Abstract
Acute myeloid leukemia (AML) is characterized by the block of differentiation, deregulated apoptosis, and an increased self-renewal of hematopoietic precursors. It is unclear whether the self-renewal of leukemic blasts results from the cumulative effects of blocked differentiation and impaired apoptosis or whether there are mechanisms directly increasing self-renewal. The AML-associated translocation products (AATPs) promyelocytic leukemia/retinoic acid receptor α (PML/RARα), promyelocytic leukemia zinc finger (PLZF)/RARα (X-RARα), and AML-1/ETO block hematopoietic differentiation. The AATPs activate the Wnt signaling by up-regulating γ-catenin. Activation of the Wnt signaling augments self-renewal of hematopoietic stem cells (HSCs). Therefore, we investigated how AATPs influence self-renewal of HSCs and evaluated the role of γ-catenin in the determination of the phenotype of HSCs expressing AATPs. Here we show that the AATPs directly activate the γ-catenin promoter. The crucial role of γ-catenin in increasing the self-renewal of HSCs upon expression of AATPs is demonstrated by (i) the abrogation of replating efficiency upon hindrance of γ-catenin expression through RNA interference, and (ii) the augmentation of replating efficiency of HSCs upon overexpression of γ-catenin itself. In addition, the inoculation of γ-catenin-transduced HSCs into irradiated recipient mice establishes the clinical picture of AML. These data provide the first evidence that the aberrant activation of Wnt signaling by the AATP decisively contributes to the pathogenesis of AML. (Blood. 2004;103:3535-3543)
Introduction
Acute myeloid leukemias (AMLs) are characterized by an abnormal accumulation of hematopoietic progenitors in the bone marrow (BM), leading to the hematopoietic insufficiency responsible for the clinical picture of AMLs. The AML phenotype seems to be maintained by an accelerated proliferation of blast cells in the BM considered to be due to a combination of 2 components: the block of differentiation-hindering progenitors to reach the postproliferative stage and to subsequently undergo apoptosis, and the increase of self-renewal of the leukemic progenitors.
AMLs are frequently associated with specific chromosomal translocations, such as t(15;17), t(11;17), and t(8;21), resulting in the generation of chimeric genes.1,2 These genes encode chimeric transcription factors, such as promyelocytic leukemia/retinoic acid receptor α (PML/RARα), promyelocytic leukemia zinc finger (PLZF)/RARα, and AML-1/ETO, which deregulate transcription of target genes of their physiologic counterparts.1-3 Accordingly, the expression of PML/RARα as well as of PLZF/RARα in animal models leads to the development of a leukemic phenotype.4-6 In contrast, the expression of AML-1/ETO alone induces alterations of hematopoiesis in vivo but does not lead to the development of leukemia.7,8
In different cell line models these AML-associated translocation products (AATPs) induce a differentiation block by interfering with chromatin remodeling through aberrant recruitment of histone-deacetylases (HDACs) to promoters of genes relevant for differentiation.9 Increased HDAC activity hinders the access of the transcription machinery to these chromatin segments.10-12 Furthermore, AATPs interact physically with transcription factors indispensable for differentiation, such as the vitamin D3 (VitD3) receptor (VDR) in VitD3-induced monocytic differentiation. As a consequence, VDR is dislocalized and becomes unable to activate its target genes.13
The contribution of impaired apoptosis to the pathogenesis of AML still remains to be defined. Wild-type PML plays a crucial role in a variety of apoptosis-signaling pathways and in the regulation of p53 activity.14-18 The PML/RARα fusion protein encoded by the t(15;17) translocation leads to the disruption of the functional PML structures, the so-called PML nuclear bodies, suggesting that at least this AATP interferes with apoptosis.14,15
The ability of leukemic progenitors for increased self-renewal may result from the combination of the differentiation block and the reduced apoptosis rate. In addition there could be factors inducing increased self-renewal independently of the differentiation block and impaired apoptosis. Recently, it has been shown that the activation of the Wnt-signaling pathway is able to promote self-renewal in hematopoietic stem cells (HSCs). Wnt3a activates β-catenin by stabilizing it in the cytosol and induces known Wnt targets such as MYC, cyclin D1, and MSX1.19 Furthermore, overexpression of β-catenin, the main downstream mediator of Wnt signaling, elevates self-renewal of HSCs through the canonical Wnt pathway mediated by the lymphoid enhancer factor 1/T-cell transcription factor (LEF-1/Tcf) complex.20 Also, the activation of Wnt signaling in human HSCs leads to an increase of self-renewal. In fact, the exposure of human HSCs to Wnt5a further augments the number of nonobese diabetic-severe combined immunodeficiency (NOD-SCID) repopulating cells.21
We have recently shown that PML/RARα, PLZF/RARα, as well as AML-1/ETO activate Wnt signaling by increasing γ-catenin expression.22 γ-Catenin is closely related to β-catenin and is able to transform cells, whereas β-catenin exhibits a transforming capacity only when it is constitutively stabilized by mutation.23 In order to delineate the contribution of γ-catenin in leukemogenesis, we studied the impact of γ-catenin in determining the phenotype caused by expression of AATPs in HSCs.
Materials and methods
Plasmids
The retroviral vectors used in this study were all derived from the bicistronic PINCO vector. PINCO-PML/RARα was kindly provided by P. G. Pelicci (European Institute of Oncology, Milan, Italy) and PINCO-γ-catenin was provided by C. Müller-Tidow (Münster University, Münster, Germany). PINCO-PLZF/RARα was created using the unique EcoRI site of PINCO. The pGL3-γ-catenin construct was kindly provided by G. Brabant (MTH, Hannover, Germany). The pGL3basic and pRL-cytomegalovirus (pRL-CMV) vectors were purchased from Promega (Mannheim, Germany). The small interference RNA (siRNA) expression vector vPGKpuroU3U6 was constructed by inserting the human U6 promoter from -265 bp to -1 bp into the U3 region of vPGKpuro vector using BbsI and HpaI restriction sites. The vPGKpuro was obtained by replacing the SV40 promoter driving the puromycin resistance by the mouse phosphoglycerate kinase 1a promoter in a pBABEpuro derivative, lacking all promoter and enhancer sequences in the long terminal repeat (LTR).
Cell lines
Mock-transfected U937 MT, PML/RARα-expressing U937 P/R9,24 PLZF/RARα-expressing U937 B412,25 and AML-1/ETO-expressing U937 A/E cells13 were maintained in RPMI1640 (Invitrogen, Karlsruhe, Germany) plus 10% fetal calf serum (FCS; Invitrogen). The 32D cells were maintained in RPMI1640 plus 10% FCS supplemented with 10 ng/mL of recombinant murine-interleukin 3 (rm-IL3; Cell Concepts, Umkirch, Germany). The ecotropic Phoenix packaging cells were cultured in Dulbecco modified Eagle medium (DMEM; Invitrogen) plus 10% FCS.
Western blotting
Western blotting was performed according to widely established protocols. Anti-RARα (C20) and anti-γ-catenin (H80) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), the anti-β-tubulin antibody (DMB1B) was from Calbiochem (Darmstadt, Germany), and secondary horseradish-peroxidase-conjugated antibodies were from Dianova (Hamburg, Germany). For “stripping,” the blots were treated with Restore Western Blot Stripping Buffer according to the manufacturer's instructions (Pierce, Rockford, IL).
Transactivation assay
The pRL-CMV plasmid (12.5 μg) was cotransfected with 40 μg pGL3-γ-catenin or pGL3basic into U937 MT, U937 P/R9, U937 B412, and U937 A/E cells by electroporation as described previoulsy.25 Sixteen hours later transgene expression was induced by the exposure of the cells to 100 μM Zn2SO4 (Sigma, Steinheim, Germany). Twenty-four hours after Zn2+ treatment luciferase activity was determined using the Dual-luciferase Reporter Assay System (Promega) according to the manufacturer's instructions.
Isolation of Sca1+/lin- HSCs
Sca1+/lin- HSCs were isolated from female C57BL/6N mice from 6 to 12 weeks of age (Charles River, Sulzfeld, Germany) killed by CO2 asphyxiation. BM was harvested from femora and tibiae by flushing the bones with a syringe and 26-gauge needle. Sca1+ cells were purified by immunomagnetic beads using the magnetic-activated cell separation (MACS) cell separation columns according to the manufacturer's instruction (Miltenyi, Bergisch-Gladbach, Germany). Sca1+ cells were “lineage depleted” by labeling the cells with biotin-conjugated lineage panel antibodies B220, CD3ϵ, Gr-1, macrophage antigen-1 (Mac-1), TER-119 (Pharmingen, San Diego, CA). Labeled cells were removed using streptavidin-loaded MACS cell separation columns (Miltenyi). Purified cells were prestimulated prior to further use for 2 days in medium containing mIL-3 (20 ng/mL), mIL-6 (20 ng/mL), and murine stem cell factor (mSCF; 100 ng/mL; Cell Concepts).
Retroviral infection
Phoenix cells were transfected with retroviral vectors as described before.13 Retroviral supernatant was collected at days 2 and 3 after transfection. Target cells were plated onto retronectin-coated (Takara-Shuzo, Shiga, Japan) nontissue culture-treated 24-well plates and exposed to the retroviral supernatant for 3 hours at 37°C in the presence of 4 μg/mL polybrene (Sigma). Cells were centrifuged at 600 g for 45 minutes. Infection was repeated 4 times and infection efficiency had to be at least 70% as assessed by the detection of green fluorescent protein (GFP)-positive cells by fluorescence-enhanced cell sorting (FACS). Differences of infection efficiency between the samples did not exceed 10%.
Colony assays, replating efficiency, differentiation
At day 5 after infection, Sca1+/lin- cells were plated at 5000 cells/mL into methylcellulose supplemented with mIL-3 (20 ng/mL), mIL-6 (20 ng/mL), and mSCF (100 ng/mL; StemCell Technologies, Vancouver, BC, Canada). On day 10 after plating, the colony number was counted. After washing out from methylcellulose, cells were cytocentrifuged for morphologic analysis and stained for the determination of surface marker expression by FACS. Five thousand cells/plate were plated again in methylcellulose determining replating efficiency by serial plating. Differentiation was assessed by May-Grünwald-Giemsa staining of the cytospins as well as by expression of c-kit, Sca-1, Gr-1, and Mac-1 (Pharmingen) by FACS analysis.
Cell cycle analysis
Sca1+/lin- cells were collected from cultures 6 days after infection and 32D cells were collected from cultures 36 hours after infection, washed in phosphate-buffered saline (PBS), and fixed with 70% ethanol at -20°C. The cells were resuspended in PBS containing propidium iodide (PI; 50 μg/mL) and RNAse (5 μg/mL; Sigma) and incubated at 37°C for 30 minutes and immediately evaluated by FACS. For the bromodeoxyuridine (BrdU) incorporation, 2 × 106 cells were incubated with 10 μM BrdU (Sigma) at 37°C for 25 minutes. Cells were fixed by ice-cold 70% ethanol, denatured by 4N HCL, stained with fluorescein isothiocyanate (FITC)-conjugated antibody against BrdU (Becton Dickinson, Heidelberg, Germany) and with PI (5 μg/mL), and then analyzed by FACS.
Small interference RNA (siRNA)
The siRNA sequences encoding inverted repeats of 21 nucleotides (nt's) separated by a 10-nt spacer were designed using publicly available software tools (www.ambion.com/techlib/misc/silencer_siRNA_template.html). The inverted repeats corresponded to 1862-1882 bp of the human γ-catenin cDNA (completely homologue to murine sequence) and had at least 3 nt differences from any other murine genes. The oligos containing HpaI and BbsI restriction sites and hairpin DNA (5′CACCGCACCATTCCCCTGTTTGTGTTGAGTACTGCACAAACAGGGGAATGGTGCTTTTT3′ 5′AAAAAGCACCATTCCCCTGTTTGTGCAGTACTCAACACAAACAGGGGAATGGTGC3′) were annealed and ligated into BbsI-HpaI-digested vPGKpuroU3U6. The construct was controlled by sequencing. The recombinant plasmid was designated as siRNA-25. Efficiency of the siRNA-25 was confirmed by Western blotting of Phoenix cell lysates cotransfected with PINCO-γ-catenin and siRNA-25 construct with the ratio of 1:20.
Transduction/transplantation model of leukemia
Female C57BL mice from 6 to 12 weeks of age (Charles River) were used as transplant recipients and donors. Sca1+/lin- were isolated as described in “Isolation of Sca1+/lin- HSCs.” Recipients were sublethally irradiated with 8.5 Gy. Transduced Sca1+/lin- HSCs (5 × 104) were inoculated into anesthetized mice by retro-orbital injection. For the engraftment control, one mouse of each group was killed and analyzed. The other mice were killed at the first appearance of signs of morbidity, loss of weight (> 10%), neurologic abnormalities, failure to thrive, or diarrhea. Isolation of BM cells was performed as described in “Isolation of Sca1+/lin- HSCs.” Spleen cells were isolated by passing the tissue through a 40-μM nylon cell strainer (Becton-Dickinson, Le Pont de Claix, France). Whole BM and spleen cells were then cytospinned on glass slides and stained with May-Grünwald-Giemsa. For surface marker analysis and protein lysate preparation, the mononuclear cells (MNCs) were enriched on a Ficoll density gradient.
Results
X-RARαs block myeloid differentiation of Sca1+/lin- HSCs
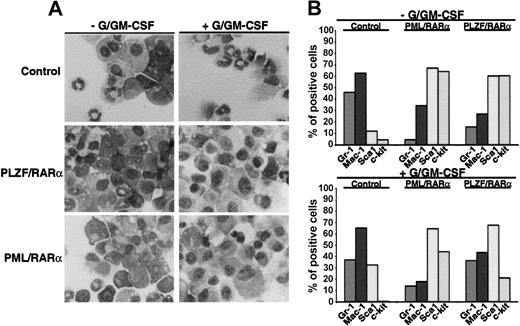
To investigate whether X-RARαs are able to inhibit differentiation of HSCs we expressed PML/RARα as well as PLZF/RARα in Sca1+/lin- HSCs by retroviral infection. The cells were seeded in semisolid medium and exposed to granulocyte/granulocyte-macrophage colony-stimulating factor (G/GM-CSF) for 10 days to induce granulocytic/monocytic differentiation. Mock-infected cells served as negative controls. Differentiation was assessed by morphology and by expression of differentiation-associated surface markers (Gr-1, Mac-1, Sca1, and c-kit). As depicted in Figure 1A, the expression of PML/RARα and of PLZF/RARα inhibited the granulocytic and monocytic differentiation of the Sca1+/lin- cells. This block of differentiation was accompanied by a reduction of Gr-1 and Mac-1 expression in PML/RARα- as well as in PLZF/RARα-expressing cells compared with the mock-transduced control cells (Figure 1B). Exposure to G/GM-CSF induced an increase of Gr-1 and Mac-1 expression nearly to the levels of control cell in the PLZF/RARα-positive cells but failed to do so in the PML/RARα-positive cells (Figure 1B). In absence of G/GM-CSF, PML/RARα- as well as PLZF/RARα-positive cells expressed high levels of the stem cell markers Sca1 and c-kit, whose expression were only slightly reduced by the exposure to G/GM-CSF, whereas c-kit expression was completely abolished in the mock-infected control cells. These data were confirmed by a reduced capacity for respiratory burst-related activity of PML/RARα- as well as of PLZF/RARα-positive cells compared with the control cells in the nitroblue tetrazolium (NBT)-reduction assay (data not shown).
Block of myeloid differentiation induced by X-RARα. Sca1+/lin- HSCs were transduced with PML/RARα or PLZF/RARα. Mock-transduced cells were used as control. The cells were seeded in semisolid medium ± G/GM-CSF for the induction of granulocytic/monocytic differentiation. At day 10 the cells were harvested and analyzed. (A) Morphologic analysis of cells stained with May-Grünwald-Giemsa. Original magnification, × 400. (B) Differentiation-specific surface marker expression. Gr-1 and Mac-1 are markers for myeloid differentiation and Sca1 and c-kit are stem cell markers. One representative experiment of 3 is given.
Block of myeloid differentiation induced by X-RARα. Sca1+/lin- HSCs were transduced with PML/RARα or PLZF/RARα. Mock-transduced cells were used as control. The cells were seeded in semisolid medium ± G/GM-CSF for the induction of granulocytic/monocytic differentiation. At day 10 the cells were harvested and analyzed. (A) Morphologic analysis of cells stained with May-Grünwald-Giemsa. Original magnification, × 400. (B) Differentiation-specific surface marker expression. Gr-1 and Mac-1 are markers for myeloid differentiation and Sca1 and c-kit are stem cell markers. One representative experiment of 3 is given.
Taken together, these data indicate that the expression of X-RARαs not only blocks G/GM-CSF-induced differentiation of Sca1+/lin- stem cells but moreover retains the cells at an early progenitor stage.
X-RARαs increase self-renewal of HSCs
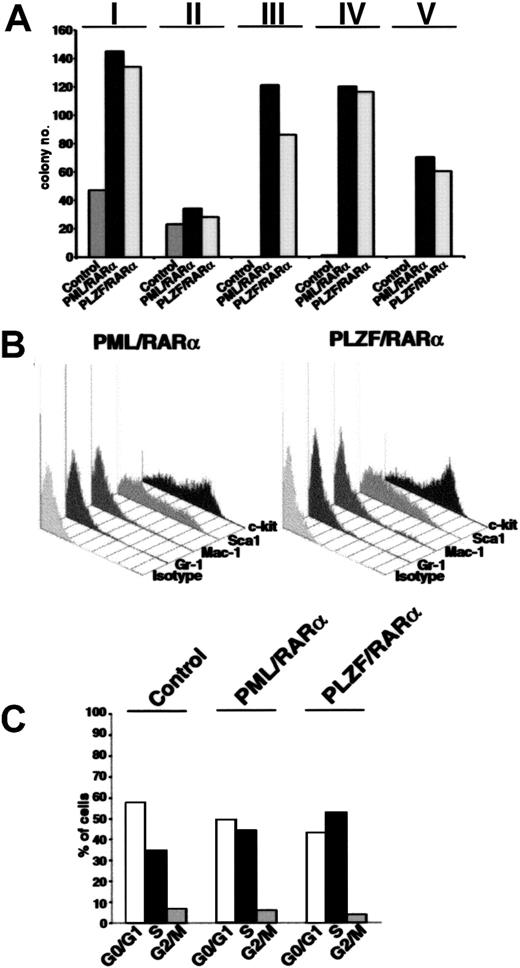
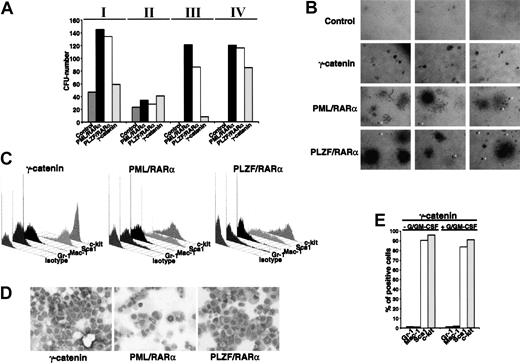
To investigate whether the differentiation block related to the expression of X-RARαs is responsible for the increased self-renewal of the leukemic stem cells, we evaluated the effect of X-RARαs on the replating efficiency of Sca1+/lin- HSCs. Therefore, PML/RARα- and PLZF/RARα-transduced Sca1+/lin- cells were plated in methylcellulose. Colony forming units (CFUs) were counted at day 10 after plating, washed out from the medium for subsequent analysis of surface marker expression, and replated under the same conditions. As shown in Figure 2A, platings 1 and 2 showed no differences between control cells and PML/RARα- or PLZF/RARα-expressing cells. All samples exhibited a strong reduction of CFUs in the second plating. Whereas control cells lost their potential to form colonies after the second plating, replating of PML/RARα- as well as of PLZF/RARα-expressing cells led to efficient colony formation at least until passage 15 (Figure 2A; data not shown). The analysis of surface markers of colonies from the third plating, the first in which control cells were no longer able to form colonies, showed a high percentage of cells expressing the stem cell markers Sca1 and c-kit (Figure 2B).
Increased self-renewal of Sca1+/lin-HSCs expressing X-RARαs. (A) Replating efficiency of Sca1+/lin- stem cells transduced with PML/RARα or PLZF/RARα. Mock-transduced cells were used as control. The cells were seeded in semisolid medium. At day 10 the cells were harvested, analyzed, and replated (I-V indicate the number of the plating round). (B) Surface marker expression of PML/RARα or PLZF/RARα expressing cells of the third plating. Gr-1 and Mac-1 were detected as markers of myeloid differentiation and Sca1 and c-kit were detected as stem cell markers. (C) Cell cycle analysis of 32D cells expressing PML/RARα or PLZF/RARα. Mock-transduced cells were used as control. Cell cycle was analyzed 36 hours after transduction by staining with propidium iodide (PI).
Increased self-renewal of Sca1+/lin-HSCs expressing X-RARαs. (A) Replating efficiency of Sca1+/lin- stem cells transduced with PML/RARα or PLZF/RARα. Mock-transduced cells were used as control. The cells were seeded in semisolid medium. At day 10 the cells were harvested, analyzed, and replated (I-V indicate the number of the plating round). (B) Surface marker expression of PML/RARα or PLZF/RARα expressing cells of the third plating. Gr-1 and Mac-1 were detected as markers of myeloid differentiation and Sca1 and c-kit were detected as stem cell markers. (C) Cell cycle analysis of 32D cells expressing PML/RARα or PLZF/RARα. Mock-transduced cells were used as control. Cell cycle was analyzed 36 hours after transduction by staining with propidium iodide (PI).
The fact that the X-RARαs considerably increased the replating efficiency of Sca1+/lin- cells raised a question: is this effect due to the differentiation block or are the X-RARαs able to influence the cell cycle progression of early hematopoietic progenitors? Thus, the involvement of PML/RARα and PLZF/RARα in the cell cycle progression of 32D cells, an early murine myeloid progenitor cell line, was determined. As shown in Figure 2C, PML/RARα-expressing cells exhibited an increase of cells in S phase (about 45%) compared with the control cells (35%), whereas the percentage of PLZF/RARα-expressing cells in the S phase reached 55%. Comparable results were obtained on Sca1+/lin- HSCs (data not shown).
In summary, these data demonstrate that the expression of X-RARα increases the potential for self-renewal of HSCs and leads to an acceleration of cell cycle progression in early progenitors.
AATPs activate the γ-catenin promoter
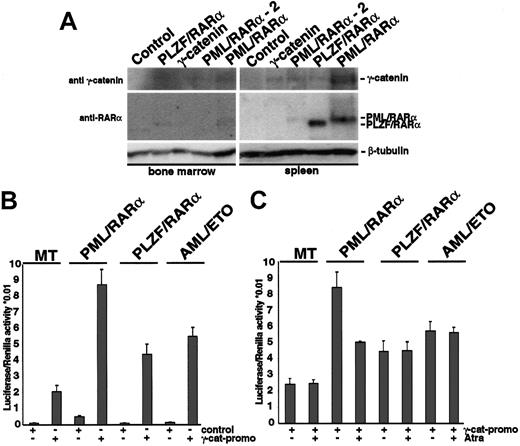
We have recently shown that expression of X-RARα or AML-1/ETO induces expression of γ-catenin, a key mediator of the Wnt-signaling pathway. Activation of Wnt signaling increases the self-renewal of HSCs. To clarify the role of the Wnt signaling mediated by γ-catenin in the X-RARα-related leukemogenesis, we investigated whether the X-RARα can induce expression of γ-catenin in vivo. Therefore, X-RARα-transduced Sca1+/lin- HSCs were inoculated into sublethally irradiated mice. We used mice that received γ-catenin-transduced cells as positive controls. To confirm the engraftment, 1 of 5 PML/RARα-positive mice, 1 of 5 PLZF/RARα-positive mice, and 1 of 5 of γ-catenin-positive mice were killed on day 10 following transplantation, and spleen and BM were analyzed for expression of γ-catenin. On day 12 another PML/RARα-positive animal showed disease symptoms and was included in the analysis. All other animals were observed for a longer period of time for possible effects on hematopoiesis (see “γ-catenin overexpression in HSCs induces myeloid leukemia”). The expression of the transgenes was detectable in the MNCs isolated from the spleen as well as from the BM of the mice that received transplants (Figure 3A). Expression of PML/RARα and PLZF/RARα in the BM was less strong, an effect most likely due to the delayed homing of inoculated stem cells in the BM with respect to that in the spleen after irradiation. Nevertheless, MNCs derived from both spleen and BM of the recipients showed expression of γ-catenin in presence of the X-RARα transgenes, which could not be detected in control animals. These data show that both PML/RARα and PLZF/RARα are able to induce γ-catenin expression in vivo.
Induction of γ-catenin by X-RARα (A) Sca1+/lin- stem cells transduced with PML/RARα, PLZF/RARα, or γ-catenin were transplanted into sublethally irradiated recipient mice. At day 10 after transplantation MNCs from the BM and the spleen were analyzed by Western blotting for the expression of X-RARα and γ-catenin. Another PML/RARα-positive animal that showed disease symptoms at day 12 after transplantation (PML/RARα-2) was included. The blots were first incubated with an anti-RARα antibody and after stripping with an anti-γ-catenin antibody. Equal protein loading was confirmed after further stripping of the membrane and staining with an anti-β-tubulin antibody. The corresponding bands are indicated. (B) Transactivation of the γ-catenin promoter by X-RARα and AML-1/ETO. The γ-catenin promoter construct was transfected by electroporation into U937 cells expressing the respective transgenes under the control of an Zn2+-inducible methallothionein 1 (MT-1) promoter. The pGL3basic was used as control. Twelve hours after transfection the transgene expression was induced by the exposure to 100 μM Zn2SO4, and luciferase expression was measured 24 hours later and normalized with Renilla activity. The average of triplicates of one representative of 3 independent experiments is given. (C) Effect of t-RA on the transactivation of the γ-catenin promoter by X-RARα and AML-1/ETO. Twelve hours after transfection the transgene expression was induced by the exposure to 100 μM Zn2SO4 for 4 hours prior to adding t-RA at a concentration of 10-6 M, and luciferase expression was measured 24 hours later. The average of triplicates of one representative of 3 independent experiments is given.
Induction of γ-catenin by X-RARα (A) Sca1+/lin- stem cells transduced with PML/RARα, PLZF/RARα, or γ-catenin were transplanted into sublethally irradiated recipient mice. At day 10 after transplantation MNCs from the BM and the spleen were analyzed by Western blotting for the expression of X-RARα and γ-catenin. Another PML/RARα-positive animal that showed disease symptoms at day 12 after transplantation (PML/RARα-2) was included. The blots were first incubated with an anti-RARα antibody and after stripping with an anti-γ-catenin antibody. Equal protein loading was confirmed after further stripping of the membrane and staining with an anti-β-tubulin antibody. The corresponding bands are indicated. (B) Transactivation of the γ-catenin promoter by X-RARα and AML-1/ETO. The γ-catenin promoter construct was transfected by electroporation into U937 cells expressing the respective transgenes under the control of an Zn2+-inducible methallothionein 1 (MT-1) promoter. The pGL3basic was used as control. Twelve hours after transfection the transgene expression was induced by the exposure to 100 μM Zn2SO4, and luciferase expression was measured 24 hours later and normalized with Renilla activity. The average of triplicates of one representative of 3 independent experiments is given. (C) Effect of t-RA on the transactivation of the γ-catenin promoter by X-RARα and AML-1/ETO. Twelve hours after transfection the transgene expression was induced by the exposure to 100 μM Zn2SO4 for 4 hours prior to adding t-RA at a concentration of 10-6 M, and luciferase expression was measured 24 hours later. The average of triplicates of one representative of 3 independent experiments is given.
To disclose how the X-RARαs activate γ-catenin expression, we investigated the regulation of the γ-catenin promoter by X-RARαs and AML-1/ETO. Therefore a luciferase reporter construct driven by the γ-catenin promoter was transfected into the U937 cells expressing either PML/RARα, PLZF/RARα, or AML-1/ETO under the control of a Zn2+-inducible methallothionein 1 (MT-1) promoter. In the control MT cells, induction of the γ-catenin promoter was detectable and further increased by the expression of PML/RARα, PLZF/RARα, as well as of AML-1/ETO (Figure 3B). In transiently transfected Hela cells we found that, in contrast to PML/RARα and PLZF/RARα, wild-type RARα was unable to activate the γ-catenin promoter (data not shown).
Treatment with all-trans retinoic acid (t-RA) is able to induce complete remissions in patients suffering from t(15;17)(PML/RARα)-positive AML-M3 but not in patients with t(11;17)(PLZF/RARα)-positive AML-M3 or t(8;21)(AML-1/ETO)-positive AMLM2. This effect on PML/RARα blasts is attributed mainly to the differentiation-inducing capacity of t-RA. Nevertheless, t-RA also increases the self-renewal of hematopoietic precursors.26 To clarify whether the effect of t-RA on PML/RARα-positive AML-M3 cells also includes regulation of γ-catenin expression we studied the effect of t-RA on the activation of the γ-catenin promoter by the AATPs. Exposure to t-RA reduced the transactivation of the γ-catenin promoter by PML/RARα in U937 cells; however, the reduction did not reach the levels of control cells lacking the translocation products (Figure 3C). In contrast, t-RA had no effect on the transactivation of the γ-catenin promoter by PLZF/RARα or AML-1/ETO, nor did it influence the basic activation of the γ-catenin promoter in the MT control cells.
Taken together, these data indicate that the AATPs up-regulate γ-catenin expression by directly transactivating the γ-catenin promoter, an effect that is at least partially reversible by t-RA in the case of the AML-M3 t(15;17)-associated translocation product PML/RARα.
γ-Catenin accelerates cell cycle progression of HSCs
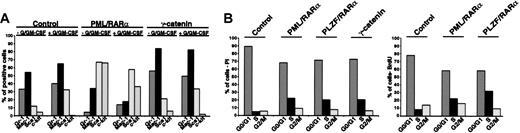
To disclose the role of the γ-catenin for the phenotype induced by the AATPs, we compared the effect of γ-catenin with that of AATPs on the differentiation of early HSCs. Therefore γ-catenin-, PML/RARα-, and PLZF/RARα-transduced Sca1+/lin- cells were cultivated in absence and presence of G/GM-CSF, and differentiation was assessed by surface marker expression as described in “X-RARαs block myeloid differentiation of Sca+/lin- HSCs.” In contrast to PML/RARα-positive cells, overexpression of γ-catenin in Sca1+/lin- failed to block myeloid differentiation independently of the exposure to G/GM-CSF (Figure 4A).
Overexpression of γ-catenin in Sca1+/lin- HSCs. (A) Differentiation of HSCs transduced with γ-catenin compared with that of PML/RARα-expressing HSCs in absence/presence of G/GM-CSF. Gr-1 and Mac-1 were detected as markers of myeloid differentiation and Sca1 and c-kit were detected as stem markers. One representative experiment of at least 3 performed is given. (B) Effect of γ-catenin and X-RARα on cell cycle progression of Sca1+/lin- HSCs. Cell cycle was analyzed 36 hours after transduction by staining the cells with propidium iodide (PI) or by BrdU incorporation (BrdU). For each assay one representative experiment of 3 is given.
Overexpression of γ-catenin in Sca1+/lin- HSCs. (A) Differentiation of HSCs transduced with γ-catenin compared with that of PML/RARα-expressing HSCs in absence/presence of G/GM-CSF. Gr-1 and Mac-1 were detected as markers of myeloid differentiation and Sca1 and c-kit were detected as stem markers. One representative experiment of at least 3 performed is given. (B) Effect of γ-catenin and X-RARα on cell cycle progression of Sca1+/lin- HSCs. Cell cycle was analyzed 36 hours after transduction by staining the cells with propidium iodide (PI) or by BrdU incorporation (BrdU). For each assay one representative experiment of 3 is given.
As γ-catenin activates target genes relevant for cell cycle progression, we compared its effect on cell cycle progression of Sca1+/lin- HSCs with that of the X-RARα's. Cell cycle progression was assessed by PI staining as well as by BrdU incorporation. As shown in Figure 4B the expression of γ-catenin increased the fraction of cells in the S phase from about 5% in the mock-infected cells to approximately 20% with a concomitant reduction of cells in G0/G1. The expression of PML/RARα as well as of PLZF/RARα had a nearly identical effect on the cell cycle progression of these cells.
In summary these data show that one of the main effects of γ-catenin in HSCs is an acceleration of cell cycle progression.
Overexpression of γ-catenin selects a subset of early HSCs unable to differentiate in presence of G/GM-CSF
The capacity of the activated Wnt signaling pathway to increase self-renewal of HSCs and the fact that γ-catenin accelerates cell cycle progression comparable to X-RARαs prompted us to investigate the influence of γ-catenin overexpression on the replating efficiency of HSCs in comparison to that of the AATPs. Thus γ-catenin-transduced Sca1+/lin- cells were compared with cells transduced with PML/RARα, PLZF/RARα, and AML-1/ETO and seeded in methylcellulose as described in “X-RARαs increase self-renewal of HSCs.” The CFUs were counted after 10 days in each plating round (Figure 5A). All groups showed a similar reduction in CFUs in the second plating. In the third plating only a few differentiated CFUs were seen in the mock-infected controls and these were not further replatable. In contrast, the AATP-positive cells yielded a high number of CFUs, whereas the number of γ-catenin-positive CFUs increased only slightly (Figure 5A). The cells of the fourth plating were further analyzed. As shown in Figure 5B, the expression of γ-catenin led to the formation of homogenous, very small, and compact colonies similar to granulocyte-erythrocyte-megakaryocyte-macrophage CFU (CFU-GEMM) but without peripheral migrating cells very similar to “blast-colonies,” indicating that these colonies were formed by a completely immature cell population. In contrast, expression of PML/RARα as well as PLZF/RARα gave origin to a mixture of CFU-GEMMs and CFU-GMs but this was observed in addition to colonies identical to those seen in the γ-catenin-expressing cells (Figure 5B arrowheads). The immature character of γ-catenin-overexpressing HSCs is confirmed by the nearly exclusive expression of c-kit and Sca1 in comparison to PML/RARα- and PLZF/RARα-expressing colonies. In fact, neither Gr-1 nor Mac-1 expression was detected in these cells (Figure 5C). In contrast to the γ-catenin-expressing CFUs, represented by one population expressing high levels of c-kit and Sca1, 2 subpopulations can be distinguished in the PML/RARα- as well as PLZF/RARα-expressing CFUs: one with high expression and one with low expression of Sca1, which is in accordance with a certain degree of myeloid differentiation. The morphology of γ-catenin-expressing cells did not exhibit any sign of myeloid differentiation, which is in contrast to the PML/RARα- as well as the PLZF/RARα-expressing cells where signs of early myeloid differentiation were seen (Figure 5D). Moreover, exposure of these cells to G/GM-CSF for a further 6 days in liquid culture was unable to induce any sign of myeloid differentiation (Figure 5E).
Colony formation of Sca1+/lin- HSCs expressing γ-catenin. (A) Replating efficiency of Sca1+/lin- stem cells transduced with γ-catenin, PML/RARα,or PLZF/RARα (Roman numerals indicate the number of the plating round). Mock-transduced cells were used as control. The cells were seeded in semisolid medium. At day 10 the cells were harvested, analyzed, and replated. (B) Morphology of the colonies at the plating IV. Original magnification, × 25. (C) Differentiation-specific surface marker expression. Gr-1 and Mac-1 are markers for myeloid differentiation and Sca1 and c-kit are stem cell markers. (D) Morphologic analysis of cells stained with May-Grünwald-Giemsa. Original magnification, × 400. (E) Differentiation of the γ-catenin-expressing cells at the plating IV in absence/presence of G/GM-CSF. One representative of at least 3 experiments is shown.
Colony formation of Sca1+/lin- HSCs expressing γ-catenin. (A) Replating efficiency of Sca1+/lin- stem cells transduced with γ-catenin, PML/RARα,or PLZF/RARα (Roman numerals indicate the number of the plating round). Mock-transduced cells were used as control. The cells were seeded in semisolid medium. At day 10 the cells were harvested, analyzed, and replated. (B) Morphology of the colonies at the plating IV. Original magnification, × 25. (C) Differentiation-specific surface marker expression. Gr-1 and Mac-1 are markers for myeloid differentiation and Sca1 and c-kit are stem cell markers. (D) Morphologic analysis of cells stained with May-Grünwald-Giemsa. Original magnification, × 400. (E) Differentiation of the γ-catenin-expressing cells at the plating IV in absence/presence of G/GM-CSF. One representative of at least 3 experiments is shown.
In summary, these data show that the overexpression of γ-catenin in HSCs leads to the selection of a subset of early stem cells that may contribute to leukemogenesis.
γ-Catenin is essential for the increased colony-forming capacity of HSCs overexpressing AATPs
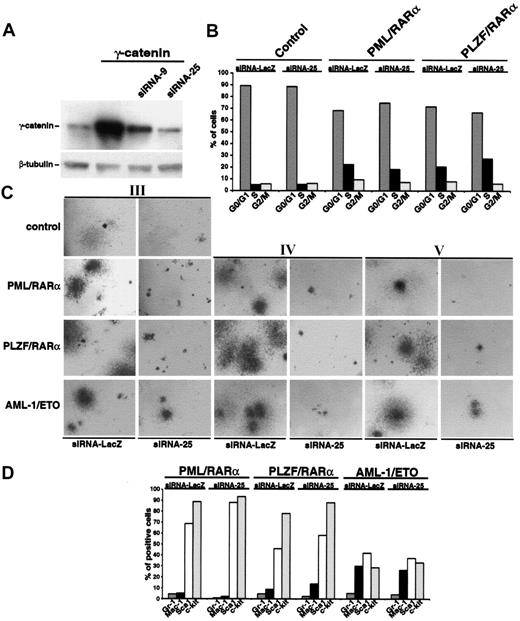
To corroborate our hypothesis that γ-catenin is crucial for the increased replating efficiency of AATP-positive CFUs, up-regulation of endogenous γ-catenin by AATPs in the HSCs was inhibited by RNA interference. Different siRNAs directed against γ-catenin were expressed from the human U6 promoter in a retroviral vector, and expression of γ-catenin was assessed by Western blotting. Figure 6A shows the effects of 2 siRNAs on γ-catenin expression. As siRNA-25 proved to be more efficient; it was used for all further experiments.
Effect of silencing the γ-catenin expression by RNA interference on the phenotype of Sca1+/lin- HSCs expressing AATPs. (A) Effect of siRNA constructs on Phoenix cells overexpressing γ-catenin. Two different siRNA constructs (siRNA-9 and siRNA25) were cotransfected into Phoenix cells together with the PINCO-γ-catenin. Thirty-six hours after transfection whole cell lysates were probed with an anti-γ-catenin antibody in Western blot analysis. Equal protein loading was confirmed after stripping of the membrane and staining with an anti-β-tubulin antibody. (B) Effect of siRNA-25 on cell cycle progression of HSCs expressing X-RARα. An siRNA directed against the β-galactosidase (siRNA-LacZ), which had no effect on γ-catenin expression (data not shown), was used as control. (C) Effect of siRNA-25 on the replating efficiency of Sca1+/lin- HSCs expressing AATPs. The siRNA-LacZ was used as control (Roman numerals indicate the number of the plating round). Original magnification, × 25. (D) Effect of siRNA-25 on the differentiation block induced by AATPs. The siRNA-LacZ was used as control. Gr-1 and Mac-1 were detected as markers of myeloid differentiation and Sca1 and c-kit were detected as stem cell markers.
Effect of silencing the γ-catenin expression by RNA interference on the phenotype of Sca1+/lin- HSCs expressing AATPs. (A) Effect of siRNA constructs on Phoenix cells overexpressing γ-catenin. Two different siRNA constructs (siRNA-9 and siRNA25) were cotransfected into Phoenix cells together with the PINCO-γ-catenin. Thirty-six hours after transfection whole cell lysates were probed with an anti-γ-catenin antibody in Western blot analysis. Equal protein loading was confirmed after stripping of the membrane and staining with an anti-β-tubulin antibody. (B) Effect of siRNA-25 on cell cycle progression of HSCs expressing X-RARα. An siRNA directed against the β-galactosidase (siRNA-LacZ), which had no effect on γ-catenin expression (data not shown), was used as control. (C) Effect of siRNA-25 on the replating efficiency of Sca1+/lin- HSCs expressing AATPs. The siRNA-LacZ was used as control (Roman numerals indicate the number of the plating round). Original magnification, × 25. (D) Effect of siRNA-25 on the differentiation block induced by AATPs. The siRNA-LacZ was used as control. Gr-1 and Mac-1 were detected as markers of myeloid differentiation and Sca1 and c-kit were detected as stem cell markers.
First we investigated the effect of hindered γ-catenin expression on cell cycle progression induced by X-RARαs by transducing X-RARα-expressing Sca1+/lin- cells with siRNA-25. As shown in Figure 6B, the presence of siRNA-25 did not significantly affect the cell cycle progression of Sca1+/lin- cells expressing the AATPs. In a comparable experimental setting we studied the effect of siRNA-25 on the replating efficiency of Sca1+/lin- stem expressing the AATPs. In contrast to the control siRNA directed against bacterial β-galactosidase (siRNA-LacZ), which did not have any influence on colony formation, the presence of siRNA-25 almost completely abolished the colony-forming capacity of the HSCs expressing the AATPs already in the third plating (Figure 6C). The presence of the siRNA-25 not only reduced the number but also the size of the colonies (Figure 6C). The effect of siRNA-25 was not only limited to the cells expressing the AATPs but also diminished the colony size of mock-infected control cells (Figure 6C). We continued the replatings for another 4 rounds and showed that the AATPs-positive cells were unable to rescue their CFU-forming capacity in presence of siRNA-25 (Figure 6C; data not shown). The analysis of surface marker expression at the second plating yielded no significant differences between cells infected with the control siRNA-LacZ or with the siRNA-25, indicating that the AATP-related block of differentiation is independent of the presence of γ-catenin (Figure 6D).
Taken together, these data indicate that γ-catenin may be important for the self-renewal of HSCs but is dispensable for the differentiation block caused by the expression of AATPs.
γ-Catenin overexpression in HSCs induces myeloid leukemia
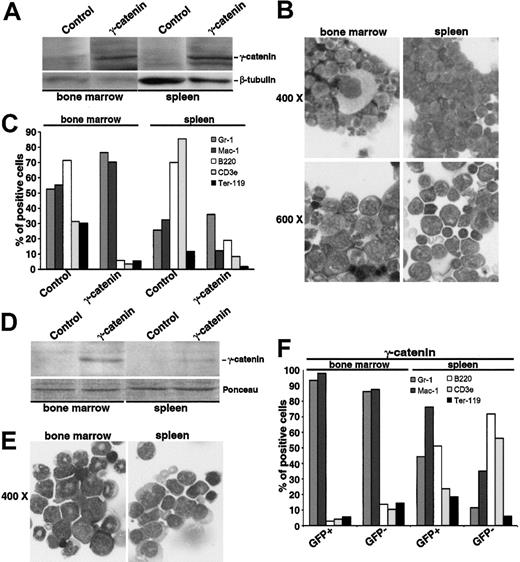
To clarify the role of γ-catenin in the X-RARα-related leukemogenesis and to explore the possibility to expand HSCs for therapeutic issues through an overexpression of γ-catenin or a pharmacologic induction of Wnt signaling, we inoculated 5 mice with Sca1+/lin- cells transduced with γ-catenin. One animal was killed to confirm engraftment on day 10 after transplantation (see above), the other 4 were observed for 12 months for the development of leukemia. In contrast to the PML/RARα- and PLZF/RARα-positive mice described in “AATPs activate the γ-catenin promoter,” which did not develop leukemia within 12 months (data not shown), one of the γ-catenin-positive mice exhibited the clinical picture of acute leukemia after 9 months. MNCs isolated from both the spleen and the BM expressed high levels of γ-catenin as determined by Western blotting (Figure 7A). There was a high rate of immature myeloid blasts in the BM as well as in the spleen (Figure 7B). These blasts expressed high levels of Gr-1 and Mac-1 in the BM but low levels of these markers in the spleen (Figure 7C), indicating that the population residing in the spleen is less differentiated than the one in the BM where nearly normal megakaryopoiesis took place (Figure 7B). Peripheral white blood cell (WBC) count was 33.75 × 109 L, whereas red blood cell (RBC) count, hemoglobin levels, and thrombocyte counts were within the normal range (data not shown). After 12 months another animal developed disease symptoms and was killed. In this mouse, GFP was still detectable, which is driven in the PINCO vector by a CMV promoter, often silenced in vivo.27 In the spleen, only the GFP-positive cells showed an expression pattern of surface markers consistent with the presence of myeloid leukemia, whereas the GFP-negative population exhibited a nearly normal pattern of surface markers. In the BM both the GFP-positive and the GFP-negative populations were constituted by leukemia cells (Figure 7C). The morphologic analysis of MNCs isolated from the spleen and the BM gave a picture of a myeloid leukemia with signs of differentiation (Figure 7D).28 The other 2 mice were killed for analysis at the end of month 12 after transplantation. Both mice showed clearly impaired hematopoiesis in the BM as well as in the spleen with a clear increase of myeloid markers (data not shown).
Leukemogenic potential of Sca1+/lin-transduced with γ-catenin and inoculated into sublethally irradiated mice. (A-C) Analysis of the mouse that developed disease symptoms after 9 months. (A) Western blot analysis of γ-catenin expression in the MNCs isolated from the BM and the spleen. Equal protein loading was confirmed after stripping of the membrane and staining with an anti-β-tubulin antibody. (B) Morphology of BM and spleen cells stained with May-Grünwald Giemsa. Two magnifications are given (× 400 and × 600). (C) Immunologic characterization of BM and spleen cells. Gr-1and Mac-1 characterize the myeloid lineage, B220 the B-lymphocytic lineage, CD3ϵ the T-lymphocytic lineage, and Ter119 the erythroid lineage. (D-F) Analysis of the mouse that developed disease symptoms after 12 months. (D) Western blot analysis of γ-catenin expression in the MNCs isolated from the BM and the spleen. Equal protein loading was confirmed by Ponceau staining of the membrane. (E) Morphology of BM and spleen cells stained with May-Grünwald Giemsa. Original magnification × 400. (F) Immunologic characterization of BM and spleen cells distinguishing between GFP-positive and -negative population. Gr-1 and Mac-1 characterize the myeloid lineage, B220 the B-lymphocytic lineage, CD3ϵ the T-lymphocytic lineage, and Ter119 the erythroid lineage.
Leukemogenic potential of Sca1+/lin-transduced with γ-catenin and inoculated into sublethally irradiated mice. (A-C) Analysis of the mouse that developed disease symptoms after 9 months. (A) Western blot analysis of γ-catenin expression in the MNCs isolated from the BM and the spleen. Equal protein loading was confirmed after stripping of the membrane and staining with an anti-β-tubulin antibody. (B) Morphology of BM and spleen cells stained with May-Grünwald Giemsa. Two magnifications are given (× 400 and × 600). (C) Immunologic characterization of BM and spleen cells. Gr-1and Mac-1 characterize the myeloid lineage, B220 the B-lymphocytic lineage, CD3ϵ the T-lymphocytic lineage, and Ter119 the erythroid lineage. (D-F) Analysis of the mouse that developed disease symptoms after 12 months. (D) Western blot analysis of γ-catenin expression in the MNCs isolated from the BM and the spleen. Equal protein loading was confirmed by Ponceau staining of the membrane. (E) Morphology of BM and spleen cells stained with May-Grünwald Giemsa. Original magnification × 400. (F) Immunologic characterization of BM and spleen cells distinguishing between GFP-positive and -negative population. Gr-1 and Mac-1 characterize the myeloid lineage, B220 the B-lymphocytic lineage, CD3ϵ the T-lymphocytic lineage, and Ter119 the erythroid lineage.
Taken together, these data indicate that the inoculation of sublethally irradiated recipient mice with HSCs overexpressing γ-catenin can establish acute myeloid leukemia.
Discussion
The aim of this study was to clarify whether the increased self-renewal of leukemic blasts is directly related to the differentiation block or if the differentiation block and self-renewal are independent features, both contributing to the leukemic phenotype. Here we propose a mechanism by which AML-M3-associated X-RARα as well as AML-M2-associated AML-1/ETO increase self-renewal of HSCs. We disclosed a crucial role of γ-catenin, a member of the Wnt-signaling cascade, for the determination of the phenotype related to the expression of AATPs.
The first indication that the increase of the self-renewal may be at least in part independent of the differentiation block is given by the different phenotypes induced by AML-M3-related X-RARα and AML-M2-related AML-1/ETO. Here we show that X-RARαs induce a phenotype in HSCs that comprises the differentiation block, increased self-renewal, and accelerated cell cycle progression. X-RARαs block differentiation at an early differentiation stage. The morphology as well as the pattern of surface marker expression of the X-RARα-positive CFUs defines early precursors, which are nevertheless already committed to myeloid differentiation. In contrast to X-RARα, AML-1/ETO seems to allow HSCs to further differentiate. This is in accordance with recent findings showing that AML-1/ETO only blocks differentiation but does not accelerate cell cycle progression.29,30 It seems that AML-1/ETO allows HSCs to reach a stage of differentiation in which the progenitor cells acquire a balance between block of differentiation and concomitant inhibition of proliferation, whereas X-RARα-positive blasts are blocked at an earlier stage associated with an increased proliferation. The contrast between the combined effect of X-RARα on differentiation as well as on cell cycle progression and the single effect of AML-1/ETO on differentiation may explain why patients with AML-M2 in remission can carry the t(8;21) translocation (AML-1/ETO) for years without relapse from their disease,31 whereas t(15;17) (PML/RAR)-positive AML-M3 patients will relapse within a few months after becoming minimal residual disease-positive.32
We recently have shown that in cell line models AATPs are able to activate the Wnt-signaling pathway by up-regulating the expression of γ-catenin. Moreover, blast cells from patients harboring either t(15;17) or t(8;21) express high levels of γ-catenin.22 The increased self-renewal of HSCs upon expression of γ-catenin is comparable to that observed in HSCs upon overexpression of β-catenin as well as upon activation of the Wnt-signaling pathway through its ligands Wnt5a and Wnt3a.19-21
The overexpression of γ-catenin is unable to completely recapitulate the AATP-induced phenotype in HSCs, because γ-catenin is unable to block differentiation of a bulk Sca1+/lin- population. This is in accordance with recent findings showing that the exposure of HSCs to Wnt3a leads to increased self-renewal in serum-free conditions, whereas differentiation is possible in a conditioned medium.19 Nevertheless, activation of the Wnt signaling through the overexpression of β-catenin seems to block differentiation.20 These differences may be explained by the fact that we used γ-catenin instead of β-catenin as a mediator of Wnt signaling.
Serial replating of HSCs overexpressing γ-catenin selects a very small population of very early HSCs. Therefore one could hypothesize that the overexpression of γ-catenin is able to block differentiation only of very early stem cells. Thus there are 2 levels in which AATP-expressing cells are blocked in their differentiation: one at a very early stem cell level due to the induction of γ-catenin and one at a more advanced level of differentiation due to other factors impaired by the AATPS, such as CCAAT/enhancer-binding protein α (C/EBPα).33 In fact, CFUs morphologically similar to the γ-catenin-positive CFUs were also frequently detectable among the AATP-expressing CFUs.
The up-regulation of γ-catenin contributes to the leukemogenesis related to AATPs. This is proven by the fact that the up-regulation of γ-catenin is indispensable for the increase of self-renewal induced by the AATPs. Hence there seems to be a limiting step during HSC development that requires activation of the Wnt-signaling pathway through γ-catenin. This role of γ-catenin is not restricted to leukemic blasts, since mock-infected stem cells were also reduced in their CFU capacity by targeting γ-catenin, indicating the existence of common signaling pathways responsible for self-renewal of both normal and leukemic stem cells.
Based on the data that purified Wnt3a retains the HSCs at a c-kit+ and Sca1+ HSCs phenotype level but fails to inhibit differentiation, it has been proposed to employ the activation of Wnt-signaling to expand human HSCs for therapeutic purposes.19,21 Such an approach has to be carefully validated. The fact that the overexpression of γ-catenin leads to a block of differentiation of a very early stem cell strongly suggests a leukemic potential of a constitutively activated Wnt signaling. In fact, mice that received HSC transplants, which constitutively overexpress γ-catenin, exhibit an impaired hematopoiesis with the possible development of AML. The late onset of the leukemia can be explained in 2 ways: (i) only a very low number of cells at a stem cell stage early enough to be blocked in differentiation by γ-catenin were inoculated into recipients; and (ii) the need to acquire additional “hits” for full transformation. These data outline the risk of the induction of a leukemic state by a long-term activation of Wnt signaling.
Taken together, our data presented here demonstrate the crucial role of γ-catenin in the increased self-renewal of HSCs related to the expression of the AATPs. This, together with the ability of γ-catenin to block the differentiation of very early stem cells, establishes γ-catenin as a potential therapeutic target for the treatment of AATP-related leukemia.
Prepublished online as Blood First Edition Paper, January 22, 2004; DOI 10.1182/blood-2003-09-3335.
Supported by grants from Deutsche Forschungsgemeinschaft (DFG RU 728/2-2; M.R.), Dr. Mildred-Scheel-Stiftung der Deutschen Krebshilfe e.V. (10-1916 Ru2; M.R.), Sander-Stiftung (2001-026.1; M.R.) and DLR (01 KV 9920; O.G.O.). T.B. is supported by a fellowship from Deutsche José Carreras Leukämie-Stiftung e.V. (DJCLS 2001/NAT-1).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal