Abstract
High gene transfer efficiencies have been difficult to achieve in hematopoietic progenitor cells (HPCs) but are important to therapeutic success of HPC gene therapy. Efficient gene transfer is especially challenging with use of column-purified vector for clinical application, as opposed to centrifuged vector commonly used for research. We investigated novel approaches to increase transduction by using a clinically applicable protocol and quantities of column-purified lentiviral vector. Recognizing the association of adenosine 5′-triphosphate (ATP)-binding cassette (ABC) transporters with HPC biology, we investigated the effect of transporter inhibitors on transduction. We found the ABC transporter inhibitor verapamil improved transduction efficiency 2- to 6-fold into CD34+ cells isolated from mobilized peripheral blood, bone marrow, and cord blood. Verapamil also improved transduction in human SCID (severe combined immunodeficient) repopulating cell (SRC) transduction 3- to 4-fold, resulting in 80% to 90% transduction levels in mice receiving primary and secondary transplants without alterations in multilineage reconstitution. Additional ABC transporter substrate inhibitors like quinidine, diltiazem, and ritonavir also enhanced transduction 2- to 3-fold, although ABC transporter inhibitors that are not substrates did not. Enhanced transduction was not observed in mature hematopoietic cells, neurospheres, mesenchymal stem cells, or hepatocytes. Enhancement of transduction in HPCs was observed with vesicular stomatitis virus-G (VSV-G)-pseudotyped lentiviral vector but not with vector pseudotyped with RD114. Thus, we present a new approach for efficient delivery to primitive HPCs by VSV-G-pseudotyped lentiviral vectors. (Blood. 2004;104:364-373)
Introduction
Stem cells possess tremendous potential for cellular and gene therapies of many human diseases. Efficient gene transfer into hematopoietic progenitor cells (HPCs), derived from the bone marrow (BM), mobilized peripheral blood (mPB), or cord blood (CB), could provide valuable therapies for genetic deficiencies, as well as for cancer and infectious diseases such as HIV. Reported retroviral transduction efficiencies into CD34+ progenitors range from 10% to 60%, and more than 75% in the nonobese diabetic-severe combined immunodeficiency (NOD-SCID) human HPC engraftment mouse model.1-8 Many factors contribute to the range in CD34+ cell transduction, including the elements in the vector, the source of cells, the culture conditions and timing of vector addition, and notably the quality and purity of the vector.
Investigators have used the method of high-speed centrifugation to increase vector titers to achieve higher transduction efficiencies. In contrast, our vector is purified by size exclusion chromatography instead of centrifugation to ensure vector safety and purity for human use, because column purification allows sterile filtering without significantly reducing vector titers.9,10 We sought to optimize lentiviral vector transduction into CD34+ progenitors by using materials and methods that are clinically relevant.
The adenosine 5′-triphosphate (ATP)-binding cassette (ABC) transporter family of proteins is well characterized for its ability to efflux a wide range of lipophilic and structurally unrelated small molecules and drugs, particularly chemotherapeutic agents and HIV protease inhibitors.11,12 There are compelling data suggesting the functional importance of ABC transporters in the biology of hematopoietic stem cells.13-20 We theorized that the high levels of ABC transporter expression may in part contribute to the lower transduction efficiency obtained in primitive hematopoietic cells, and that an ABC transporter inhibitor may increase CD34+ progenitor transduction efficiency.
High reproducible transduction efficiencies are paramount for therapeutic success of stem cell gene therapy. We present here that ABC transporter substrate inhibitors increase transduction of vesicular stomatitis virus-G (VSV-G)-pseudotyped lentiviral vectors in CD34+ progenitor cells, and we investigate the mechanism for this phenomenon. The data describe a clinically applicable approach for ensuring efficient lentivirus vector transduction into hematopoietic progenitor cells, with implications for efficient gene delivery for treatment of genetic and infectious disease.
Materials and methods
Reagents
Verapamil (V4629), diltiazem (D2521), probenecid (P8761), quinidine (Q0875), sodium orthovanadate (S6508), α-tocopherol (T3251), arsenic trioxide (A1010), and reserpine (R0875) were purchased from Sigma Aldrich (Bedford, MA) as United States Pharmacopeia (USP) grade reagents when available. Ritonavir (Norvir) was obtained from Abbott Laboratories (Abbott Park, IL).
Protamine sulfate (P4505) and polybrene (H2968) were purchased from Sigma. The anti-transforming growth factor (TGF)β antibody (clone AB-100-NA) was purchased from R&D Systems (Minneapolis, MN).
Vector production
VRX494 is a safety-modified, gutted HIV vector that expresses the enhanced green fluorescent protein (eGFP) cDNA from the endogenous HIV long terminal repeat (LTR). The clinical trial lentivector VRX496 is identical to VRX494, except that it does not encode eGFP, but instead a 186-base truncated tag region derived from eGFP. Vector was produced by calcium phosphate-mediated transfection of 2 plasmids: the vector construct and the helper construct, VIRPAC, which produce higher titers than a 3-plasmid system. This vector production system has been meticulously designed for safety to avoid generation of a replication competent lentivirus (RCL).21 Laboratory scale, or high-speed centrifuged vector, was produced by calcium phosphate-mediated transfection of vector plasmid with a VSV-G-containing packaging construct into 293 cells. Supernatant was collected every 12 hours from 24 to 48 hours after transfection, pooled, then concentrated by high-speed centrifugation at 10 000g for 12 hours. The vector was resuspended in storage buffer at approximately 0.0025 of its original volume and then frozen at -80°C. The titer of the centrifuged vector was determined by the frequency of GFP expression on HeLa-tat cells and was found to range between 1 × 109 and 5 × 109 transducing units (TUs) per mL.
A scalable purification process was developed for clinical or manufacturing-grade vector. Supernatant of plasmid-transfected 293 cells was harvested at 24, 36, and 48 hours after transfection. The supernatant was filtered through a series of cartridge filters of decreasing pore diameter down to 0.22 μm, then concentrated by ultrafiltration. Buffer was exchanged, and benzonase was added to destroy contaminating plasmid and cellular DNA. The vector was then purified by size exclusion chromatography and formulated in storage buffer.9 The final vector preparation was sterilized by filtration through a 0.22-μm cartridge filter. Vector titers were determined by TaqMan polymerase chain reaction (PCR) for copy number in HeLa-tat cells,10 and the vector was proven free of replication-competent lentivirus.10 Final vector titers achieved were more than 108 TU/mL.
Cells
Frozen CD34+ progenitors (> 98% purity) derived from adult bone marrow, adult granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood, and cord blood were purchased from AllCells (Berkeley, CA) under a protocol approved by the institutional review board (IRB). Additional peripheral blood CD34+ cells mobilized by cyclophosphamide and G-CSF were generous gifts from Dr Christian Chabannon (Cellular Therapy Institute, Cancer Center, Marseilles, France). Rhesus macaque CD34+ cells derived from bone marrow were kindly provided by Dr Boris Camels (National Institutes of Health [NIH], Bethesda, MD).
Transduction
CD34+ progenitors were thawed according to manufacturer's specifications, then cultured at 37°C for 1 hour. Cells were cultured with the inhibitor at 37°C for 0 to 90 minutes before addition of vector. Vector (25 TU/cell) was added to plates coated with 1.5 μg/cm2 Retronectin (Takara, Japan), then cells were added to the plate and cultured at 37°C. Twenty-four hours after inhibitor addition, the cells were washed free of vector and drug and were resuspended in fresh medium containing 25 TU/cell vector and cultured at 37°C. CD34+ progenitors were cultured in Iscove modified Dulbecco medium (IMDM; Invitrogen, Carlsbad, CA) supplemented with 1 × BIT (serum substitute containing albumin-insulin-transferrin; Stem Cell Technologies, Vancouver, BC, Canada) and 25 to 100 ng/mL thrombopoietin (TPO), Flt-3 ligand (Flt-3L), and stem cell factor (SCF; R&D Systems). Cells used in in vitro assays were washed free of vector 4 days after the start of transduction. Cells transplanted into mice were washed free of vector 2 days after the start of transduction and immediately transplanted into mice. Cells (100 000) were analyzed by Taqman PCR to determine vector copy number.
Culture of CD34+ progenitor cells
CD34+ progenitors were maintained in liquid culture in IMDM plus 1 × BIT supplemented with 25 ng/mL TPO, SCF, and Flt-3L, or plated in colony-forming unit (CFU) and long-term culture-initiating cell (LTC-IC) assays. Cells (500-1000) were plated in methylcellulose (H3231; Stem Cell Technologies) supplemented with 50 μg/mL SCF, 10 μg/mL interleukin 3 (IL3), 10 μg/mL granulocyte-macrophage colony-stimulating factor (GMCSF), and 1 U/mL erythropoietin (EPO). Colony growth was scored 2 weeks after plating and was analyzed by flow cytometry for eGFP expression. For the LTC-IC assay, 1 to 3 × 105 cells were overlaid on MS-5 stromal cells. Cells underwent demidepopulation weekly and at each maintenance point were analyzed by flow cytometry. After 5 weeks, LTC cells were plated into methylcellulose supplemented as described earlier for formation of secondary CFUs. Colonies were scored 2 weeks after plating and analyzed for eGFP expression by flow cytometry.
Mature hematopoietic cell transduction and culture
CD4+ T lymphocytes were purified from peripheral blood mononuclear cells (PBMCs) by positive selection and cultured in X-vivo 15 (Cambrex, Walkersville, MD) supplemented with 100 U/mL IL2 and anti-CD3/CD28-conjugated beads.22,23 T cells were transduced with 10 TU/cell VRX494 in the presence or absence of 5 to 50 μM ritonavir or 10 to 50 μg/mL verapamil. PBMCs were transduced such that vector comprised 5% of the medium in the presence or absence of 0.5 to 1 mM probenecid or 10 to 50 μg/mL verapamil. Cells were washed 24 hours after drug addition to remove the drugs, followed by a second addition of vector at the same dose.
Transduction in primary cells and nonhematopoietic stem cell progenitors
Frozen neurospheres were obtained from Clonetics (Cambrex) and were cultured according to manufacturer's protocol. Cells (50 000) were plated onto polyethylenimine (PEI)-coated chamber slides and allowed to attach overnight. Cells were transduced at 20 to 50 TU/cell VRX494 in the presence or absence of 50 μg/mL verapamil, 100 μM diltiazem, or 50 μM quinidine for 24 hours, washed; a second dose of VRX494 was added. After 2 weeks, neurosphere progeny were stained by immunohistochemistry for NeuN (Chemicon, Temecula, CA; MAB377), GFAP (Chemicon; MAB3402), and O4mAb (Chemicon; MAB345).
Frozen mesenchymal stem cells (MSCs) were obtained from Clonetics and cultured according to manufacturer's protocol. After one passage, 1 × 105 cells were replated onto 6-well plates and allowed to attach for 6 hours. Cells were cultured with 10 or 100 TU/cell VRX494 in the presence or absence of 25 or 50 μg/mL verapamil or 100 or 200 μM diltiazem for 24 hours, washed; a second dose of VRX494 was added without ABC transporter substrates. MSCs were analyzed for transduction efficiency 10 days later.
Frozen hepatocytes were obtained from Clonetics and cultured according to the manufacturer's protocol. Cells (1-2 × 105) were plated onto 10 μg/cm2 collagen type I-coated 24-well plates and were allowed to attach for 6 hours. Cells were cultured with 10 to 50 TU/cell VRX494 in the presence or absence of 25 to 50 μg/mL verapamil, 100 μM diltiazem, or 30 μM ritonavir. Five days later, cells were analyzed for transduction efficiency.
Rhodamine 123 efflux assay
One million cells were incubated with 0.5 μg/mL Rhodamine 123 (Sigma) in X-vivo 10 (Cambrex) medium for 20 minutes at 37°C in the presence or absence of ABC transporter substrates. Cells were washed twice with X-vivo 10 then resuspended in X-vivo 10 in the presence or absence of ABC transporter substrates as appropriate. Retention of Rhodamine 123 in cells was measured by flow cytometry. Aliquots were measured immediately and at various time points thereafter. Efflux rates were determined by changes in the mean fluorescence intensity (MFI) over 90 minutes. Fold-difference was determined by comparisons of the rate of change between cells exposed to ABC transporter substrates and no drug controls.
Mice
NOD.CB17-Prkdcscid/J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in microisolator cages at Bioqual (Rockville, MD). All experiments were approved by the Institutional Animal Care and Use Committee (IACUC). Six- to 7-week-old mice were sublethally irradiated with 350 cGy (137Cs source), injected with antimouse CD122 (IL2-Rβ) antibody purchased from Pharmingen (San Jose, CA), and then received transplants the next day with 2 × 105 cord blood CD34+ cells. Mice killed at 6 to 8 weeks after engraftment were tested for eGFP expression in CD45+, CD34+, CD33+, CD19+, and CD3+ subsets from the bone marrow by using phycoerythrin (PE)-conjugated antibodies (clones HI30, 581, WM53, HIB19, UCHT1, respectively; Pharmingen, San Diego, CA) by flow cytometry (FACSCalibur; Becton Dickinson). Mice killed at 14 weeks after engraftment were analyzed for eGFP expression in the CD45+, CD34+, CD33+, CD19+, and CD3+ subsets in the marrow, spleen, and thymus. For secondary transplantation, half of the total marrow recovered from the 14-week primary mouse was transplanted into 350-cGy-irradiated secondary recipient NOD.CB17-Prkdcscid/J mice. Mice were killed 8 weeks after engraftment and analyzed for eGFP expression in the CD45+, CD33+, and CD19+ subsets from the bone marrow.
Results
Efficient CD34+ transduction can be achieved with high levels of centrifuged vector
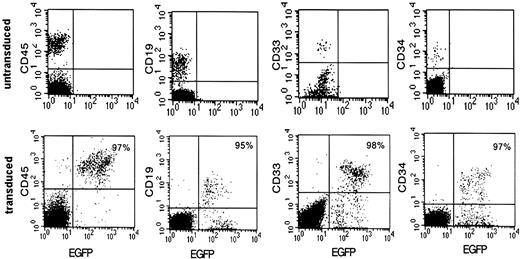
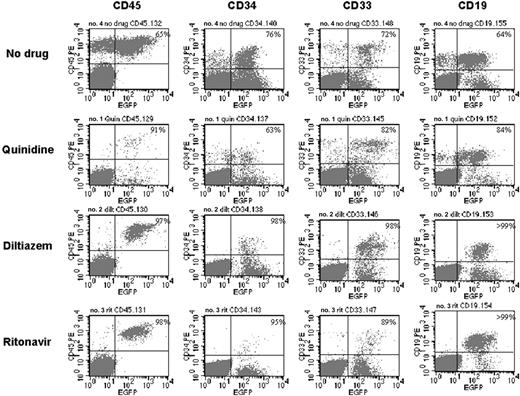
NOD-SCID mice received transplants with cord blood CD34+ cells transduced at 42% efficiency and were subsequently killed 6 to 8 weeks after transplantation. Bone marrow was recovered, and eGFP expression was analyzed among cells expressing the panhuman hematopoietic marker CD45, the primitive cell marker CD34, a myeloid cell marker CD33, and a lymphoid cell marker CD19 (Figure 1). Total human cells could be transduced up to 97% as measured by eGFP CD45+ cells in one mouse. High levels of transgene expression were preserved in the CD34+ lineage and in cells differentiating into myeloid and lymphoid lineages (Figure 1 middle panels). Total eGFP expression in CD45+ cells isolated from the bone marrow of 2 additional mice reconstituted in parallel with the one shown in Figure 1 was 88% and 89%. However, very high levels of vector were required to achieve this efficient level of transduction, as explained later.
Efficient HPC transduction with use of high levels of vector. Transduction efficiencies in a NOD-SCID mouse 8 weeks after infusion with CD34+ progenitors transduced at approximately 500 TU/cell with high-speed centrifuged vector for 4 consecutive days. Cells isolated from the bone marrow were analyzed by flow cytometry. Percentages of eGFP-expressing human cells are shown in the upper right corner.
Efficient HPC transduction with use of high levels of vector. Transduction efficiencies in a NOD-SCID mouse 8 weeks after infusion with CD34+ progenitors transduced at approximately 500 TU/cell with high-speed centrifuged vector for 4 consecutive days. Cells isolated from the bone marrow were analyzed by flow cytometry. Percentages of eGFP-expressing human cells are shown in the upper right corner.
Investigators have generally titered GFP-expressing vectors on cells by measuring GFP fluorescence.24 Because vectors that are used in the clinic will not express GFP, such as our clinical vector VRX496, alternate methods for vector titration are required. We and others have found that the titer of centrifuged vector determined by GFP expression can underestimate the level of transduction by about 10-fold when the cells are analyzed for vector copy number by TaqMan PCR.25 Therefore, for the experiment presented in Figure 1, CD34+ cells were transduced with centrifuged vector at 50 transducing units (TU) per cell as measured by GFP titer in HeLa-tat cells for 4 consecutive days, resulting in a dose of 200 TU/cell. Taking into account the underestimation of titer by GFP (confirmed by retrospective assessment by copy number on the vector), these cells were actually transduced at approximately 500 TU/cell on each of the 4 consecutive days. This high level of vector required for efficient transduction is unrealistic for large-scale clinical application.
CD34 transduction using column-purified VRX494
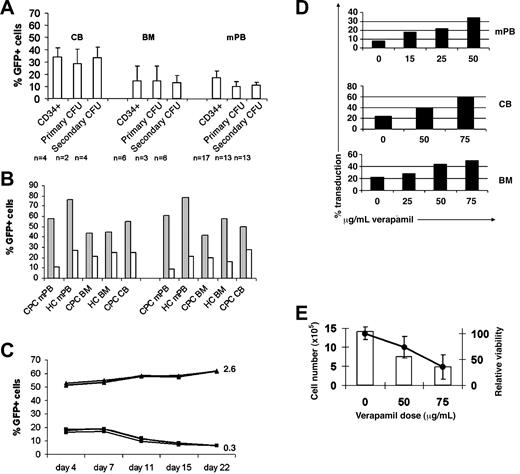
To investigate a dose and protocol that would be feasible to use at a clinical scale, CD34+ cells were transduced at 50 TU/cell for 2 consecutive days. For this experiment and all others presented in this report, titer was determined not by GFP expression but by copy number in HeLa-tat cells.20 This method yielded transduction levels of 22% to 50% in CB, 10% to 32% in mPB, and 15% to 22% in BM (Figure 2A). Expression of eGFP stabilized between day 7 and day 11, similar to a pattern observed by Haas et al.26 LTC-ICs were transduced at the same frequency as more committed progenitors as defined by primary CFUs.
High CD34+ progenitor cell transduction efficiencies by using clinically relevant amounts of vector with verapamil. (A) Average vector transduction efficiencies in CB, BM, and mPB CD34+ progenitors in liquid culture 11 days after transduction, primary CFU, and secondary CFU (LTC-IC) cultures. CD34+ progenitors were transduced at 50 TU/cell for each of 2 consecutive days with column-purified vector. CFUs were pooled and analyzed by flow cytometry. Standard error bars are shown, and the population for each experiment is shown below the graph. (B) Stable eGFP expression in mPB CD34 (left) and LTC-IC (right) in the presence (▦) or absence (□) of 75 μg/mL verapamil after transduction with 25 TU/cell for each of 2 consecutive days with high-speed centrifuged (HC) or column-purified (CPC) vector. Pooled CFUs were analyzed by flow cytometry. (C) Stability of eGFP expression in 3 separate CB CD34+ stroma-free liquid cultures after transduction with (▴) or without (▪) verapamil using 25 TU/cell column-purified vector for 2 days. Average vector copy number per cell at day 11 after transduction is displayed to the right of the graph for each culture. (D) Verapamil dose-dependent increase in CD34+ cell transduction efficiency in mPB, CB, and BM cultures using column-purified vector. (E) Total cell number averaged between mPB, BM, and CB cultures (□) and relative cellular viability (•) 4 days after transduction at increasing doses of verapamil. Of note, verapamil-induced decrease in SRC viability is not observed. Error bars indicate SD.
High CD34+ progenitor cell transduction efficiencies by using clinically relevant amounts of vector with verapamil. (A) Average vector transduction efficiencies in CB, BM, and mPB CD34+ progenitors in liquid culture 11 days after transduction, primary CFU, and secondary CFU (LTC-IC) cultures. CD34+ progenitors were transduced at 50 TU/cell for each of 2 consecutive days with column-purified vector. CFUs were pooled and analyzed by flow cytometry. Standard error bars are shown, and the population for each experiment is shown below the graph. (B) Stable eGFP expression in mPB CD34 (left) and LTC-IC (right) in the presence (▦) or absence (□) of 75 μg/mL verapamil after transduction with 25 TU/cell for each of 2 consecutive days with high-speed centrifuged (HC) or column-purified (CPC) vector. Pooled CFUs were analyzed by flow cytometry. (C) Stability of eGFP expression in 3 separate CB CD34+ stroma-free liquid cultures after transduction with (▴) or without (▪) verapamil using 25 TU/cell column-purified vector for 2 days. Average vector copy number per cell at day 11 after transduction is displayed to the right of the graph for each culture. (D) Verapamil dose-dependent increase in CD34+ cell transduction efficiency in mPB, CB, and BM cultures using column-purified vector. (E) Total cell number averaged between mPB, BM, and CB cultures (□) and relative cellular viability (•) 4 days after transduction at increasing doses of verapamil. Of note, verapamil-induced decrease in SRC viability is not observed. Error bars indicate SD.
A number of strategies, including spinoculation, sequential vector additions, cytokine prestimulation, absence of cytokine costimulation, use of serum substitutes, use of polybrene and protamine sulfate, and use of a TGF-β antibody to modulate cell cycle, were directly compared in attempts to increase CD34+ cell transduction efficiency. However, these approaches resulted in only marginal increases, ranging from 2% to 10% above baseline (data not shown).
ABC transporter inhibitor, verapamil, improves lentivector transduction in hematopoietic progenitor cells
We observed consistently higher efficiencies when transduction occurred in the presence of verapamil compared with control cultures. Improved transduction was observed with the column-purified clinical (CPC) vector VRX494, as well as laboratory high-speed centrifuged (HC) vector. VRX494 transduction of mPB CD34+ cells at 25 TU/cell for 2 days with CPC vector increased transduction from 10% efficiency in the absence of verapamil to 60% efficiency in the presence of 75 μg/mL verapamil. Verapamil-dependent increases in transduction were also observed in BM and CB CD34+ cultures (Figure 2B). Equivalent increases in eGFP expression were observed in long-term culture (LTC-IC) representing more primitive progenitor cells. Transduction levels remained stable over a 22-day culture after transduction (Figure 2C). Verapamil also improved vector copy numbers in transduced cells from 0.3 to 2.6 as evaluated on day 11 after transduction.
Increasing concentrations of verapamil directly modulated transduction efficiency (Figure 2D). Verapamil improved CD34+ mPB transduction efficiency in a dose-dependent manner, ranging from 8% at baseline to 32% in the presence of 50 μg/mL drug. No significant improvements in transduction were observed with doses below 25 μg/mL. CD34+ CB cells were transduced at 25% efficiency without verapamil compared with 40% with 50 μg/mL verapamil and 60% with 75 μg/mL verapamil. CD34+ BM cells were transduced at 20% efficiency without verapamil compared with 45% efficiency in 50 μg/mL verapamil.
There were no differences in total CFU production in the absence or presence of verapamil (142 ± 39 versus 187 ± 51 total CFUs per 1000 cells plated, respectively). In the absence of drug, our culture conditions produced an approximate 1:2 ratio of erythroid burst-forming unit (BFU-E) to granulocyte-macrophage colony-forming unit (CFU-GM). However, after verapamil exposure, the ratio changed to 4:1. Because total CFU counts were not altered, verapamil does not appear to be selectively toxic to CFUs.
Verapamil resulted in a dose-dependent decrease in cellular viability and total cell numbers, although the severity of the decrease was highly variable between experiments (Figure 2E). At 4 days after transduction, the total cell number was reduced by 2- to 3-fold compared with cells transduced in the absence of verapamil. Verapamil exerted slightly less toxicity toward CB than against BM- or mPB-derived cells.
Verapamil improves transduction of primary and secondary SCID-repopulating cells
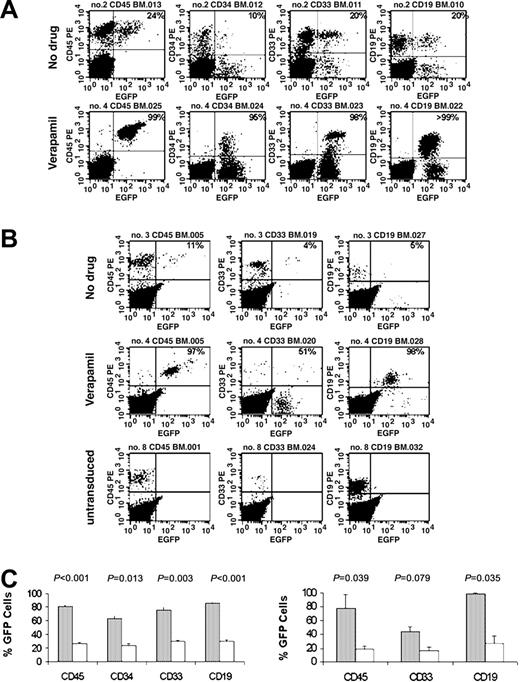
NOD-SCID mice received transplants with 2 × 105 CB CD34+ progenitors that had been transduced with 25 TU/cell with CPC VRX494 in the presence or absence of verapamil. Transplanted CD34+ cells were transduced at 23% without verapamil and 51% with verapamil. Mice were killed 6 to 8 weeks after transplantation, and cells were recovered from bone marrow and spleen. Expression of eGFP was observed in an average of 31% ± 15% of CD45+ cells recovered from mice in the control group. In contrast, eGFP expression was observed in an average of 81% ± 22% of CD45+ cells recovered from the mice in the verapamil group (Figure 3A; Table 1). Multilineage eGFP expression was observed in both cohorts, with statistically greater expression in all lineages from mice in the verapamil cohort. Differences in human cell engraftment and in the proportion of each lineage between the verapamil and control cohorts were statistically insignificant (Table 1).
Verapamil enhancement of SRC transduction. (A) Representative flow cytometric analysis at 6 to 8 weeks after primary transplant with stem cells that were transduced with 25 TU column-purified vector for 2 days in 75 μg/mL verapamil or without verapamil. (B) Flow cytometric analysis of a mouse that received a secondary transplant infused with cells that either were untransduced or were transduced with 25 TU column-purified vector for 2 days in 75 μg/mL verapamil or without verapamil. (C) Average percentage of transgene-expressing repopulating cells from mice during primary transplantation (left; n = 7, ▦;n = 8, □; ± SEM) and secondary transplantation (right; n = 2, ▦;n = 3, □; ± SD) in the presence (gray bars) or absence (open bars) of verapamil.
Verapamil enhancement of SRC transduction. (A) Representative flow cytometric analysis at 6 to 8 weeks after primary transplant with stem cells that were transduced with 25 TU column-purified vector for 2 days in 75 μg/mL verapamil or without verapamil. (B) Flow cytometric analysis of a mouse that received a secondary transplant infused with cells that either were untransduced or were transduced with 25 TU column-purified vector for 2 days in 75 μg/mL verapamil or without verapamil. (C) Average percentage of transgene-expressing repopulating cells from mice during primary transplantation (left; n = 7, ▦;n = 8, □; ± SEM) and secondary transplantation (right; n = 2, ▦;n = 3, □; ± SD) in the presence (gray bars) or absence (open bars) of verapamil.
Percentage of human cell engraftment and transduction in NOD-SCID mice
. | Engraftment . | . | . | Transduction . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | No verapamil . | Verapamil . | P . | No verapamil . | Verapamil . | P . | ||||
| 6-8 wk after primary transplantation | ||||||||||
| BM CD45 | 1.2 ± 0.8 | 2.2 ± 1.1 | .439 | 31 ± 15 | 81 ± 22 | .003 | ||||
| BM CD34 | 0.3 ± 0.2 | 0.3 ± 0.0 | .312 | 26 ± 24 | 55 ± 35 | .170 | ||||
| BM CD33 | 0.6 ± 0.6 | 0.6 ± 0.3 | .315 | 29 ± 24 | 77 ± 37 | .038 | ||||
| BM CD19 | 0.7 ± 0.5 | 1.5 ± 1.0 | .459 | 31 ± 18 | 85 ± 12 | .001 | ||||
| 14 wk after primary transplantation + αIL-2Rβ Ab | ||||||||||
| BM CD45 | 71 ± 23 | 70 ± 4 | .987 | 19 ± 9 | 81 ± 9 | .005 | ||||
| BM CD34 | 18 ± 5 | 13 ± 1 | .335 | 20 ± 13 | 85 ± 12 | .011 | ||||
| BM CD33 | 9 ± 2 | 12 ± 2 | .279 | 29 ± 8 | 73 ± 13 | .017 | ||||
| BM CD19 | 50 ± 16 | 51 ± 1 | .977 | 27 ± 19 | 89 ± 3 | .022 | ||||
| Spl CD45 | 24 ± 20 | 17 ± 2 | .737 | 34 ± 8 | 79 ± 5 | .006 | ||||
| Spl CD19 | 27 ± 19 | 28 ± 13 | .946 | 35 ± 8 | 97 ± 3 | .002 | ||||
| 8 wk after secondary transplantation | ||||||||||
| BM CD45 | 0.2 ± 0.2 | 0.1 ± 0.2 | .936 | 19 ± 13 | 78 ± 27 | .039 | ||||
| BM CD33 | 0.1 ± 0.1 | 0.0 ± 0.0 | .495 | 16 ± 13 | 44 ± 10 | .079 | ||||
| BM CD19 | 0.1 ± 0.1 | 0.1 ± 0.1 | .724 | 27 ± 26 | 99 ± 1 | .035 | ||||
. | Engraftment . | . | . | Transduction . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | No verapamil . | Verapamil . | P . | No verapamil . | Verapamil . | P . | ||||
| 6-8 wk after primary transplantation | ||||||||||
| BM CD45 | 1.2 ± 0.8 | 2.2 ± 1.1 | .439 | 31 ± 15 | 81 ± 22 | .003 | ||||
| BM CD34 | 0.3 ± 0.2 | 0.3 ± 0.0 | .312 | 26 ± 24 | 55 ± 35 | .170 | ||||
| BM CD33 | 0.6 ± 0.6 | 0.6 ± 0.3 | .315 | 29 ± 24 | 77 ± 37 | .038 | ||||
| BM CD19 | 0.7 ± 0.5 | 1.5 ± 1.0 | .459 | 31 ± 18 | 85 ± 12 | .001 | ||||
| 14 wk after primary transplantation + αIL-2Rβ Ab | ||||||||||
| BM CD45 | 71 ± 23 | 70 ± 4 | .987 | 19 ± 9 | 81 ± 9 | .005 | ||||
| BM CD34 | 18 ± 5 | 13 ± 1 | .335 | 20 ± 13 | 85 ± 12 | .011 | ||||
| BM CD33 | 9 ± 2 | 12 ± 2 | .279 | 29 ± 8 | 73 ± 13 | .017 | ||||
| BM CD19 | 50 ± 16 | 51 ± 1 | .977 | 27 ± 19 | 89 ± 3 | .022 | ||||
| Spl CD45 | 24 ± 20 | 17 ± 2 | .737 | 34 ± 8 | 79 ± 5 | .006 | ||||
| Spl CD19 | 27 ± 19 | 28 ± 13 | .946 | 35 ± 8 | 97 ± 3 | .002 | ||||
| 8 wk after secondary transplantation | ||||||||||
| BM CD45 | 0.2 ± 0.2 | 0.1 ± 0.2 | .936 | 19 ± 13 | 78 ± 27 | .039 | ||||
| BM CD33 | 0.1 ± 0.1 | 0.0 ± 0.0 | .495 | 16 ± 13 | 44 ± 10 | .079 | ||||
| BM CD19 | 0.1 ± 0.1 | 0.1 ± 0.1 | .724 | 27 ± 26 | 99 ± 1 | .035 | ||||
Data are presented as mean ± SD. Ab indicates antibody; and Spl, spleen.
A second cohort of mice was injected with an antibody against the murine IL-2Rβ chain, which reduces natural killer (NK) cell activity to increase human cell engraftment and to improve thymic repopulation,27 prior to transplantation with 2 × 105 CD34+ CB cells. The mice were killed 14 weeks after transplantation. Human cell engraftment was on average 70% in these mice, and differences in engraftment between the verapamil and control groups were statistically insignificant. EGFP expression in CD45+ cells was an average of 19% ± 9% from mice in the control group compared with an average of 81% ± 9% for mice from the verapamil group. Compared with the control group, the verapamil group had significantly higher eGFP expression in the CD34+, CD33+, and CD19+ lineages. Mice from the verapamil cohort also had statistically greater eGFP expression in splenocytes (Table 1). Only one mouse exhibited thymic reconstitution, with eGFP expression in 47% of CD45+ cells and in 23% of CD3+ cells. These data demonstrate myeloid and B- and T-lymphoid differentiation of lentiviral-transduced SCID repopulating cells (SRCs).
Finally, we tested whether transduced cells could reconstitute NOD-SCID mice during secondary transplantation. Fifteen to 20 million total bone marrow cells (consisting of a mix approximately 70% human and 30% murine cells) obtained from the 14-week αIL-2Rβ antibody-treated mice presented earlier received were transplanted into 8-week-old recipient NOD-SCID mice, which were killed 8 weeks later. Human cell engraftment in these mice was very low, but human cells were detected. Similar transduction levels were observed between primary and secondary transplantation (Figure 3B; Table 1). An average of 78% ± 27% of CD45+ cells expressed eGFP in the verapamil cohort, compared with an average of 19% ± 13% of CD45+ cells in the marrow of control mice. Remarkably, one mouse that received a secondary transplant expressed eGFP in 97% of CD45+ cells. Transduction levels in CD33+ and CD19+ cells were consistently greater in the verapamil cohort than in the control cohort. Figure 3C represents graphically average SRC transduction levels from the primary and secondary transplantation experiments in Figure 3A-B. These data demonstrate that verapamil increases the transduction of multipotential human hematopoietic progenitor cells with no alterations in multilineage reconstitution.
Structurally diverse ABC transporter substrates improve CD34+ progenitor transduction
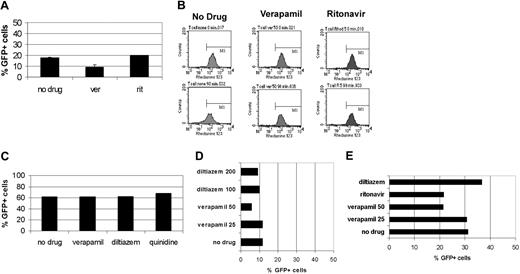
A panel of substrates and inhibitors of ABC transporters were systematically tested for their ability to increase transduction into CD34+ progenitors derived from mPB or CB. The p-glycoprotein substrate inhibitors quinidine (a sodium channel inhibitor) and diltiazem (a calcium channel inhibitor) increased transduction levels into CD34+ progenitors an average of 3.0- and 2.5-fold above baseline, respectively (Table 2; Figure 4A-B). The HIV protease inhibitor ritonavir, an ABC transporter inhibitor substrate, improved CD34+ progenitor transduction an average of 2.0-fold above baseline. In comparison, verapamil improved transduction an average of 3.4-fold above baseline in these experiments. The multidrug resistance-associated protein (MRP) inhibitor probenecid increased CD34+ progenitor transduction levels 1.5-fold above baseline. No conclusions from the differences between drug-mediated enhancements of transduction may be made because we did not optimize dosing; therefore, it may be possible to further improve on the transduction efficiency above the level described here. The level of transduction in LTC-IC was consistent with the transduction rate into CD34+ progenitor cells. In contrast, the agents tested that are ABC transporter inhibitors but not substrates had no effect on transduction efficiency. Reserpine and the adenosine 5′-triphosphatase (ATPase) inhibitor sodium vanadate did not improve CD34+ progenitor transduction efficiency (Figure 4).
Effect of various ABC transporter inhibitors on human CD34+ cell transduction
. | Fold change vs no drug control . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | LTC-IC transduction . | CD34+ transduction . | CD34+ viability . | 4-day fold expansion . | |||
| Untransduced | NA | NA | 1.15 ± 0.14 | 2.8 ± 1.0 | |||
| No drug | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.00 ± 0.00 | 1.6 ± 0.7 | |||
| Verapamil | 3.7 ± 1.9 | 3.4 ± 1.6 | 0.52 ± 0.19 | 1.4 ± 1.1 | |||
| Diltiazem | 2.8 ± 1.2 | 2.5 ± 0.9 | 0.76 ± 0.21 | 1.2 ± 0.8 | |||
| Quinidine | 3.6 ± 1.6 | 3.0 ± 1.7 | 0.43 ± 0.23 | 0.7 ± 0.6 | |||
| Ritonavir | 2.2 ± 1.4 | 2.0 ± 1.1 | 0.72 ± 0.32 | 1.2 ± 1.1 | |||
| Probenecid | 1.5 ± 0.0 | 1.5 ± 0.2 | 0.85 ± 0.30 | 1.5 ± 0.7 | |||
| Reserpine | 0.8 ± 0.3 | 1.0 ± 0.0 | 0.54 ± 0.06 | 1.0 ± 0.2 | |||
| Sodium vanadate | 0.6* | 0.9* | 0.46* | 0.5* | |||
| Arsenic trioxide | 2.0* | 2.0* | 0.85* | 1.4* | |||
| α-tocopherol | 1.2* | 1.2* | 0.93* | 1.0* | |||
| Quinidine + verapamil | 2.9* | 5.1* | 0.39* | 0.6* | |||
| Diltiazem + verapamil | 3.7* | 3.6* | 0.43* | 0.9* | |||
| Quinidine + diltiazem | 4.5* | 3.1* | 0.43* | 0.5* | |||
| Quinidine + ritonavir | 4.4* | 5.1* | 0.34* | 1.0* | |||
| Ritonavir + verapamil | ND | 2.9* | 0.56* | 0.7* | |||
| Diltiazem + ritonavir | ND | 6.1* | 0.44* | 0.4* | |||
. | Fold change vs no drug control . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | LTC-IC transduction . | CD34+ transduction . | CD34+ viability . | 4-day fold expansion . | |||
| Untransduced | NA | NA | 1.15 ± 0.14 | 2.8 ± 1.0 | |||
| No drug | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.00 ± 0.00 | 1.6 ± 0.7 | |||
| Verapamil | 3.7 ± 1.9 | 3.4 ± 1.6 | 0.52 ± 0.19 | 1.4 ± 1.1 | |||
| Diltiazem | 2.8 ± 1.2 | 2.5 ± 0.9 | 0.76 ± 0.21 | 1.2 ± 0.8 | |||
| Quinidine | 3.6 ± 1.6 | 3.0 ± 1.7 | 0.43 ± 0.23 | 0.7 ± 0.6 | |||
| Ritonavir | 2.2 ± 1.4 | 2.0 ± 1.1 | 0.72 ± 0.32 | 1.2 ± 1.1 | |||
| Probenecid | 1.5 ± 0.0 | 1.5 ± 0.2 | 0.85 ± 0.30 | 1.5 ± 0.7 | |||
| Reserpine | 0.8 ± 0.3 | 1.0 ± 0.0 | 0.54 ± 0.06 | 1.0 ± 0.2 | |||
| Sodium vanadate | 0.6* | 0.9* | 0.46* | 0.5* | |||
| Arsenic trioxide | 2.0* | 2.0* | 0.85* | 1.4* | |||
| α-tocopherol | 1.2* | 1.2* | 0.93* | 1.0* | |||
| Quinidine + verapamil | 2.9* | 5.1* | 0.39* | 0.6* | |||
| Diltiazem + verapamil | 3.7* | 3.6* | 0.43* | 0.9* | |||
| Quinidine + diltiazem | 4.5* | 3.1* | 0.43* | 0.5* | |||
| Quinidine + ritonavir | 4.4* | 5.1* | 0.34* | 1.0* | |||
| Ritonavir + verapamil | ND | 2.9* | 0.56* | 0.7* | |||
| Diltiazem + ritonavir | ND | 6.1* | 0.44* | 0.4* | |||
Data are presented as mean ± SD. See also Figure 4. NA indicates not applicable; and ND, not done.
Single experiment.
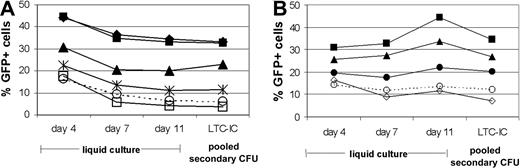
Many ABC transporter inhibitors stably enhance transduction in mPB CD34+ progenitors. (A) Cells were transduced with VRX494 in the presence of 50μg/mL verapamil (▪), 50 μM quinidine (♦), 100 μM diltiazem (▴), 1 mM probenecid (asterisk), 25 μM reserpine (□), and no drug (○ and dashed line). (B) Cells were transduced with VRX494 in the presence of 50 μg/mL verapamil (▪), 100 μM diltiazem (▴), 20 μM ritonavir (•), 250 μM sodium vanadate (⋄), and no drug (○ and dashed line).
Many ABC transporter inhibitors stably enhance transduction in mPB CD34+ progenitors. (A) Cells were transduced with VRX494 in the presence of 50μg/mL verapamil (▪), 50 μM quinidine (♦), 100 μM diltiazem (▴), 1 mM probenecid (asterisk), 25 μM reserpine (□), and no drug (○ and dashed line). (B) Cells were transduced with VRX494 in the presence of 50 μg/mL verapamil (▪), 100 μM diltiazem (▴), 20 μM ritonavir (•), 250 μM sodium vanadate (⋄), and no drug (○ and dashed line).
There was dose-dependent toxicity observed with all drugs tested; viability fell to 43% to 85% relative to nondrug-treated controls (Table 2). Likewise, the expansion of cells was reduced up to 2-fold in the presence of these drugs compared with transduction in the absence of drugs. CD34+ progenitors differentiate during culture in cytokines, and the proportion of cells expressing the CD34 antigen decreases over time. After 7 days, the proportion of cells remaining CD34+ was similar for all conditions tested, and similar CFU counts were measured among the different drug conditions. This finding indicates that at this level of culture, no drug-mediated selection could be detected.
Combinations of functionally dissimilar ABC transporter substrates were cocultured to determine whether transduction levels could be improved synergistically. Near-complete cytotoxicity was observed when 2 substrates were cocultured at the conventional dose used in the experiments mentioned earlier. Reduced cytotoxicity was observed when 2 substrates were cocultured at half their conventional doses, but transduction efficiencies were similar to the level obtained with use of a single substrate at its conventional dose. This suggests an additive but not synergistic effect during coculture of functionally dissimilar ABC transporter substrates (Table 2).
Structurally diverse ABC transporter substrates increase transduction of human NOD-SCID repopulating cells
To test whether additional ABC transporter substrates similarly improve SRC transduction, NOD-SCID mice received transplants with 2 × 105 cord blood CD34+ progenitors that had been transduced with clinical grade VRX494 without ABC transporter substrates (n = 14) or in the presence of verapamil (n = 13), quinidine (n = 4), diltiazem (n = 6), or ritonavir (n = 7). Mice were killed 6 to 14 weeks after transplantation. Cells recovered from bone marrow were tested for eGFP expression among cells expressing CD45, CD34, CD33, or CD19. As evident from Table 3, the assay has inherent variability between mice within a single cohort. Expression of eGFP was observed in an average of 47% of CD45+ cells recovered from mice in the control group and 76% for verapamil, 60% for quinidine, 65% for diltiazem, and 52% for ritonavir (Figure 5; Table 3). Cells transduced in the presence of ABC transporter substrates exhibited higher SRC transduction. In particular, verapamil, diltiazem, and ritonavir caused the greatest increases in transduction in CD19+ cells compared with no drug controls. Further, increased median CD45+ cell transduction was observed for all drugs compared with the control. The proportion of each lineage was similar when transduced in the presence or absence of ABC transporter substrates. These data suggest that SRCs are transduced at a higher rate in the presence of ABC transporter substrates, with no apparent toxicity toward subsequent repopulation potential.
Percentage of multilineage differentiation in NOD-SCID mice of human CD34+ cells transduced in the presence of ABC transporter inhibitors
Mouse* . | No drug . | Verapamil . | Quinidine . | Diltiazem . | Ritonavir . |
|---|---|---|---|---|---|
| CD45 | |||||
| 1 | 8 | 21 | 24 | 24 | 11 |
| 2 | 12 | 22 | 65 | 32 | 39 |
| 3 | 16 | 43 | 91 | 78 | 40 |
| 4 | 24 | 75 | 94 | 94 | 55 |
| 5 | 30 | 86 | — | 97 | 59 |
| 6 | 37 | 86 | — | — | 61 |
| 7 | 40 | 87 | — | — | 98 |
| 8 | 46 | 88 | — | — | — |
| 9 | 47 | 91 | — | — | — |
| 10 | 57 | 94 | — | — | — |
| 11 | 65 | 97 | — | — | — |
| 12 | 71 | 99 | — | — | — |
| 13 | 76 | 99 | — | — | — |
| 14 | 79 | — | — | — | — |
| Mean | 44 | 76 | 69 | 65 | 52 |
| SD | 24 | 28 | 32 | 35 | 27 |
| Median | 43 | 87 | 78 | 78 | 55 |
| CD34 | |||||
| 1 | 2 | 9 | 20 | 27 | 13 |
| 2 | 10 | 32 | 45 | 42 | 27 |
| 3 | 10 | 33 | 63 | 65 | 29 |
| 4 | 13 | 38 | 97 | 67 | 38 |
| 5 | 16 | 55 | — | 70 | 48 |
| 6 | 23 | 76 | — | 98 | 95 |
| 7 | 30 | 82 | — | — | 97 |
| 8 | 34 | 84 | — | — | — |
| 9 | 50 | 93 | — | — | — |
| 10 | 53 | 95 | — | — | — |
| 11 | 54 | 97 | — | — | — |
| 12 | 60 | 99 | — | — | — |
| 13 | 76 | — | — | — | — |
| 14 | 80 | — | — | — | — |
| Mean | 36 | 66 | 57 | 62 | 49 |
| SD | 26 | 31 | 33 | 25 | 34 |
| Median | 32 | 79 | 54 | 66 | 38 |
| CD33 | |||||
| 1 | 7 | 14 | 20 | 11 | 31 |
| 2 | 7 | 14 | 45 | 22 | 57 |
| 3 | 20 | 63 | 50 | 56 | 63 |
| 4 | 20 | 63 | 82 | 69 | 67 |
| 5 | 32 | 67 | — | 71 | 89 |
| 6 | 35 | 75 | — | 97 | 95 |
| 7 | 42 | 79 | — | — | — |
| 8 | 48 | 82 | — | — | — |
| 9 | 52 | 93 | — | — | — |
| 10 | 60 | 95 | — | — | — |
| 11 | 71 | 98 | — | — | — |
| 12 | 72 | 99 | — | — | — |
| 13 | 79 | 100 | — | — | — |
| 14 | 83 | — | — | — | — |
| 15 | 94 | — | — | — | — |
| Mean | 45 | 73 | 49 | 54 | 67 |
| SD | 26 | 29 | 26 | 33 | 23 |
| Median | 45 | 79 | 47 | 62 | 65 |
| CD19 | |||||
| 1 | 8 | 33 | 12 | 44 | 33 |
| 2 | 13 | 70 | 24 | 50 | 67 |
| 3 | 20 | 76 | 50 | 57 | 75 |
| 4 | 20 | 81 | 84 | 94 | 81 |
| 5 | 31 | 82 | — | 95 | 97 |
| 6 | 42 | 83 | — | 100 | 100 |
| 7 | 47 | 87 | — | — | 100 |
| 8 | 49 | 91 | — | — | — |
| 9 | 50 | 95 | — | — | — |
| 10 | 62 | 97 | — | — | — |
| 11 | 63 | 100 | — | — | — |
| 12 | 64 | 100 | — | — | — |
| 13 | 70 | 100 | — | — | — |
| 14 | 75 | — | — | — | — |
| 15 | 83 | — | — | — | — |
| Mean | 44 | 84 | 42 | 73 | 79 |
| SD | 22 | 18 | 32 | 26 | 24 |
| Median | 48 | 87 | 37 | 75 | 81 |
Mouse* . | No drug . | Verapamil . | Quinidine . | Diltiazem . | Ritonavir . |
|---|---|---|---|---|---|
| CD45 | |||||
| 1 | 8 | 21 | 24 | 24 | 11 |
| 2 | 12 | 22 | 65 | 32 | 39 |
| 3 | 16 | 43 | 91 | 78 | 40 |
| 4 | 24 | 75 | 94 | 94 | 55 |
| 5 | 30 | 86 | — | 97 | 59 |
| 6 | 37 | 86 | — | — | 61 |
| 7 | 40 | 87 | — | — | 98 |
| 8 | 46 | 88 | — | — | — |
| 9 | 47 | 91 | — | — | — |
| 10 | 57 | 94 | — | — | — |
| 11 | 65 | 97 | — | — | — |
| 12 | 71 | 99 | — | — | — |
| 13 | 76 | 99 | — | — | — |
| 14 | 79 | — | — | — | — |
| Mean | 44 | 76 | 69 | 65 | 52 |
| SD | 24 | 28 | 32 | 35 | 27 |
| Median | 43 | 87 | 78 | 78 | 55 |
| CD34 | |||||
| 1 | 2 | 9 | 20 | 27 | 13 |
| 2 | 10 | 32 | 45 | 42 | 27 |
| 3 | 10 | 33 | 63 | 65 | 29 |
| 4 | 13 | 38 | 97 | 67 | 38 |
| 5 | 16 | 55 | — | 70 | 48 |
| 6 | 23 | 76 | — | 98 | 95 |
| 7 | 30 | 82 | — | — | 97 |
| 8 | 34 | 84 | — | — | — |
| 9 | 50 | 93 | — | — | — |
| 10 | 53 | 95 | — | — | — |
| 11 | 54 | 97 | — | — | — |
| 12 | 60 | 99 | — | — | — |
| 13 | 76 | — | — | — | — |
| 14 | 80 | — | — | — | — |
| Mean | 36 | 66 | 57 | 62 | 49 |
| SD | 26 | 31 | 33 | 25 | 34 |
| Median | 32 | 79 | 54 | 66 | 38 |
| CD33 | |||||
| 1 | 7 | 14 | 20 | 11 | 31 |
| 2 | 7 | 14 | 45 | 22 | 57 |
| 3 | 20 | 63 | 50 | 56 | 63 |
| 4 | 20 | 63 | 82 | 69 | 67 |
| 5 | 32 | 67 | — | 71 | 89 |
| 6 | 35 | 75 | — | 97 | 95 |
| 7 | 42 | 79 | — | — | — |
| 8 | 48 | 82 | — | — | — |
| 9 | 52 | 93 | — | — | — |
| 10 | 60 | 95 | — | — | — |
| 11 | 71 | 98 | — | — | — |
| 12 | 72 | 99 | — | — | — |
| 13 | 79 | 100 | — | — | — |
| 14 | 83 | — | — | — | — |
| 15 | 94 | — | — | — | — |
| Mean | 45 | 73 | 49 | 54 | 67 |
| SD | 26 | 29 | 26 | 33 | 23 |
| Median | 45 | 79 | 47 | 62 | 65 |
| CD19 | |||||
| 1 | 8 | 33 | 12 | 44 | 33 |
| 2 | 13 | 70 | 24 | 50 | 67 |
| 3 | 20 | 76 | 50 | 57 | 75 |
| 4 | 20 | 81 | 84 | 94 | 81 |
| 5 | 31 | 82 | — | 95 | 97 |
| 6 | 42 | 83 | — | 100 | 100 |
| 7 | 47 | 87 | — | — | 100 |
| 8 | 49 | 91 | — | — | — |
| 9 | 50 | 95 | — | — | — |
| 10 | 62 | 97 | — | — | — |
| 11 | 63 | 100 | — | — | — |
| 12 | 64 | 100 | — | — | — |
| 13 | 70 | 100 | — | — | — |
| 14 | 75 | — | — | — | — |
| 15 | 83 | — | — | — | — |
| Mean | 44 | 84 | 42 | 73 | 79 |
| SD | 22 | 18 | 32 | 26 | 24 |
| Median | 48 | 87 | 37 | 75 | 81 |
See also Figure 5. —indicates no mice used.
Each numbered row contains data for 1 individual mouse.
Multilineage differentiation in representative NOD/SCID mice receiving transplants of CD34+ progenitors transduced in the presence of various ABC transporter inhibitors. Flow cytometric analysis of cells isolated from the bone marrow of mice that received transplants of human CD34+ progenitor cells transduced with no drug or in the presence of quinidine, diltiazem, or ritonavir. The proportions of GFP-positive human cells are depicted in the top right quadrant.
Multilineage differentiation in representative NOD/SCID mice receiving transplants of CD34+ progenitors transduced in the presence of various ABC transporter inhibitors. Flow cytometric analysis of cells isolated from the bone marrow of mice that received transplants of human CD34+ progenitor cells transduced with no drug or in the presence of quinidine, diltiazem, or ritonavir. The proportions of GFP-positive human cells are depicted in the top right quadrant.
ABC transporter substrates do not improve transduction in every cell type
We were interested whether this phenomenon was hematopoietic progenitor specific. To investigate whether ABC transporter inhibitor substrates might improve transduction in mature hematopoietic cells, or in stem cells of nonhematopoietic origin, we transduced cells with low levels of vector that would prevent saturation and masking of an inhibitor-specific enhancement of transduction.
Of particular interest for T lymphocytes was whether transduction was increased in the presence of the HIV protease inhibitor ritonavir, as this drug could serve a dual role by retarding viral replication in HIV-1-infected T cells. Primary CD4+ T lymphocytes were transduced at 10 TU/cell. No differences in CD4+ T-lymphocyte transduction levels were observed in the presence or absence of verapamil or ritonavir in 2 independent experiments (Figure 6A). Although there was no evidence of improved transduction, ritonavir and verapamil inhibited rhodamine efflux 3- to 4-fold compared with no drug, verifying the functional activity of the ABC transporter substrates at these doses (Figure 6 B).
ABC transporter substrates that are inhibitors do not increase transduction in T cells, hepatocytes, or nonhematopoietic progenitor cells. (A) Primary CD4+ T lymphocytes suboptimally transduced at 10 TU/cell alone or in the presence of 50 μg/mL verapamil or 50 μM ritonavir. Error bars indicate SD. (B) Rhodamine efflux from the lymphocytes as shown in panel A. Shown are time zero and 90 minutes. (C) Percentage transduction in neurosphere colonies after transduction at a total of 40 TU/cell alone or in the presence of 50 μg/mL verapamil, 100 μM diltiazem, or 50 μM quinidine. (D) Percentage transduction in mesenchymal progenitors (MSCs) after transduction at a total of 20 TU/cell alone or in the presence of 25 or 50 μg/mL verapamil, 100 μM diltiazem, or 50 μM quinidine. (E) Transduction efficiency in primary hepatocyte culture after addition of 10 TU/cell alone or in the presence of 25 or 50 μg/mL verapamil, 100 μm diltiazem, or 30 μM ritonavir.
ABC transporter substrates that are inhibitors do not increase transduction in T cells, hepatocytes, or nonhematopoietic progenitor cells. (A) Primary CD4+ T lymphocytes suboptimally transduced at 10 TU/cell alone or in the presence of 50 μg/mL verapamil or 50 μM ritonavir. Error bars indicate SD. (B) Rhodamine efflux from the lymphocytes as shown in panel A. Shown are time zero and 90 minutes. (C) Percentage transduction in neurosphere colonies after transduction at a total of 40 TU/cell alone or in the presence of 50 μg/mL verapamil, 100 μM diltiazem, or 50 μM quinidine. (D) Percentage transduction in mesenchymal progenitors (MSCs) after transduction at a total of 20 TU/cell alone or in the presence of 25 or 50 μg/mL verapamil, 100 μM diltiazem, or 50 μM quinidine. (E) Transduction efficiency in primary hepatocyte culture after addition of 10 TU/cell alone or in the presence of 25 or 50 μg/mL verapamil, 100 μm diltiazem, or 30 μM ritonavir.
We next tested whether transduction of human neuronal progenitors (neurospheres) or mesenchymal progenitors (MSCs) could be enhanced by ABC transporter substrates, as well as hepatocytes, which strongly express ABC transporters.28,29 Neurospheres were transduced in the presence of 20 TU/cell VRX494 after attachment to plates. Sixty percent to 80% transduction was observed 4 days after transduction. Immunohistochemistry characterized the majority of progeny cells as astrocytes, with some neurons, and very few oligodendrocytes. After 2 weeks, we observed 61% transduction in neurosphere progeny cells. Verapamil, diltiazem, and quinidine did not improve neurosphere progeny transduction at doses that improve HSC transduction (Figure 6C). No significant cytotoxicity was noted at the doses tested (data not shown).
MSCs were transduced with 10 TU/cell, resulting in 7.5% transduction. The addition of ABC transporter inhibitors did not improve and slightly inhibited transduction efficiency (Figure 6D). Verapamil reduced viability to 60%, but no cytotoxicity was observed with use of the other substrates.
Hepatocytes were transduced with 10 TU/cell, resulting in approximately 30% transduction. Surprisingly, because hepatocytes express high levels of ABC transporters, ABC transporter substrates did not improve hepatocyte transduction efficiency and were instead slightly inhibitory (Figure 6E). No significant cytotoxicity was noted at the doses tested. These data suggest that strong ABC transporter expression is not sufficient for the transduction enhancement of ABC transporter substrates and that there is a distinct factor present in primitive hematopoietic progenitors that is absent in mature hematopoietic cells, neuronal, mesenchymal, and hepatic cells.
Inhibitors do not increase transduction of RD114-pseudotyped vectors
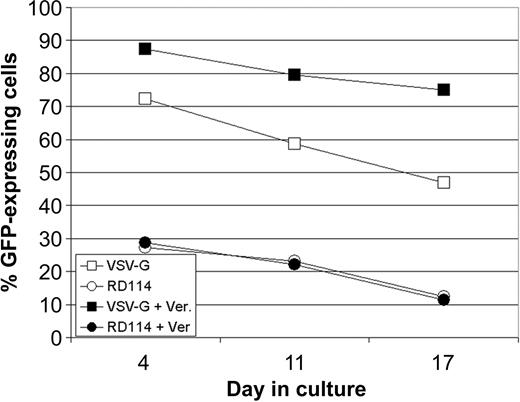
Verapamil did not enhance transduction of lentivirus vectors into primary T cells, despite robust inhibition of rhodamine efflux. These data made us consider that the increase in transduction might result from a unique interaction between the lentivirus vector and the stem cell. To examine if the vector envelope might be playing a role in this interaction, we examined in parallel the transduction of CD34+ hematopoietic progenitors with lentivirus vectors pseudotyped with either VSV-G or RD114, in the presence and absence of verapamil (Figure 7). Without verapamil, VSV-G-pseudotyped vectors transduced cells between 2.5- and 4-fold better than did RD114-pseudotyped vectors. In the presence of verapamil, transduction was improved for VSV-G-pseudotyped vectors. In contrast, RD114-pseudotyped vector transduction was entirely unaffected. These data provide suggestive evidence that enhancement of transduction in hematopoietic cells with lentivirus vectors is specific to the cell biology and the vector envelope.
Effect of ABC transporter substrate inhibitors on transduction using lentivirus vectors pseudotyped with RD114. CD34+ hematopoietic progenitor cells were transduced at 25 TU/cell in the presence or absence of 50 μg/mL verapamil with VSV-G- or RD114-pseudotyped lentivirus vectors. Transduction efficiency was measured on days 4, 11, and 17 after transduction.
Effect of ABC transporter substrate inhibitors on transduction using lentivirus vectors pseudotyped with RD114. CD34+ hematopoietic progenitor cells were transduced at 25 TU/cell in the presence or absence of 50 μg/mL verapamil with VSV-G- or RD114-pseudotyped lentivirus vectors. Transduction efficiency was measured on days 4, 11, and 17 after transduction.
Discussion
We describe in this report a novel approach for improving lentiviral gene transfer into human hematopoietic progenitors by exposing cells to verapamil during transduction. Relative to cells transduced under standard conditions, verapamil increased transduction efficiencies 2- to 6-fold, resulting in stable transduction levels of 80% to 90% in cells recovered from primary and secondary transplantation in NOD/SCID mice. Other ABC transporter inhibitor substrates also improved transduction but less efficiently than verapamil, and nonsubstrate inhibitors showed no effect.
Importantly, clinically relevant vector used to attain this high level of transduction was column purified and not centrifuged. High-speed centrifugation has been a method for concentrating vector to achieve higher transduction efficiencies reported elsewhere. High-speed centrifugation does not result in a clinically applicable vector because it cannot be sterile filtered, which is a necessary step to ensure sterility prior to use in humans. Therefore, we report for the first time high lentiviral transduction efficiencies in CD34+ hematopoietic progenitor cells by using a grade of vector that is clinically applicable.
Verapamil is a clinically approved drug, used for the treatment of arrhythmia, hypertension, angina, cardiomyopathy, and migraine headaches. Verapamil is an L-type calcium ion influx inhibitor and is a potent vasodilator of coronary vessels. In addition to calcium channel-related therapies, verapamil interferes with ABC transporter activity, including p-glycoprotein. Thus, verapamil and functionally similar drugs have been considered for anticancer therapies against multidrug-resistant tumors.
Transduction in the presence of verapamil, quinidine, diltiazem, and ritonavir provided no adverse effect on engraftment of SRCs and subsequent multilineage reconstitution. Engraftment levels and differentiation patterns remained similar in primary and secondary transplantations between cohorts of mice given cells transduced in the presence or absence of verapamil. We observed an increase in the BFU-E/CFU-GM ratio from 1:2 to 4:1 in the presence of verapamil. Although the reason for this is unknown, we are looking into GATA-1 expression after drug treatment.30
These data demonstrate that a variety of structurally and mechanistically diverse ABC transporter substrates improve lentiviral transduction efficiency into human CD34+ (CFC, LTC-IC, and SRC). Furthermore, ABC transporter substrates improve transduction into rhesus macaque CD34+ progenitors (data not shown), suggesting a conserved mechanism between the 2 species. ABC transporter substrates do not improve transduction into mature hematopoietic cells, suggesting there may be cellular factors unique to stem cells that allow this effect.
The mechanism of verapamil-enhanced transduction remains unknown. We know that primitive cells express higher levels of ABC transporters and have been relatively difficult to transduce compared with more mature hematopoietic progenitors. One hypothesis is that verapamil may serve to block the efflux of critical cellular factors required for efficient viral vector integration or reverse transcription. Many ABC transporters are ion channels; therefore, inhibition of transporter activity might alter the cellular pH or intracellular ionic concentrations of Na+, Ca2+, or K+. Another interesting possibility is an effect on deoxynucleosides (dNSs) and their precursors, which directly affect transduction efficiency by increasing the rate of reverse transcription. Indeed, we found that verapamil and dNS addition had a cumulative improvement on transduction in HPCs (data not shown).
It is possible that biologically relevant small molecules could be associated with the vector, either internalized within the vector, on the surface of the vector, or in the vector suspension buffer. Interestingly, we found that if we pseudotyped our vector with RD114, transduction could not be improved by verapamil addition in 2 separate experiments by using a VSV-G-pseudotyped vector as the positive control. This may be because RD114 is somehow insensitive to changes in membrane polarization, or because the effect of ABC transporter inhibitor substrates is specific to VSV-G only.
The transduction enhancement effect does not appear to be due to ATPase inhibition, given the ineffectiveness of sodium vanadate and the lack of transduction enhancement in primary T cells. ABC transporter substrates may facilitate a change in the lipid bilayer or a protein component in the cellular membrane that encourages interactions with the lentiviral envelope, which is VSV-G in our case. VSV-G is a unique vector envelope in that it is thought to interact with the plasma membrane instead of a specific receptor. This might be a reason that enhancement of transduction did not occur with RD114. The possibility remains that there is a particular feature about RD114 that prevents it from interacting with, or being affected by, ABC transporter inhibitor substrates.
We present a lentivirus-based vector acceptable for use for human gene therapy, which efficiently transduces stem cells, resulting in stable gene expression before and after differentiation in vivo. We further describe a novel approach for increasing transduction efficiencies into these cells, which reduces the amount of vector necessary for efficient transduction, and provides a useful tool for future therapeutic stem cell clinical trials.
Prepublished online as Blood First Edition Paper, April 1, 2004; DOI 10.1182/blood-2003-07-2363.
Supported in full by private funds.
Each of the authors of this study is or was employed by VIRxSYS Corp, whose potential vector was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Randall Merling, Andrew Worden, Kathy Schonely, Reuben Cohen, Bing Jiang, Eden Deausen, David Berlinger, Mechelle Bray, Wei Han, and Danlan Wei for their contributions to this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal