN SCL-transduced HSCs was slightly greater than that of Mock GFP-transduced HSCs when the chimerism was accessed shortly after transplantation (Figure 2A). However, the ratios of GFP+ cells gradually decreased, and, at 12 weeks after transplantation, they were as low as approximately 20%, irrespective of introduced genes (Figure 2A). We previously compared in detail the number of the GFP+ cells and congenic marker (Ly5.1+) cells at different time points after transplantation. Silencing of GFP expression did occur, but its ratio was less than 20% by 3 months after transplantation (data not shown). Thus, the decrease in GFP+ cells in the current study is attributed mainly to the decrease in donor-derived cells rather than to the silencing of GFP expression, indicating that the long-term repopulating capacity of retrovirally transduced (ie, GFP+) HSCs was less than that of nontransduced HSCs, regardless of whether WT SCL, DN SCL, or Mock virus was introduced.
Effects of retrovirally mediated WT SCL and DN SCL on stem cell activities. (A) Analysis of total peripheral blood after competitive reconstitution assay. (B) Lineage-specific contribution in peripheral blood from recipient mice. (left) Percentage of GFP+ cells in the donor-derived Mac1+/Gr1+ cells. (right) Percentage of GFP+ cells in the donor-derived Thy1.2+/B220+ cells (percentage of GFP+/CD45.1+/Mac1+/Gr1+ or CD45.1+/Thy1.2+/B220+ cells of recipient mice). Numbers of recipient mice in each group were between 6 and 9 in panels A and B. Plots are shown as the mean ± SD. *P < .1; **P < .01; and ***P < .0001.
Effects of retrovirally mediated WT SCL and DN SCL on stem cell activities. (A) Analysis of total peripheral blood after competitive reconstitution assay. (B) Lineage-specific contribution in peripheral blood from recipient mice. (left) Percentage of GFP+ cells in the donor-derived Mac1+/Gr1+ cells. (right) Percentage of GFP+ cells in the donor-derived Thy1.2+/B220+ cells (percentage of GFP+/CD45.1+/Mac1+/Gr1+ or CD45.1+/Thy1.2+/B220+ cells of recipient mice). Numbers of recipient mice in each group were between 6 and 9 in panels A and B. Plots are shown as the mean ± SD. *P < .1; **P < .01; and ***P < .0001.
We found, however, that the contribution of WT SCL-transduced cells was greater than that of the DN SCL- or the Mock GFP-transduced cells in the myeloid lineage (Figure 2B, left) and, conversely, that the contribution of DN SCL-transduced cells was greater than that of the WT SCL- or the Mock GFP-transduced cells in the lymphoid lineage (Figure 2B, right) until 12 weeks after transplantation if the chimerism was separately characterized in myeloid (Mac1+ or Gr1+) and lymphoid (Thy1.2+ or B220+) lineages.
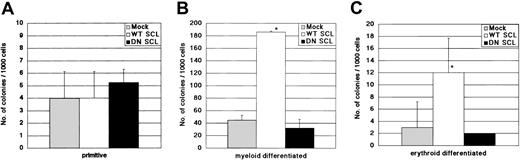
In a methylcellulose colony-forming assay using the retrovirally transduced GFP+ cells, we found that neither WT SCL nor DN SCL affected the number of immature progenitor-derived colonies compared with the Mock GFP vector (Figure 3A). WT SCL-transduced cells, however, gave rise to progenitors that formed 4 times as many mature myeloid and erythroid colonies than those of Mock GFP-transduced cells (Figure 3B-C).
In vitro colony-forming potential of retrovirally gene-transduced cells. Numbers of mixed (A), myeloid (B), and erythroid (C) colonies are shown. Data show the mean ± SD of triplicates. Similar data were obtained in 2 independent experiments.*P < .1.
In vitro colony-forming potential of retrovirally gene-transduced cells. Numbers of mixed (A), myeloid (B), and erythroid (C) colonies are shown. Data show the mean ± SD of triplicates. Similar data were obtained in 2 independent experiments.*P < .1.
Up-regulation or down-regulation of SCL in HSCs influences the distribution of progeny in recipient mice
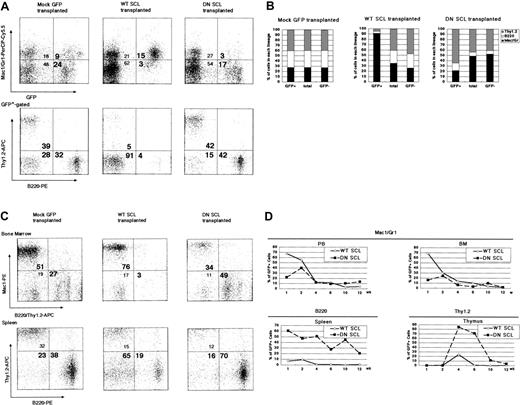
CLTR assay results suggested that SCL had no effect on the maintenance of multipotent HSCs but that it affected the extent of contribution to mature cells from myeloid or lymphoid lineage progenitors. To focus on the lineage commitment of HSCs, we next performed a noncompetitive reconstitution assay. In the blood from recipients of transplanted Mock GFP-transduced HSCs, the ratio of Mac1+ or Gr1+ (hereafter Mac1+/Gr1+) cells in the GFP+ cells was essentially the same as that in total blood cells 6 weeks after transplantation (Figure 4A, upper left panel). In GFP+ cells from recipients of WT SCL-transduced HSCs, however, the Mac1+/Gr1+ cell ratio was markedly higher than that in the total blood cells (Figure 4A, upper middle panel). This corresponded to the decreased ratio of the sum of B220+ cells and Thy1.2+ cells (Figure 4A, lower middle panel). In contrast, the ratio of Mac1+/Gr1+ cells in the GFP+ cells was markedly lower than that in the total blood cells from the recipients of transplanted DN SCL-transduced HSCs (Figure 4A, upper right panel), which corresponded to the increase in the sum of B220+ cells and Thy1.2+ cells (Figure 4A, lower right panel). These results were consistent in all recipients (Figure 4B).
Contribution of WT SCL- and DN SCL-transduced donor-derived cells in myeloid and lymphoid lineages 6 weeks after transplantation. (A) Results of representative FACS analysis of peripheral blood. Profiles for GFP and Mac1 or Gr1 in peripheral blood cells (upper panels) and B220 and Thy1.2 in GFP+-gated peripheral blood subpopulations (lower panels) are shown. (B) Mean ratios of T, B, and myeloid lineage cells in GFP+-gated, nongated, or GFP--gated peripheral blood subpopulations in recipient mice receiving Mock GFP-transduced cells (SD = 0.02-0.04), WT SCL-transduced cells (SD = 0.19-0.60), and DN SCL-transduced-cells (SD = 0.21-0.22). (C) Results of representative FACS analysis of bone marrow and spleen. (D) Chimerism of GFP+ cells in myeloid cells from peripheral blood and bone marrow (upper panels), in B220+ cells from spleen (lower left), and in Thy1.2+ cells from thymus (lower right). (A-D) Three, 7, and 5 recipient mice were analyzed during Mock GFP-transduced, WT SCL-transduced, and DN SCL-transduced cell transplantation, respectively. Numbers in each quadrant (A,C) represent the percentage of cells in the respective quadrants.
Contribution of WT SCL- and DN SCL-transduced donor-derived cells in myeloid and lymphoid lineages 6 weeks after transplantation. (A) Results of representative FACS analysis of peripheral blood. Profiles for GFP and Mac1 or Gr1 in peripheral blood cells (upper panels) and B220 and Thy1.2 in GFP+-gated peripheral blood subpopulations (lower panels) are shown. (B) Mean ratios of T, B, and myeloid lineage cells in GFP+-gated, nongated, or GFP--gated peripheral blood subpopulations in recipient mice receiving Mock GFP-transduced cells (SD = 0.02-0.04), WT SCL-transduced cells (SD = 0.19-0.60), and DN SCL-transduced-cells (SD = 0.21-0.22). (C) Results of representative FACS analysis of bone marrow and spleen. (D) Chimerism of GFP+ cells in myeloid cells from peripheral blood and bone marrow (upper panels), in B220+ cells from spleen (lower left), and in Thy1.2+ cells from thymus (lower right). (A-D) Three, 7, and 5 recipient mice were analyzed during Mock GFP-transduced, WT SCL-transduced, and DN SCL-transduced cell transplantation, respectively. Numbers in each quadrant (A,C) represent the percentage of cells in the respective quadrants.
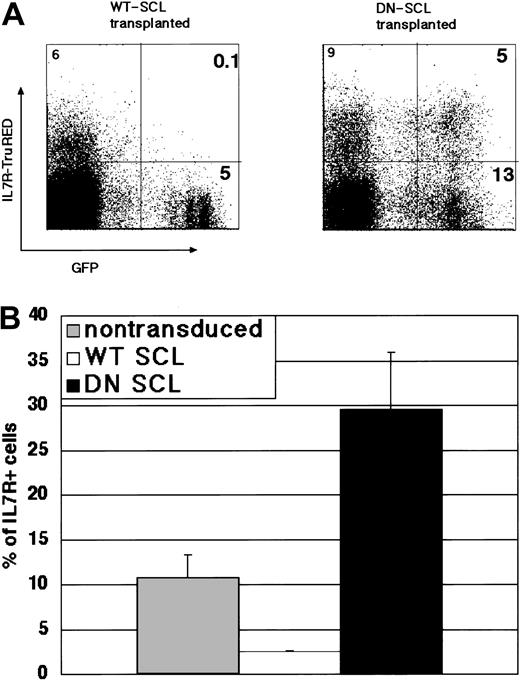
Next, we examined the myeloid and lymphoid cell populations in each hematopoietic organ. In the GFP+ bone marrow cells 6 weeks after transplantation, more than 75% Mac1+/Gr1+ cells—less than 3% B220+ or Thy1.2+ (B220+/Thy1.2+) cells and less than 35% Mac1+/Gr1+ cells—more than 45% B220+/Thy1.2+ cells were observed in the recipients of transplanted WT SCL- and DN SCL-transduced HSCs, respectively, whereas 51% Mac1+/Gr1+ and 27% B220+/Thy1.2+ cells were in the GFP+ cells from Mock GFP-transduced HSCs (Figure 4C, upper panel). In most of the recipients of DN SCL-transduced HSCs, the ratio of B220+ cells was increased in the GFP+ population compared with that in the total population of spleen cells (Figure 4C, lower panel). Changes in the ratios of Mac1+/Gr1+ cells in the blood and bone marrow, B220+ cells in the spleen, and Thy1.2+ cells in the thymus, collectively, showed that WT SCL and DN SCL facilitated the contributions from donor cells to myeloid and lymphoid lineages, respectively (Figure 4D). It was suggested that persistent up-regulation or down-regulation of SCL in HSCs influences their commitment to myeloid or lymphoid progenitors. Furthermore, we found marked decreases and increases in the number of cells expressing IL-7Rα in the GFP+ bone marrow cells from recipients receiving WT SCL- and DN SCL-transduced HSCs (Figure 5A-B). This observation supports the possibility that SCL influences the cell fate of an HSC at the bifurcation of myeloid and lymphoid lineages because IL-7Rα is expressed in immature progenitor cells and because IL-7Rα is one of the key markers discriminating CLPs from CMPs.
IL-7Rα-expressing cells in the donor-derived cells from bone marrow of recipients of transplanted WT SCL- and DN SCL-transduced HSCs. (A) Results of representative FACS analysis 10 weeks after transplantation. Numbers in quadrants represent the ratio of cells in the respective quadrants. (B) Mean of IL-7α-expressing cells in GFP+-gated or nongated subpopulations in lineage-depleted bone marrow cells from recipients. Data show the mean ± SD of 2 to 4 mice from 2 independent experiments.
IL-7Rα-expressing cells in the donor-derived cells from bone marrow of recipients of transplanted WT SCL- and DN SCL-transduced HSCs. (A) Results of representative FACS analysis 10 weeks after transplantation. Numbers in quadrants represent the ratio of cells in the respective quadrants. (B) Mean of IL-7α-expressing cells in GFP+-gated or nongated subpopulations in lineage-depleted bone marrow cells from recipients. Data show the mean ± SD of 2 to 4 mice from 2 independent experiments.
In vitro single-cell progenitor assay demonstrates that WT SCL and DN SCL direct HSCs to opposite lineages
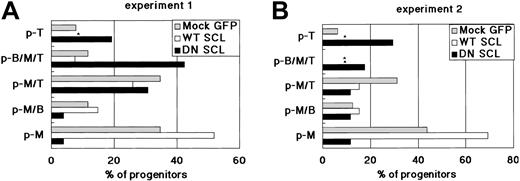
To obtain further evidence that SCL affects the commitment process of HSCs rather than the expansion of myeloid progenitors, we examined the developmental capacity of individual hematopoietic progenitors to generate T, B, and myeloid (M) cells using an MLP assay.23 Cell growth sufficient to perform fluorescence-activated cell sorter (FACS) analysis was achieved in approximately 50% of the wells (data not shown), in each of which a single sorted cell was cultured together with a dGuo-treated fetal thymic lobe. A single cell was designated as p-M, p-T, p-B, p-MT, p-MB, p-TB, or p-MTB if the cell population in the well expressed only Mac1/Gr1, Thy1.2, B220, Mac1/Gr1 and Thy1.2, Mac1/Gr1 and B220, Thy1.2 and B220, or Mac1/Gr1, Thy1.2, and B220, respectively. WT SCL-transduced HSCs generated a higher number of p-M and a lower number of p-T than Mock GFP-transduced HSCs (Figure 6A-B). Indeed, p-T was not detected in the cells derived from WT SCL-transduced HSCs in repeated experiments. Conversely, DN SCL-transduced HSCs generated a higher number of p-T and a lower number of p-M than Mock GFP-transduced HSCs (Figure 6A-B). Generation of p-MT and p-MB was not affected by the introduction of WT SCL or DN SCL into HSCs. If only the expansion of myeloid or lymphoid progenitors takes place and the myeloid or lymphoid commitment is not affected by the up-regulation or down-regulation of SCL, the ratio of the number of p-M-containing wells to that of p-T-containing wells would not be altered, and only the cell number of each well would be affected. Results of the MLP assay combined with those of the reconstitution assay described here strongly suggest that the up-regulation of SCL actively promotes an HSC to the myeloid lineage and that the down-regulation of SCL results in the facilitated lymphoid commitment of HSCs.
Frequency of different types of progenitors in WT SCL- and DN SCL-transduced HSCs. Progenitor types were determined by analyzing the cells generated from a single cell. Results of 2 independent experiments (A-B) are shown. Asterisks indicate the wells in which the indicated type of progenitor was undetectable. At least 3000 hematopoietic cells per well were analyzed using FACS.
Frequency of different types of progenitors in WT SCL- and DN SCL-transduced HSCs. Progenitor types were determined by analyzing the cells generated from a single cell. Results of 2 independent experiments (A-B) are shown. Asterisks indicate the wells in which the indicated type of progenitor was undetectable. At least 3000 hematopoietic cells per well were analyzed using FACS.
Erythroblast maturation of transduced HSCs in recipient bone marrow
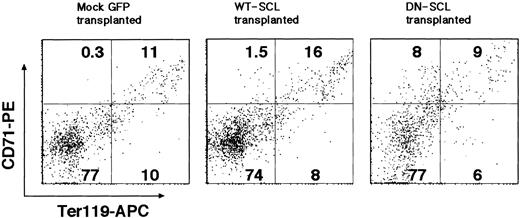
SCL expression is closely associated with erythroid maturation. We analyzed bone marrow cells from mice that underwent transplantation for erythroid differentiation markers. We found an increase in the frequency of Ter119low/- CD71+ cells representing an immature erythroid population24 in GFP+ bone marrow cells from recipients receiving DN SCL-transduced HSCs (Figure 7). In GFP+ cells derived from WT SCL-transduced HSCs, however, the frequency of the Ter119+CD71+ cells, more mature erythroid cells, was slightly increased (Figure 7), suggesting that SCL also affects the maturation of erythroid cells, as expected from the previous report.18,19
Perturbed erythropoiesis in bone marrow of recipients of transplanted DN SCL-transduced HSCs. FACS analysis of single-cell suspensions of GFP+-gated bone marrow cells immunostained with Ter119 and CD71 antibodies is shown. Numbers in quadrants represent the ratio of cells in the respective quadrants.
Perturbed erythropoiesis in bone marrow of recipients of transplanted DN SCL-transduced HSCs. FACS analysis of single-cell suspensions of GFP+-gated bone marrow cells immunostained with Ter119 and CD71 antibodies is shown. Numbers in quadrants represent the ratio of cells in the respective quadrants.
The hypothesis of lineage commitment of an HSC through SCL signaling is consistent with the expression pattern of SCL, which is positive in HSCs, CMPs, and their progeny but is negative in CLPs.2 The results demonstrated in this report suggest that SCL is the most obvious candidate molecule determining the cell fate of HSCs toward CMPs or CLPs.
Discussion
Mice without the SCL gene in adult HSCs were recently established by a conditional gene-targeting method.18,19 In these studies, SCL was shown to be dispensable for HSC properties, such as long-term repopulating activity and multipotency, but not for proper erythroid and megakaryocyte generation. In the current study, we observed similar abnormalities induced by DN SCL during erythroid differentiation (Figure 7), but megakaryocytic differentiation was not changed by the transduction of WT SCL or DN SCL, as judged by the expression of CD61 (data not shown). Our major observation that the down-regulation of SCL results in predominant lymphoid commitment is also supported by results of a conditional knockout (cKO) mouse study.19 Cre-mediated excision of SCL resulted in a significant increase in the number of T cells in the peripheral blood of HSC recipients. The number of B cells did not appear to be influenced in the cKO mice, and this result could represent a difference from our result. However, the number of mice analyzed in the cKO study18,19 does not appear to be sufficiently large. Therefore, we speculate that the decrease in the number of B cells in the cKO mice might be observed if the number of mice examined were large enough, given that we observed large mouse-to-mouse variation in T- and B-cell ratios in the GFP+ population. If so, data described in the cKO studies are very similar to ours using DN SCL-transduced HSCs, including the findings that continued expression of SCL is not essential for the maintenance of HSCs.
Our study, however, is the first to shed light on the fact that SCL influences the polarity of an HSC toward myeloid or lymphoid commitment because WT SCL and DN SCL guide HSCs to opposing commitment pathways. Previously, the exogenous expression of SCL was reported to support proliferation and to inhibit apoptosis of 32D myeloid cells17 and CD34+ bone marrow cells11,12 or to stimulate erythromegakaryocytic lineage progenitor cells and to inhibit monomyelocytic lineage cell differentiation. These data and ours apparently show a discrepancy. We used the most highly purified HSCs from bone marrow, which might be a plausible cause of the discrepancies, while others used less purified mouse cells or human cells. It is likely that we observed early differentiation events from HSCs rather than mature lineage differentiation events from progenitors.
Thus, we propose that SCL controls the generation of CMPs and CLPs from HSCs without affecting the self-renewal activity of HSCs. It is of interest whether other transcription factors such as GATA-2, NF-E2, and C/EBPα, which are expressed in CMP but not in CLP, and Aiolos and GATA-3, which are expressed in CLP but not in CMP, play a similar role.
The authors of the reports describing SCL cKO mouse characterization did not conclude that lymphoid commitment was facilitated at the expense of myeloid commitment.18,19 This could suggest that DN SCL might be stronger than SCL cKO in influencing HSCs. If so, it is possible that DN SCL interferes with bHLH motif-containing transcription factors other than SCL and that such transcription factors substitute for the function of SCL in SCL cKO mice. Direct comparison of mice receiving DN SCL-transduced HSCs with SCL cKO mouse-derived HSCs could help to address this issue.
Although CMPs and CLPs as the direct progeny of HSCs has been described, no molecular backgrounds controlling the transition from HSC to progenitor has been characterized. Changes in the expression profiles of transcription factors, including bHLH motif-containing ones, must represent the most important determinant. Our data indicate that a change in SCL activity is the first candidate of such a determinant.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-06-1935.
Supported by KAKENHI nos. 13307029 and 13218021 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT); KAKENHI no. 14370300 from the Japan Society for the Promotion of Science; Special Coordination Funds for Promoting Science and Technology from MEXT; and Health and Labor Sciences Research Grants on Pharmaceutical and Medical Safety from the Ministry of Health, Labor, and Welfare of Japan.
Performed through Special Coordination Funds for Promoting Science and Technology, MEXT, Japan; Grant-in-Aid for Scientific Research on Priority Areas, KAKENHI (13218021) from MEXT, Japan; Grant-in-Aid for Scientific Research, KAKENHI (13557080 and 14370300 JSPS).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank H. Nakauchi (Institute of Medical Science, University of Tokyo) for the Ly5.1 mice, T. Kitamura (Institute of Medical Science, University of Tokyo) for the pMY retrovirus, K. Nagao (Kirin Brewery Research Laboratory) for the mouse embryo cDNA library, T. Yoshimatsu (Wakunaga Pharmaceutical Co.) for the ψMP34, and H. Kawamoto (University of Kyoto) for advice regarding the MLP assay.
Author notes
Hisamaru Hirai died suddenly on August 23, 2003. His students, fellows, and colleagues will greatly miss his energetic and nurturing leadership in the field of hematology. We dedicate this paper in his memory.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal