Abstract
BACKGROUND: Children with acute lymphoblastic leukemia treated with L-asparaginase (ASP) have a prevalence of thrombosis of 37% (95%CI 24–48%). The increased risk for thrombosis in these children dictates the need for primary prophylaxis clinical studies. However, the question of the optimum anticoagulant to be tested is an issue. The mechanism for increased risk of thrombosis in this population is related to the acquired antithrombin (AT) deficiency associated with ASP. Owing to the acquired AT deficiency, low-molecular-weight heparins (LMWHs) are unlikely to be a good option. Ximelagatran is a new anticoagulant that has been shown to be efficacious and well tolerated in primary prophylaxis in adults. The active metabolite of ximelagatran, melagatran, is a direct thrombin inhibitor and does not rely on AT for interaction and subsequent effect. Therefore, ximelagatran may become the drug of choice for this population. Before initiation of large clinical studies, the effect of acquired AT deficiency on the anticoagulant effect of melagatran needs to be determined ex vivo.
OBJECTIVES: To determine the interaction of an ASP-induced acquired AT deficiency on the anticoagulant effects of melagatran and LMWH.
STUDY POPULATION: Newly diagnosed children with ALL being treated with ASP during induction chemotherapy. Plasma was drawn pre-therapy and during the 4 weeks of induction chemotherapy. Five sets of pooled samples from a minimum of 5 children per pool were used for the study. AT levels were decreased (0.53 U/mL) in the pooled samples.
METHODS: Prophylactic and therapeutic levels of melagatran (0.3/05 μM) (Exanta ®, AstraZeneca AB, Sweden) or LMWH (0.3/0.7 U/ml) (Fragmin, Pfizer, USA) were added to plasma pools. Pools with added-back anticoagulant were divided, and in one portion purified human AT was added back to nomalize plasma AT levels to 1.0 u/mL. Samples with melagatran and LMWH were run neat and with added- back AT. Endogenous thrombin generation capacity was assessed using a continuous optical density recording of cleaving chromogenic substrate. The endogenous thrombin generation capacity was derived by calculation of area under the curve. Results are reported as a percentage of the neat sample. Statistical comparison was performed by a one-way ANOVA.
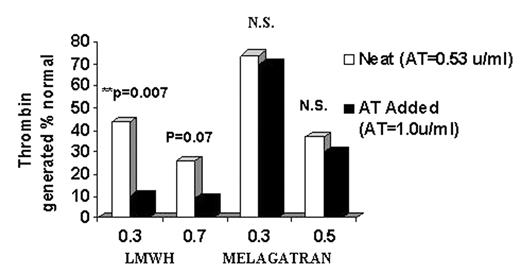
RESULTS: In the LMWH 0.3u/mL group, there was a statistically significant difference in thrombin generated between the neat and AT-added-back groups (Figure 1); there was a trend, but no significant difference, in the 0.7 u/mL LMWH group. In contrast, there was no difference in thrombin generation with ximelagatran 0.3 μ M and 0.5 μM without and with AT added.
CONCLUSION: In children with acquired AT deficiency related to ASP, the anticoagulant effects of LMWH are profoundly affected by endogenous AT levels. In contrast, the anticoagulant effects of melagatran are completely independent of endogenous AT levels. Therefore, the anticoagulant response of melagatran will be far more predictable in these children. Ximelagatran is a suitable candidate for further assessment in prevention of thrombosis in children with ALL.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal