Abstract
Duchenne muscular dystrophy (DMD) is caused by mutations in the dystrophin gene on the X-chromosome that result in skeletal and cardiac muscle damage and premature death. Studies in mice, including the mdx mouse model of DMD, have demonstrated that circulating bone marrow–derived cells can participate in skeletal muscle regeneration, but the potential clinical utility of treating human DMD by allogeneic marrow transplantation from a healthy donor remains unknown. To assess whether allogeneic hematopoietic cell transplantation (HCT) provides clinically relevant levels of donor muscle cell contribution in dogs with canine X-linked muscular dystrophy (c-xmd), 7 xmd dogs were given hematopoietic cell (HC) transplants from nonaffected littermates. Compared with the pretransplantation baseline, the number of dystrophin-positive fibers and the amount of wild-type dystrophin RNA did not increase after HCT, with observation periods ranging from 28 to 417 days. Similar results were obtained when the recipient dogs were given granulocyte colony-stimulating factor (G-CSF) after their initial transplantation to mobilize the cells. Despite successful allogeneic HCT and a permissive environment for donor muscle engraftment, there was no detectable contribution of bone marrow–derived cells to either skeletal muscle or muscle precursor cells assayed by clonal analyses at a level of sensitivity that should detect as little as 0.1% donor contribution.
Introduction
Duchenne muscular dystrophy (DMD), the most frequent and severe form of muscular dystrophy, is caused by complete or partial lack of dystrophin, a component of a multiprotein complex linking the cytoskeleton of the muscle fiber to the extracellular matrix, the dystrophin-associated glycoprotein complex (DAG).1 In the absence of dystrophin, the complex is functionally impaired and this results in degeneration of skeletal muscle fibers.2 The clinical course of DMD is severe and progressive: affected individuals can be detected at birth based on high serum levels of muscle enzymes; they exhibit muscle weakness by the age of 5 years, lose independent ambulation, and succumb to respiratory failure or cardiomyopathy in their late teens or early twenties.3,4 The skeletal muscle fibers of DMD patients undergo degeneration and necrosis, and although robust muscle regeneration is evident early in the disease, muscle tissue is eventually replaced by connective tissue and adipose cells.4,5
Identification of dystrophin gene mutations as the cause of human DMD led to the identification of animal models, such as the mdx mouse6 and the c-xmd dog.7,8 A splice acceptor site mutation in the dog8 results in severe dystrophin deficiency with low levels (∼ 0.01%) of “revertant” fibers that express dystrophin. Although a mild phenotype is observed in the mdx mouse, the c-xmd dog has a clinical course that is very similar to human DMD, characterized by progressive muscle atrophy, degeneration, fibrosis, and a shortened life span.
No curative or ameliorative therapies exist for human DMD; however, the mdx mouse disease phenotype can be improved by a dystrophin transgene and by virally transduced dystrophin,9-11 and recent studies in mice have demonstrated that hematopoietic stem cells can contribute to skeletal muscle regeneration.12-15 In normal and mdx mice, bone marrow–derived cells were shown to participate in skeletal muscle repair after crush-induced or chemically induced damage.16-19 There has been a great deal of controversy, however, regarding the clinical utility of hematopoietic cell transplantation (HCT) for muscular dystrophies, with some groups doubting the significance20,21 and others initiating human trials of allogeneic HCT for DMD. Given the striking clinical and pathologic similarities between canine and human DMD and the established predictive value of the preclinical canine model of allogeneic HCT,22-24 we evaluated whether hematopoietic cell (HC) transplants from healthy DLA-identical littermates contributed to skeletal muscle repair at clinically significant levels in the c-xmd dogs.
Materials and methods
Dogs, dog leukocyte antigen (DLA) matching of littermates, and DMD typing
Female golden retrievers carrying the xmd mutation were obtained from Cornell University (Dr Barry Cooper, Ithaca, NY) and from the University of Missouri (Dr Joe Kornegay, Columbia, MO). Litters of golden retrievers crossed with beagles, mongrels, or golden retrievers were raised at the Fred Hutchinson Cancer Research Center (Seattle, WA). The genotypes of wild-type, carrier, and xmd-affected dogs were determined as described8 (Figure 1). There were 7 DLA-identical littermate donor/recipient pairs chosen on the basis of identity for highly polymorphic major histocompatibility complex (MHC) class I and class II microsatellite markers.25-28 Specific DLA-DRB1 allelic identity was determined by sequencing as described.28 All dogs were enrolled in a veterinary preventive medicine program that included routine anthelmintics and a standard immunization series against canine distemper, parvovirus, adenovirus type 2, parainfluenza virus, and coronavirus. In addition, 2 intradermal doses of a custom-prepared formalin-inactivated canine papilloma virus vaccine were administered. Dogs weighed between 7.7 and 12.6 kg (median, 10.2 kg) and were between 132 and 166 days (median, 139 days) old at the time of transplantation. Elevated enclosed runs were used for housing, and dogs were maintained in social groups wherever possible. This study was approved by the Institutional Animal Care and Use Committee at Fred Hutchinson Cancer Research Center, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
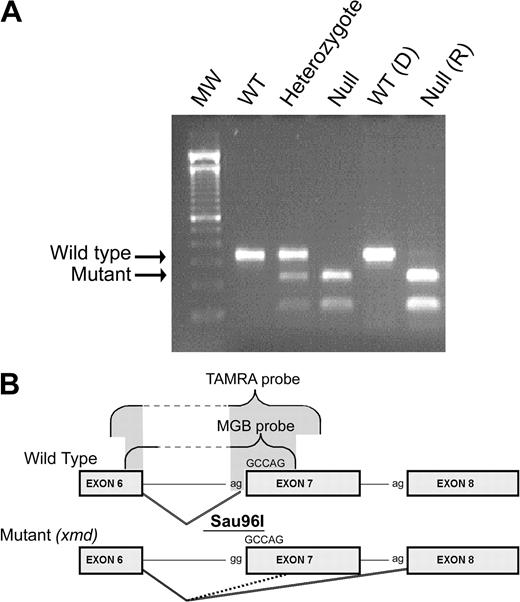
DMD genotyping. (A) Genomic DNA extracted from blood of wild-type (WT), heterozygote (carrier), and null (affected or xmd) dogs was used for PCR amplification.8 The PCR product containing the polymorphic Sau96I site (B) created by the mutation in the dystrophin gene was digested, electrophoresed, and visualized with ethidium bromide. The genotype of one DLA-matched pair of donor and recipient is shown here. D indicates donor; R, recipient. The wild-type band (310 bp) and the mutant band (150 bp) are marked with arrows. (B) Schematic of the wild-type and xmd mutant alleles. The cxmd allele has a point mutation (a to g) in the consensus splice acceptor site of intron 6. As a consequence, alternative splicing either skips exon 7 entirely (solid line) or splices into a cryptic site 5 nucleotides 3-prime of the original splice site (dashed line). Both of these predominant splice variants introduce frame-shifts and early termination codons. Exon probes (TAMRA and MGB) were designed to hybridize to the wild-type junction between exons 6 and 7 and to distinguish between normally spliced mRNA and the splice variants created by the cxmd mutation.
DMD genotyping. (A) Genomic DNA extracted from blood of wild-type (WT), heterozygote (carrier), and null (affected or xmd) dogs was used for PCR amplification.8 The PCR product containing the polymorphic Sau96I site (B) created by the mutation in the dystrophin gene was digested, electrophoresed, and visualized with ethidium bromide. The genotype of one DLA-matched pair of donor and recipient is shown here. D indicates donor; R, recipient. The wild-type band (310 bp) and the mutant band (150 bp) are marked with arrows. (B) Schematic of the wild-type and xmd mutant alleles. The cxmd allele has a point mutation (a to g) in the consensus splice acceptor site of intron 6. As a consequence, alternative splicing either skips exon 7 entirely (solid line) or splices into a cryptic site 5 nucleotides 3-prime of the original splice site (dashed line). Both of these predominant splice variants introduce frame-shifts and early termination codons. Exon probes (TAMRA and MGB) were designed to hybridize to the wild-type junction between exons 6 and 7 and to distinguish between normally spliced mRNA and the splice variants created by the cxmd mutation.
Clinical xmd status
Serum creatine kinase (CK) levels were determined on all dogs within 24 hours of birth, on the day of transplantation (day 0), and at various time points after transplantation as clinically indicated. Progressive weakness; excessive salivation; progressive enlargement of the base of the tongue; inability to fully open the jaw; dysphagia; signs of pharyngeal and esophageal dysfunction; marked muscle atrophy of truncal and limb muscles accompanied by fibrosis and contractures; decreased growth rate; plantigrade stance; an abnormal stiff-limbed, short-strided, and shuffling gait; and exercise intolerance were apparent in all male xmd dogs. The dogs' clinical status was assessed twice daily and compared with the dog's own baseline status (day -30 of transplantation).
Total body irradiation (TBI), hematopoietic cell transplantation (HCT), and supportive care
The day of hematopoietic cell grafting was designated as day 0 (Figure 2). Clinically unaffected carrier females or wild-type (male and female) dogs served as HC transplant donors for affected littermates. DLA-identical donor marrow cells were aspirated 4 weeks before HCT (day -30) under general anesthesia through needles inserted into the humeri and femora, collected in heparinized tissue culture medium at 4°C, and then cryopreserved as described.29 On days -5 to -1, donors were treated by subcutaneous injections of recombinant canine granulocyte colony-stimulating factor (rcG-CSF; gift from Amgen, Thousand Oaks, CA), at 5 μg/kg twice daily. Leukaphereses for collection of G-CSF–“mobilized” peripheral blood mononuclear cells (G-PBMCs) were performed on day 0 using either an arterio-veinous shunt30 or a percutaneous central dual-lumen catheter31 and a continuous flow blood separator (Cobe 2997; Cobe BCT, Lakewood, CO).30 On day 0, recipients were administered TBI at a single dose of 920 cGy (n = 6) or 200 cGy (n = 1) delivered at 7 cGy/min from a linear accelerator (Varian CLINAC 4, Palo Alto, CA).29 The lower TBI dose was chosen because of the poor clinical condition of the recipient dog (G122). Within 4 hours of TBI, half of the previously cryopreserved marrow cells at doses of 1.8 to 5.6 (median 3.4) × 108 nucleated cells/kg and freshly isolated G-PBMCs (ranging from 1.8 to 18.3 [median, 7.7] × 108/kg) were infused intravenously; the remaining marrow and G-PBMC product was infused the following day. Postgrafting immunosuppression consisted of oral cyclosporine (CSP; a kind gift of Sangstat, Fremont, CA), 15 mg/kg twice daily, on days -2to35.32 Blood CSP levels were measured using the Cyclosporine Monoclonal Whole Blood Assay (Abbott Laboratories, Abbott Park, IL) on days 7 and 21 to ensure adequate immunosuppression was achieved in all recipients. All dogs were given standard supportive care that included subcutaneous fluids with electrolytes, systemic antibiotics (enrofloxacin [Baytril]), oral nonabsorbable antibiotics, neomycin sulfate, and polymyxin sulfate, from day -5 until the day of white cell count recovery to more than 1000/μL.32 Complete blood counts were obtained daily before and for the first 4 weeks after transplantation, twice weekly until week 26, and then once weekly thereafter (Figure 3). Serum chemistry levels were obtained once weekly to monitor kidney and liver changes. Each dog's clinical status was assessed twice daily. The end-points of the study were sustained allogeneic hematopoietic engraftment and donor marrow cell contribution to skeletal muscle. Completion time of the study was determined when the dogs died of natural DMD-related causes or when they were euthanized because of poor condition due to transplantation and/or DMD-related complications. When euthanized, the dogs underwent complete necropsies with histologic examinations of tissue samples.
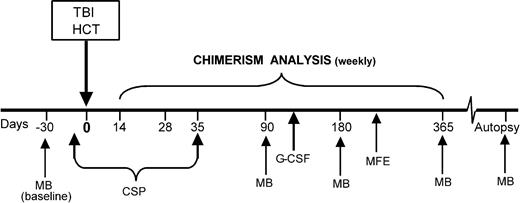
Transplant scheme. The xmd dogs were given 920 cGy or 200 cGy TBI on day 0, followed by HCT with G-CSF–mobilized PBMCs and bone marrow from DLA-identical littermates. Following one muscle biopsy, 4 recipients were given G-CSF for 5 days. Myofiber transplant study was used in 3 recipient dogs 9 months after HCT. Muscle biopsies were performed before and after HCT at different time points. CSP was used for postgrafting immunosuppression. TBI indicates total body irradiation; HC transplant source, G-CSF–mobilized PBMCs and bone marrow cells from DLA-identical littermate; MB, muscle biopsy; CSP, cyclosporine, 15 mg/kg orally twice a day, days -2 through +35; G-CSF, granulocyte colony-stimulating factor, 5 mg/kg subcutaneously twice a day for 5 days; and MFE, muscle fiber transplant.
Transplant scheme. The xmd dogs were given 920 cGy or 200 cGy TBI on day 0, followed by HCT with G-CSF–mobilized PBMCs and bone marrow from DLA-identical littermates. Following one muscle biopsy, 4 recipients were given G-CSF for 5 days. Myofiber transplant study was used in 3 recipient dogs 9 months after HCT. Muscle biopsies were performed before and after HCT at different time points. CSP was used for postgrafting immunosuppression. TBI indicates total body irradiation; HC transplant source, G-CSF–mobilized PBMCs and bone marrow cells from DLA-identical littermate; MB, muscle biopsy; CSP, cyclosporine, 15 mg/kg orally twice a day, days -2 through +35; G-CSF, granulocyte colony-stimulating factor, 5 mg/kg subcutaneously twice a day for 5 days; and MFE, muscle fiber transplant.
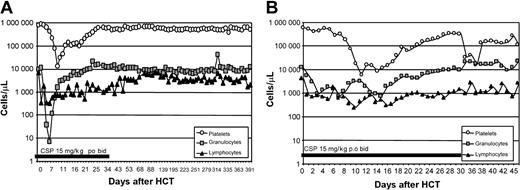
Hematopoietic cell recovery after HCT. Representative example of peripheral blood platelet (○), granulocyte (▪), and lymphocyte (▴) changes in the xmd dogs conditioned with 920 cGy (A) and 200 cGy (B) TBI and given a hematopoietic cell graft from DLA-identical littermates on day 0.
Hematopoietic cell recovery after HCT. Representative example of peripheral blood platelet (○), granulocyte (▪), and lymphocyte (▴) changes in the xmd dogs conditioned with 920 cGy (A) and 200 cGy (B) TBI and given a hematopoietic cell graft from DLA-identical littermates on day 0.
Over a period of 5 days, 4 recipient dogs were given rcG-CSF at 5 μg/kg subcutaneously, twice a day, between days +87 and +323 after HCT, as a means of “mobilizing” hematopoietic stem cells from the marrow into the bloodstream and the muscle.29
Engraftment and chimerism
Hematopoietic engraftment was assessed by sustained increases in granulocyte and platelet counts after the postirradiation nadir, by documentation of donor cells using microsatellite marker polymorphism analyses in specimens from peripheral blood and marrow,25 and by histologic features of the marrow from biopsy or autopsy specimens. The percent donor contribution to recipient hematopoiesis was quantified by estimating the proportion of donor-specific DNA among host DNA using phosphorimaging analyses33 or by eye approximation after autoradiography. This technique allowed detection of 2.5% to 97.5% donor cell chimerism. All donor hematopoietic chimerism was defined by a complete absence of a host-specific band for more than 24 weeks after transplantation.32
Muscle biopsy
Sartorious muscle biopsy samples from the recipients and donors were obtained 30 days before HCT (day -30) to serve as baselines. Biopsies were subsequently taken from recipients on days +90, +180, and +360 after HCT, and after muscle fiber transplantation. Each biopsy was divided into 3 pieces: the first piece was reserved for histologic analyses and prepared by embedding in optimal cutting temperature compound and freezing in liquid nitrogen–cooled isopentane; the second one was used for molecular analyses and prepared by snap freezing in liquid nitrogen; and the third sample was reserved for clonal analysis. The first 2 samples were stored at -80°C.
Immunofluorescence
Cryostat sections (6 μm) were cut for staining. Slides showing cross-orientation of the muscle fibers were selected for examination. Nonfixed sections were blocked in 3% bovine serum albumin in 1× Dulbecco phosphate-buffered saline (PBS; Gibco, Grand Island, NY) and incubated for 60 minutes at room temperature with the following primary antibodies: mouse anti–human dystrophin monoclonal antibody (NCL-DYS2, 20 μg/mL; Novocastra Laboratories); rabbit anti–human utrophin (UTR-103, 10 μg/mL; a kind gift from Stanley Froehner PhD, University of Washington, Seattle); and mouse anti–rat fetal myosin (NCL-MHCd; Novocastra Laboratories). Sections were then rinsed 3 times for 15 minutes in PBS and incubated in the dark for 30 minutes at room temperature with either rabbit anti–mouse fluorescein isothiocyanate (FITC)–conjugated or goat anti–rabbit Rhodamine-conjugated secondary antibody (1:100 dilution; Invitrogen, Frederick, MD). Preparations were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI; Sigma, St Louis, MO). Sections were then rinsed in PBS (3 × 15 minutes) and mounted in Vectashield (Vector Laboratories, Burlingame, CA). Normal mouse and rabbit isotype (Invitrogen) antibodies were used as negative controls. Muscle biopsy samples taken from wild-type dogs were used as positive controls. Staining was examined and photographed using a Nikon Eclipse 800 fluorescent microscope (Nikon, Tokyo, Japan). Images were acquired using a Plan Apo 20 ×/0.75 NA objective and were processed using Metamorph (Universal Imaging, West Chester, PA).
Quantitative reverse transcription–polymerase chain reaction (RT-PCR)
Total RNA was extracted by adding TRIzol solution (Invitrogen) to the muscle biopsy samples. The mixture of the aqueous phase and ethanol was then processed following the Rneasy Mini kit (QIAGEN, Valencia, CA). RNA from each sample (3 200-ng aliquots) was used for random hexamer-primed cDNA synthesis using Moloney murine leukemia virus reverse transcriptase (RT; Invitrogen). Primers and probes for canine dystrophin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, used as endogenous control) were designed using Primer Express software (Applied Biosystems, Foster City, CA). The probes were placed at the junction of 2 different exons. Both minor grove binder (MGB)– and 6-carboxy tetramethyl-rhodamine (TAMRA)–labeled probes were used for dystrophin, and a TAMRA probe was used for GAPDH. The primer pairs and probes for dystrophin and GAPDH were as follows: dystrophin forward primer, 5′-gcc tgg ctt tga acg ctc t-3′ and reverse primer, 5′-gct ggc aaa cca cac tat tcc-3′; TAMRA probe, 6FAM-tcc aca gtc ata ggc cag acc tgt ttg a-TAMRA and MGB probe, 6FAM-tca tag gcc aga cct gt-MGB; GAPDH forward primer, 5′-ggc aca gtc aag gct gag aac-3′ and reverse primer, 5′-cca gca tca ccc cat ttg at-3′; and TAMRA probe, 6FAM-ctt cca gga gcg aga tcc cgc c-TAMRA. All RT reactions were performed in triplicate on ABI Prism 7700 Sequence Detection System (Applied Biosystems). From each of the 3 RT reaction mixtures generated from each sample, 3 μL was used per 50 μL PCR (1X TaqMan Mastermix [Applied Biosystems], 800 nM of each primer and 250 nM of probe). TaqMan PCR conditions were 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Total RNA from normal muscle was used for establishing a standard curve. Threshold cycles (Ct) values for dystrophin were converted to arbitrary units and normalized to GAPDH, followed by normalization to the calibrator. The 72-bp and 101-bp PCR amplicons for dystrophin and GAPDH, respectively, were sequenced for sequence verification. Results are presented as means ± SEM for each set of triplicate RNA aliquots analyzed from each sample. Data were analyzed using a 2-tailed Student t test for comparison between data points. P < .05 was considered statistically significant.
Clonal analysis of canine muscle
Muscle biopsy specimens from HC transplant recipients approximately 2.25 cm3 in size were washed in PBS with penicillin-streptomycin (0.005%; Gibco Invitrogen), separated from fat and connective tissue, mechanically minced, and digested at 37°C with Collagenase type IV (Gibco Invitrogen) in 0.2% in PBS, for 45 minutes, and then with Collagenase and Trypsin–EDTA (ethylenediaminetetraacetic acid, 0.005%; Gibco Invitrogen) for an additional 45 minutes. Samples were then passed through successive 100-μm filters, collected, and centrifuged for 5 minutes at 1200 rpm. Cells were plated at a range of 10-fold dilutions on 100-mm gelatin-coated tissue culture dishes containing 10 mL growth medium. The initial medium contained Ham F10 nutrients supplemented with 0.8 mM calcium chloride (F10C), 15% preselected horse serum, 6 ng/mL human recombinant basic fibroblast growth factor (FGF), 2% embryonic day–12 (E12) chicken embryo extract, gentamycin, and fungizone. An additional 6 ng/mL basic FGF (bFGF) was added approximately every 12 hours. At 5 days, cultures were given a complete medium change without the addition of embryo extract, and 6 ng/mL bFGF continued to be added at 12-hour intervals for the next week. By this time, short myotubes began forming in many of the clones. The cultures were then aspirated, rinsed twice with Saline G, and replenished with medium lacking bFGF and containing 1 micromolar insulin. Clones containing many multinucleated myotubes became visible within the next week. These were marked, isolated with cloning cylinders, dissociated with trypsin, and the cell pellets were used for variable number of tandem repeat (VNTR) analysis in order to distinguish cells of HC transplant donor and recipient origin. Fibroblast clones, colonies exhibiting no obvious fusion, were similarly isolated and harvested.
Muscle fiber transplant study
At 245 to 280 days after HCT, excisional biopsies of the quadriceps muscle measuring 4 cm by 3 cm were obtained from both the HC transplant recipients (G123, G141, and G142) and their respective marrow donors. These were performed under general anesthesia observing sterile operative conditions. HC transplant recipients received portions of their donor's muscle biopsies, which were divided into 6 3-mm by 3-mm pieces and placed into the quadriceps defect and closed with absorbable suture. The location of the donor muscle fiber transplant was marked by nonabsorbable sutures. Following the procedure, the wound was closed with absorbable sutures and surgical staples. The area of biopsy and muscle fiber transplant was removed at the time of death (at necropsy) or 5 months after placement of the muscle transplant. This tissue was analyzed to determine both clonality and donor versus recipient origin using RT-PCR and VNTR analyses. At the time of biopsy, the site of the muscle fiber transplant was identified by the suture and was removed. Samples were also taken at 3 sites directly distal to the muscle transplant site (noted in Table 3 as adjacent sites 0, 1, and 2).
Percentage expression of wild-type dystrophin in recipient dog muscles before and after experimental manipulations using real-time PCR
Recipient's ID no. . | Before HCT (± SE) . | After HCT (± SE) . | After G-CSF (± SE) . | Muscle fiber transplants (± SE) . | Adjacent 0 (± SE) . | Adjacent 1 (± SE) . | Adjacent 2 (± SE) . |
|---|---|---|---|---|---|---|---|
| G121 | 0.01 (0.01) | < 0.01 | NA | ND | ND | ND | ND |
| G122 | < 0.01 | 0.03 (0.04) | NA | ND | ND | ND | ND |
| G125 | 0.05 (0.08) | 0.21 (0.25) | NA | ND | ND | ND | ND |
| G019 | 0.03 (0.05) | 0.15 (0.04) | 0.39 (0.17) | ND | ND | ND | ND |
| G123 | 0.03 (0.03) | 0.03 (0.03) | 0.12 (0.04) | 0.19 (0.30) | ND | ND | ND |
| G141 | 0.69 (0.36) | 0.02 (0.03) | 0.03 (0.02) | 20.67 (5.1)* | 0.08 (0.05) | 0.16 (0.04) | 6.10 (2.8)* |
| G142 | 0.03 (0.02) | 0.05 (0.08) | 0.02 (0.01) | 0.02 (0.03) | 1.9 (0.68)* | 0.02 (0.06) | < 0.01 |
Recipient's ID no. . | Before HCT (± SE) . | After HCT (± SE) . | After G-CSF (± SE) . | Muscle fiber transplants (± SE) . | Adjacent 0 (± SE) . | Adjacent 1 (± SE) . | Adjacent 2 (± SE) . |
|---|---|---|---|---|---|---|---|
| G121 | 0.01 (0.01) | < 0.01 | NA | ND | ND | ND | ND |
| G122 | < 0.01 | 0.03 (0.04) | NA | ND | ND | ND | ND |
| G125 | 0.05 (0.08) | 0.21 (0.25) | NA | ND | ND | ND | ND |
| G019 | 0.03 (0.05) | 0.15 (0.04) | 0.39 (0.17) | ND | ND | ND | ND |
| G123 | 0.03 (0.03) | 0.03 (0.03) | 0.12 (0.04) | 0.19 (0.30) | ND | ND | ND |
| G141 | 0.69 (0.36) | 0.02 (0.03) | 0.03 (0.02) | 20.67 (5.1)* | 0.08 (0.05) | 0.16 (0.04) | 6.10 (2.8)* |
| G142 | 0.03 (0.02) | 0.05 (0.08) | 0.02 (0.01) | 0.02 (0.03) | 1.9 (0.68)* | 0.02 (0.06) | < 0.01 |
Muscle fiber transplants refer to muscle biopsy samples taken from original marrow donors and implanted into muscle of HC transplant recipients; adjacent 0, 1 and 2 refer to successive sections of recipient muscle taken distal from the site of the muscle fiber transplant.
For before HCT/after HCT, P = .6; before HCT/after G-CSF, P = .7; and after HCT/after G-CSF, P = .3. All P values reflect the average of all dogs.
HCT indicates hematopoietic cell transplantation; G-CSF, granulocyte colony stimulating factor; NA, not applicable; ND, not determined.
‡ The number of wild-type dystrophin mRNA molecules (those containing the exon 6/exon 7 splice junction) was first standardized relative to GAPDH mRNA molecules in the same samples and then expressed as a percentage relative to dystrophin expression in a wild-type dog (100%).
Statistically significant difference between sample and pre-HCT sample
Results
HCT and clinical course
Each dog was genotyped based on a restriction endonuclease site polymorphism generated by the mutation (Figure 1). There were 6 xmd dogs that received myeloablative and 1 that received nonmyeloablative conditioning followed by HC transplants from unaffected DLA-matched littermates based on the protocol illustrated in Figure 2. All HC transplant recipients achieved complete or near-complete and sustained engraftment of donor hematopoietic cells in lymphoid and myeloid compartments in the peripheral blood and marrow as determined by microsatellite marker polymorphism analyses (Figures 3, 4; Table 1). The main clinical features related to c-xmd were evident in all affected dogs at the time of HCT, and their clinical status continued to worsen after HCT as evidenced by progressive weakness. Between days 27 and 46 after HCT, 3 dogs died due to either ileocecal intussusception in the absence of histologic evidence of intestinal GVHD (G121 and G125) or to hypercalcemia that did not respond to medical therapy (G122). There were 2 recipients that died on days 406 (G019) and 306 (G123) after HCT from heart and respiratory failure, respectively, the most common causes of death in DMD. More than 2 years after HCT, 2 dogs are alive (G142 and G141), but they have not exhibited improved muscle function following treatment.
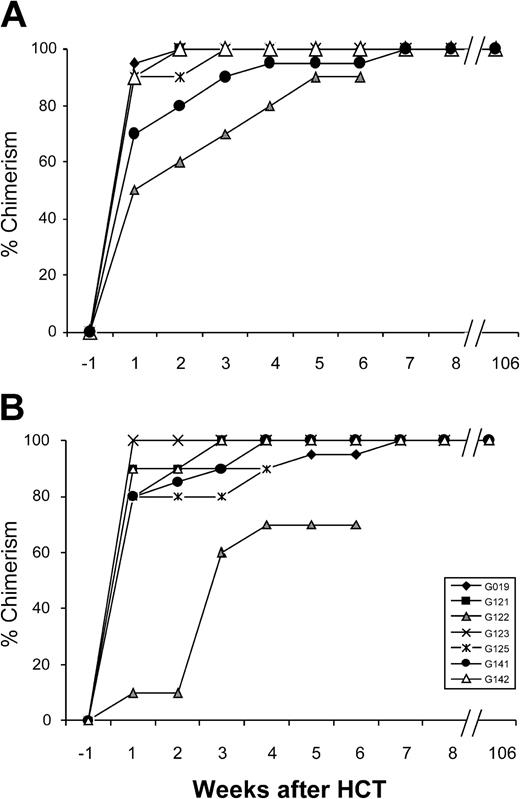
Hematopoietic donor chimerism in 7 dogs. Percent donor peripheral granulocytes (A) and mononuclear cells (B) after HCT measured weekly in 7 xmd dogs. All dogs achieved sustained and stable chimerism after 5 weeks.
Hematopoietic donor chimerism in 7 dogs. Percent donor peripheral granulocytes (A) and mononuclear cells (B) after HCT measured weekly in 7 xmd dogs. All dogs achieved sustained and stable chimerism after 5 weeks.
Clinical features of xmd dogs before and after HCT
Donor no./recipient no. . | Parents . | Age at HCT, d . | TBI, cGy . | % donor peripheral blood after HCT,*lymphocytes/monocytes . | Status after HCT . |
|---|---|---|---|---|---|
| G017 carrier/G019 | GR/Beagle | 135 | 920 | 100/100 | Dead d 406 (heart failure) |
| G119/G121 | GR/Beagle/Mini-Mongrel | 139 | 920 | 100/100 | Euthanized d 27 (intussusception) |
| G120 carrier/G122 | GR/Beagle/Mini-Mongrel | 153 | 200 | 90/70 | Euthanized d 31 (hypercalcemia) |
| G124/G123 | GR/Beagle/Mini-Mongrel | 166 | 920 | 100/100 | Euthanized d 306 (respiratory failure) |
| G124/G125 | GR/Beagle/Mini-Mongrel | 132 | 920 | 100/100 | Euthanized d 46 (intussusception) |
| G139 carrier/G141 | GR/GR | 145 | 920 | 100/90 | Alive d 745 (clinical disease progression) |
| G143/G142 | GR/GR | 137 | 920 | 100/100 | Alive d 750 (clinical disease progression) |
Donor no./recipient no. . | Parents . | Age at HCT, d . | TBI, cGy . | % donor peripheral blood after HCT,*lymphocytes/monocytes . | Status after HCT . |
|---|---|---|---|---|---|
| G017 carrier/G019 | GR/Beagle | 135 | 920 | 100/100 | Dead d 406 (heart failure) |
| G119/G121 | GR/Beagle/Mini-Mongrel | 139 | 920 | 100/100 | Euthanized d 27 (intussusception) |
| G120 carrier/G122 | GR/Beagle/Mini-Mongrel | 153 | 200 | 90/70 | Euthanized d 31 (hypercalcemia) |
| G124/G123 | GR/Beagle/Mini-Mongrel | 166 | 920 | 100/100 | Euthanized d 306 (respiratory failure) |
| G124/G125 | GR/Beagle/Mini-Mongrel | 132 | 920 | 100/100 | Euthanized d 46 (intussusception) |
| G139 carrier/G141 | GR/GR | 145 | 920 | 100/90 | Alive d 745 (clinical disease progression) |
| G143/G142 | GR/GR | 137 | 920 | 100/100 | Alive d 750 (clinical disease progression) |
No dog had graft-versus-host disease (GVHD).
HCT indicates hematopoietic cell transplantation; TBI, total body irradiation; GR, golden retriever; and d, day.
Percent donor chimerism was measured on a weekly basis after HCT. For the majority of dogs, values shown are those measured at autopsy. For G141 and G142, the chimerism was last evaluated on days 745 and 750 after HCT, respectively. C-xmd dogs were given DLA-identical HC transplants from their matched wild-type or carrier littermate. The graft consisted of donor marrow and G-CSF–mobilized peripheral blood
Dystrophin protein expression in xmd dogs
One month before HCT, skeletal muscle biopsies were taken from the left sartorius muscles to determine the baseline level of dystrophin expression. There were 2 different dystrophin antibodies recognizing the amino terminus and carboxy terminus of the protein used for immunostaining, and they gave similar results. Surprisingly, the number of dystrophin-positive fibers (so-called revertant fibers) varied considerably among dogs and showed a bimodal distribution (Table 2). Offspring from golden retrievers had very low levels of dystrophin-positive fibers (between 0% and 0.002% of the total fibers counted), consistent with previous publications.8 In contrast, some affected offspring (G121, G122, G125) from a mixed genetic background (crossing a Golden retriever with a Beagle/mini-Mongrel) had very high levels of dystrophin-positive fibers (ranging from 30%-60% of the total fibers counted). An exception was dog G123 that had a very low frequency of dystrophin-positive fibers (0.002%) compared with the very high levels (30%-60%) of its littermates G121, G122, and G125. This bimodal distribution of dystrophin-positive revertant fibers within the same outbred litter strongly suggested that an autosomal modifier locus (or loci) determined the abundance of revertant fibers. All dogs with high levels of dystrophin-positive fibers were clinically severely affected and died shortly after the HCT; therefore the high levels of dystrophin probably did not represent full-length protein but rather could be truncated dystrophin forms that are not functional. The influence of genetic background differences on revertant fiber frequencies is under further investigation, but while not yet understood, it did not affect the outcome of our analysis. The expression of utrophin, a protein that is known to compensate for lack of dystrophin in mouse models, was uniformly high in the all the dogs (75%-90% utrophin-positive muscle fibers).
Frequency of dystrophin and utrophin protein before transplantation, after G-CSF administration, and at necropsy in the muscle fibers of xmd HC transplant recipients as determined by immunofluorescence
. | % dystrophin-positive fibers . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Dystrophin background and recipient no. . | . | After HCT . | . | . | % utrophin-positive fibers before HCT . | |||
| . | Before HCT . | 3 mo . | After G-CSF . | Necropsy . | . | |||
| Low | ||||||||
| G019 | 0 | 0 | 0 | 0.07 | 90 | |||
| G123 | 0.23 | 0.08 | 0.41 | 0.03 | 75 | |||
| G141 | 0.05 | 0 | 0.04 | ND | 80 | |||
| G142 | 0.15 | 0.56 | 0.09 | ND | 85 | |||
| High | ||||||||
| G121 | 60 | ND | ND | 20 | 87 | |||
| G122 | 50 | ND | ND | 50 | 90 | |||
| G125 | 30 | ND | ND | 30 | 88 | |||
. | % dystrophin-positive fibers . | . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Dystrophin background and recipient no. . | . | After HCT . | . | . | % utrophin-positive fibers before HCT . | |||
| . | Before HCT . | 3 mo . | After G-CSF . | Necropsy . | . | |||
| Low | ||||||||
| G019 | 0 | 0 | 0 | 0.07 | 90 | |||
| G123 | 0.23 | 0.08 | 0.41 | 0.03 | 75 | |||
| G141 | 0.05 | 0 | 0.04 | ND | 80 | |||
| G142 | 0.15 | 0.56 | 0.09 | ND | 85 | |||
| High | ||||||||
| G121 | 60 | ND | ND | 20 | 87 | |||
| G122 | 50 | ND | ND | 50 | 90 | |||
| G125 | 30 | ND | ND | 30 | 88 | |||
HCT indicates hematopoietic cell transplantation; G-CSF, granulocyte colony-stimulating factor; and ND, not determined.
Dystrophin and utrophin expression were examined by immunofluorescence. Mouse anti–human dystrophin and rabbit anti–human utrophin antibodies were used.
To determine whether HCT contributed stem cells to skeletal muscle, the contralateral sartorius muscles were biopsied approximately 3 months after HCT to assess dystrophin expression. Because others have shown that muscle damage facilitates the contribution of marrow-derived cells to skeletal muscle repair,17 4 dogs were treated with G-CSF for 5 days after the first muscle biopsies to provide maximum access of the “mobilized” marrow stem cells to the regenerating region of the second muscle biopsy sites. After an additional 3 months, repeat muscle biopsies were performed at the same sites. In addition, necropsies with a wide sampling of skeletal muscle tissue were performed in the 3 dogs that died of complications following HCT. In both sets of post-HCT biopsies and in the necropsy samples, the number of dystrophin-positive fibers did not show significant differences from the pretransplantation biopsies (Table 2; Figure 5), remaining at levels of approximately 0.1% in the low revertant dogs and 20% to 50% in the high revertant dogs. In addition, heart sections from 2 dogs that died from cardiac complications (G019 and G123) were examined for dystrophin protein expression at autopsy. As was observed in skeletal muscle, the occasional rare (< 1/10 000) cell expressed dystrophin, but there was no definitive interpretation made because no adequate control (pretransplantation cardiac muscle biopsy) was taken for comparison. Thus, there was no evidence to suggest donor cell contribution to cardiac muscle.
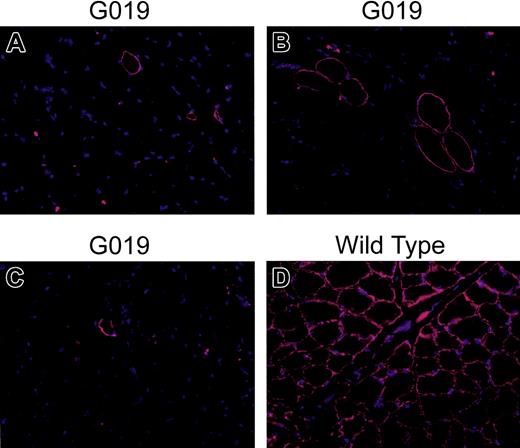
Dystrophin-positive fibers before and after HCT and at autopsy. Immunofluorescence staining was performed on muscle tissue sections from pre- and posttransplantation biopsies; necropsy and wild-type dogs were used as positive controls. Sartorius from (A) before HCT and (B) bicep from necropsy; (C) diaphragm from necropsy; (D) sartorius muscle from wild-type dog. Normal mouse immunoglobulin G (IgG; Invitrogen) was used as negative control (not shown). Nonfixed sections were blocked in 3% bovine serum albumin in 1 × Dulbecco phosphate-buffered saline (PBS; Gibco) and incubated for 60 minutes at room temperature with mouse anti–human dystrophin monoclonal antibody (NCL-DYS2, 20 μg/mL; Novocastra Laboratories). Sections were then rinsed 3 times for 15 minutes in PBS and incubated in the dark for 30 minutes at room temperature with rabbit anti–mouse Rhodamine-conjugated secondary antibody (1:100 dilution; Invitrogen). Preparations were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI; Sigma). Sections were then rinsed in PBS (3 × 15 minutes) and mounted in Vectashield (Vector Laboratories). Fields were chosen to show rare positive fibers. Original magnification × 20.
Dystrophin-positive fibers before and after HCT and at autopsy. Immunofluorescence staining was performed on muscle tissue sections from pre- and posttransplantation biopsies; necropsy and wild-type dogs were used as positive controls. Sartorius from (A) before HCT and (B) bicep from necropsy; (C) diaphragm from necropsy; (D) sartorius muscle from wild-type dog. Normal mouse immunoglobulin G (IgG; Invitrogen) was used as negative control (not shown). Nonfixed sections were blocked in 3% bovine serum albumin in 1 × Dulbecco phosphate-buffered saline (PBS; Gibco) and incubated for 60 minutes at room temperature with mouse anti–human dystrophin monoclonal antibody (NCL-DYS2, 20 μg/mL; Novocastra Laboratories). Sections were then rinsed 3 times for 15 minutes in PBS and incubated in the dark for 30 minutes at room temperature with rabbit anti–mouse Rhodamine-conjugated secondary antibody (1:100 dilution; Invitrogen). Preparations were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI; Sigma). Sections were then rinsed in PBS (3 × 15 minutes) and mounted in Vectashield (Vector Laboratories). Fields were chosen to show rare positive fibers. Original magnification × 20.
Dystrophin RNA expression
The intronic mutation at the exon 7 splice acceptor site results predominantly in mRNA species that either skip exon 7 or splice at a cryptic site 5 bases into exon 7, both events producing out-of-frame junctions and truncated proteins. To determine the contribution of donor (wild type) hematopoietic cells to recipient (xmd) muscle, the amount of wild-type dystrophin mRNA was analyzed by real-time PCR with 17 base and 28 base probes complementary to the exon 6 and exon 7 junction of wild-type dystrophin (Figure 1B). Similar results were obtained with each probe; only the MGB probe data are shown in Table 3. When normalized to the levels of GAPDH, the amount of wild-type dystrophin RNA in the pre-HCT biopsies of the affected dogs was between 100- and 10 000-fold lower than the amount in comparable muscle biopsies from normal dogs. Dogs with high levels of dystrophin-positive fibers by immunostaining did not have higher amounts of correctly spliced dystrophin mRNA nor could we identify alternative splice forms of RNA that correlated with the high expressing phenotype, indicating that the increased staining likely reflected an alteration in dystrophin protein stability or localization.
There was no significant change in the amount of dystrophin RNA in either the first post-HCT biopsies or after G-CSF administration, consistent with the lack of changes in the numbers of dystrophin-positive fibers. The low levels of dystrophin mRNA detected by the probes could be due to low levels of normally spliced transcript that occurred despite the mutation, or to a low level of hybridization of the probe to the aberrantly spliced transcript. Either way, we concluded that the amounts of wild-type dystrophin RNA were not altered by HCT under experimental conditions that could have detected a change of between 0.1% and 1%.
Clonal analysis of muscle satellite cells
The dystrophin protein and RNA analyses indicated that marrow-derived donor cells did not detectably contribute to the mature skeletal muscle fibers in the affected dogs that underwent transplantation. To determine whether donor stem cells were contributing to the satellite cell population, we clonally cultured cells derived from skeletal muscle biopsies. We obtained the biopsies approximately 12 weeks after HCT and 6 to 8 weeks after G-CSF mobilization. Muscle biopsy–derived cells were plated at low density and clones were visually scored after approximately 2 to 3 weeks of culture: myogenic clones consisted of small, round cells and contained multinucleated myotubes (Figure 6B); fibroblast clones contained densely growing flattened cells without fusion (Figure 6A). Muscle and fibroblast clones were isolated, and VNTR analyses were performed on the DNA from each clone to determine whether they were of donor or host origin (Figure 6C for an example of the VNTR analysis of select clones) and the relative percentage contribution of each genome. A total of 251 muscle clones were analyzed (Table 4). Of the muscle clones, 86% were entirely of the host genotype, and none was entirely of the donor genotype. Only 13.5% of the muscle clones had a minor representation (2%-35%) of the donor genotype. Similar percentages were found in pure fibroblast clones and in clones with mixed muscle and fibroblast phenotype (Table 4). Therefore, none of the isolated satellite cell clones was 100% donor genotype as would be expected from a donor-derived clone. The partial donor genotype in some clones is most likely due to cross-contamination between colonies that were caused by the frequent feeding schedule required for these cultures. Mixed donor/host clone genotypes might also be due to fusion of a circulating donor cell with a host cell in vivo. In this regard, 3 clones had a small round cell phenotype but without evidence of myotubes or muscle differentiation, and all 3 of these had relatively high donor DNA contribution (Table 4, undetermined/nonfused), perhaps reflecting a replicative postfusion cell with a chimeric phenotype. Based on the working definition that a satellite cell would give rise to a myogenic colony in our clonal assay, we did not have any evidence of donor-derived satellite cells following hematopoietic stem cell (HSC) transplantation and we could be 95% confident that the true probability of donor-derived satellite cells was less than 2%.
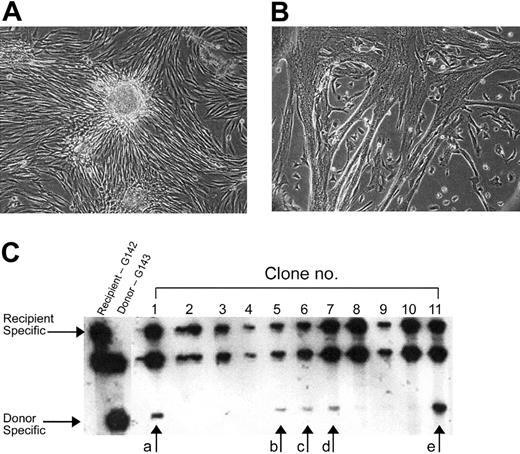
Clonal analyses of muscle-derived cells from HC transplant xmd recipients. Donor or recipient origin of the muscle-derived cell clones was determined by VNTR analyses. Fibroblast (A) or muscle clones (B) were isolated, harvested, and assayed by VNTR analyses (C). The arrows in panel C refer to the donor-specific bands in the satellite cell clones revealing a mixed genotype in 5 of the clones with donor contributions ranging from 2% to 35% (a = 10%, b ≤ 5%, c ≤ 5%, d ≤ 5%, and e = 20%). Original magnification × 40 (A,B).
Clonal analyses of muscle-derived cells from HC transplant xmd recipients. Donor or recipient origin of the muscle-derived cell clones was determined by VNTR analyses. Fibroblast (A) or muscle clones (B) were isolated, harvested, and assayed by VNTR analyses (C). The arrows in panel C refer to the donor-specific bands in the satellite cell clones revealing a mixed genotype in 5 of the clones with donor contributions ranging from 2% to 35% (a = 10%, b ≤ 5%, c ≤ 5%, d ≤ 5%, and e = 20%). Original magnification × 40 (A,B).
Clonal analysis of muscle-derived cells from xmd recipients of HC transplants
. | Percent donor DNA in muscle-derived clones from the dogs that received HCTs, no. clones . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Clone type . | 0% . | 1-10% . | 11-20% . | 21-35% . | 36-65% . | ||||
| Muscle | 164 | 19 | 4 | 1 | 0 | ||||
| Fibroblast | 22 | 3 | 0 | 0 | 0 | ||||
| Muscle and fibroblast | 29 | 4 | 2 | 0 | 0 | ||||
| Undertermined/nonfused | 0 | 0 | 0 | 1 | 2 | ||||
. | Percent donor DNA in muscle-derived clones from the dogs that received HCTs, no. clones . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|
| Clone type . | 0% . | 1-10% . | 11-20% . | 21-35% . | 36-65% . | ||||
| Muscle | 164 | 19 | 4 | 1 | 0 | ||||
| Fibroblast | 22 | 3 | 0 | 0 | 0 | ||||
| Muscle and fibroblast | 29 | 4 | 2 | 0 | 0 | ||||
| Undertermined/nonfused | 0 | 0 | 0 | 1 | 2 | ||||
The percentage of donor DNA in muscle and fibroblast clones as assessed by VNTR analysis. Total number of clones = 251.
Transplantation of donor muscle cells into affected HC transplant recipients
Although the recipient dogs should be tolerant of HC transplant donor muscle cells, we performed direct muscle cell transplantation to demonstrate that the donor cells could engraft, differentiate, and persist in the host. In 3 dogs, avascular teased fragments from donor muscle biopsies were directly transplanted. It was anticipated that muscle fibers would degenerate, and adherent satellite cells would have an opportunity to repopulate the host muscles in the areas of the transplants. Histologic examination at 3 weeks after the cell transplantations showed strong expression of dystrophin within fibers that were positive for fetal myosin in all 3 dogs, indicating recent regeneration (Figure 7), whereas biopsies 5 months later showed scattered dystrophin-positive fibers that were more abundant than before transplantation (Figure 7). These fibers did not express dystrophin uniformly, and we assumed that this was because of the continued fusion with mutant host satellite cells that diluted-out the contribution of the wild-type donor nuclei. Even at the later time point, however, the real-time PCR analyses also showed areas with increased amounts of dystrophin RNA at or near the site of the muscle transplant (Table 3), indicating that the donor myoblasts had the capacity to participate in muscle regeneration and form stable myofibers that were not subject to immune rejection.
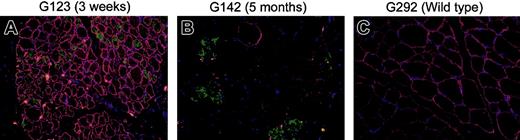
Dystrophin and fetal myosin staining in muscle fiber transplants in xmd hematopoietic chimeras at 3 weeks and 5 months after transplantation of muscle. Myofiber transplant from original HC donors to the xmd HC transplant recipients was performed on 3 pairs of dogs. Muscle biopsies were taken at 3 weeks (G123) and 5 months (G141 and G142) after transplantation and costained with dystrophin (red), fetal myosin (green, indicating regeneration), and DAPI (blue, showing nuclear staining). Immunofluorescence staining was performed as described in Figure 5, with the following primary antibodies: rabbit anti–mouse dystrophin (1:400, a kind gift from Jeff Chamberlain, University of Washington, Seattle) and mouse anti–rat fetal myosin (1:20, NCL-MHCd; Novocastra Laboratories). Donkey anti–rabbit Rhodamine-conjugated secondary antibody and rabbit anti–mouse FITC-conjugated secondary antibody (1:100 dilution; Invitrogen) were used for detection. Normal mouse and rabbit isotype (Invitrogen) antibodies were used as negative controls. Focal areas of brightly stained dystrophin-positive fibers were noted in panel A and were also positive for fetal myosin staining. Scattered staining for dystrophin and fetal myosin was observed in panel B after 5 months. However, dystrophin-positive fibers were now negative for fetal myosin. Wild-type muscle from G292 was used as control.
Dystrophin and fetal myosin staining in muscle fiber transplants in xmd hematopoietic chimeras at 3 weeks and 5 months after transplantation of muscle. Myofiber transplant from original HC donors to the xmd HC transplant recipients was performed on 3 pairs of dogs. Muscle biopsies were taken at 3 weeks (G123) and 5 months (G141 and G142) after transplantation and costained with dystrophin (red), fetal myosin (green, indicating regeneration), and DAPI (blue, showing nuclear staining). Immunofluorescence staining was performed as described in Figure 5, with the following primary antibodies: rabbit anti–mouse dystrophin (1:400, a kind gift from Jeff Chamberlain, University of Washington, Seattle) and mouse anti–rat fetal myosin (1:20, NCL-MHCd; Novocastra Laboratories). Donkey anti–rabbit Rhodamine-conjugated secondary antibody and rabbit anti–mouse FITC-conjugated secondary antibody (1:100 dilution; Invitrogen) were used for detection. Normal mouse and rabbit isotype (Invitrogen) antibodies were used as negative controls. Focal areas of brightly stained dystrophin-positive fibers were noted in panel A and were also positive for fetal myosin staining. Scattered staining for dystrophin and fetal myosin was observed in panel B after 5 months. However, dystrophin-positive fibers were now negative for fetal myosin. Wild-type muscle from G292 was used as control.
Discussion
Based on a number of reports that demonstrated the capacity of adult murine hematopoietic stem cells to participate in skeletal muscle regeneration, we tested the clinical utility of this proposed therapy in the canine xmd model. Studies in mice showed that bone marrow–derived cells could contribute to skeletal muscle fibers and potentially to the satellite cell compartment at levels that ranged from less than 1% to almost 10% of the fibers.7,16,19,34-36 From these studies, it was difficult to accurately predict the degree of contribution that might be obtained in a larger animal with both a longer life span and a greater demand for muscle repair. In fact, this uncertainty has been used to justify clinical trials of allogeneic bone marrow transplantation in human DMD. The canine xmd model has a similar clinical course to humans and has also been a validated model for preclinical studies of bone marrow transplantation. This model could thus be used to assess whether bone marrow transplantation, either alone or with adjunctive therapies to stimulate stem cell entry into skeletal muscle, would have benefit in human studies in patients with DMD.
In this study, we demonstrated that near-complete or complete donor hematopoietic chimerism in physically active dogs with DMD did not provide any clinical benefit, nor show any significant contribution of donor cells to the diseased skeletal muscle. Specifically, disease progression occurred in all of the dogs that underwent transplantation. Further, there were no significant increases in the percentages of dystrophin-positive fibers or the amount of wild-type dystrophin mRNA after HCT. Also, the use of G-CSF to mobilize donor hematopoietic stem cells from the marrow into the blood combined with the physical disruption of the blood/muscle barrier by surgical incision biopsy did not show evidence of donor reconstitution in the recipient's muscle, either at the level of myofibrils or satellite cells. The lack of donor muscle cells was not due to rejection of the dystrophin-positive cells because the allogeneic HCT induced tolerance for dystrophin in the host, as demonstrated by the fact that direct donor muscle cell transplantation showed engraftment and survival in the host. Therefore, allogeneic bone marrow transplantation, even with adjuvant procedures to disrupt the blood/muscle barrier and mobilize hematopoietic stem cells from the marrow into the bloodstream, did not show any significant contribution of donor cells to skeletal muscle in a clinically relevant large-animal model of DMD, indicating that this would not be a viable therapy for humans in its current form.
Not only did the dystrophin protein and RNA analyses indicate that marrow-derived donor cells did not detectably contribute to the mature skeletal muscle fibers in the xmd dogs, VNTR analyses performed on the DNA from each muscle-derived clone to determine whether they were of donor or host origin indicated that none of the isolated satellite cell clones was of donor origin. The partial donor genotype in some clones might be due to mixed colonies, as well as to the possible fusion of a circulating donor cell with a host cell. Only 3 clones had relatively high donor DNA contribution, perhaps a result of a postfusion cell with a chimeric phenotype. However, based on our clonal analyses, there was no evidence for donor-derived satellite cells following HCT. These findings are in agreement with those by one group of investigators using the mouse model showing that there is no donor-derived marrow cell contribution to satellite cells,37 but our canine data are in contrast to data from other mouse studies.36
It is possible that improved understanding of muscle cell regeneration might lead to insights that could improve stem cell delivery to skeletal muscle. For example, 2 recent studies suggested that populations of muscle stem cells could be isolated from adult muscle and used for therapy. The first showed that Wnt signaling from damaged muscle recruited a CD45+/Sca1+ cell into the myogenic lineage,38 whereas the second identified a population of stem cells associated with blood vessels in skeletal muscle, termed mesoangioblasts, that could be expanded in vitro and delivered to muscle through intra-arterial injection.39 Although we did not identify direct contributions of donor bone marrow–derived cells to the host skeletal muscle in our study, dogs with stable chimerism would be ideal models in which to study muscle stem cell transplantation. It will thus be of great interest to determine whether muscle-derived stem cells or mesoangioblasts from the hematopoietic cell donor dogs can reconstitute muscle in stable chimeric recipients.
Prepublished online as Blood First Edition Paper, August 24, 2004; DOI 10.1182/blood-2004-06-2247.
Supported by grants from Muscular Dystrophy Association–USA and Parent Project Muscular Dystrophy, and grants HD47175 (Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center), CA78902, DK56465, CA15704, and AR18860 from the National Institutes of Health, Bethesda, MD.
C.D.'A. and Z.W. contributed equally to this work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Alix Joslyn and Brian Steinmetz and their technicians at the Fred Hutchinson Cancer Research Center, as well as technicians in hematology and pathology, for their expertise. Benjamin Weigler, DVM PhD, and Michelle Spector, DVM, are thanked for their care of the dogs. The authors are grateful to Connie Chan, Sue Carbonneau, Helen Crawford, Bonnie Larson, Karen Carbonneau, and Deborah Gayle for their administrative assistance with these studies and the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal