Comment on Lou et al, page 752
A major limitation of idiotype vaccination is the requirement for patient-specific reagents. This could be overcome using shared framework region peptides. Knowing that this does not lead to loss of normal B-cell responses is important in bringing this approach to clinical trials.
In murine lymphoma models, humoral and cellular mechanisms against idiotype (Id) determinants are effective in inducing tumor regression in the tumor-bearing host, and, in clinical trials, vaccination with patient-specific Id has demonstrated efficacy with induction of largely humoral responses. Previous studies have suggested that effective antitumor responses require induction of efficient major histocompatibility complex (MHC)–restricted CD4+ and CD8+ T cells.1 Although a number of studies have suggested that tolerance exists to germ line–derived heavy chain (VH)sequences, CD4+ T-cell responses and more rarely CD8 responses against Id-derived peptides have been demonstrated. With advances in knowledge of the requirements for peptide binding to specific MHC class I molecules, it was demonstrated that peptides from Id could bind to MHC class I and induce effective CD8 T-cell responses. The surprising finding was that these peptides are often not patient specific but derived from framework regions, often shared among patients. T cells generated against these peptides could kill not only antigen-presenting cells pulsed with the Id-derived peptide but also the primary tumor cells from which the Id was derived.2 These data suggest that tolerance against these regions either does not exist or can easily be broken. If tolerance can be overcome against such germ line region peptides, why then is there no ongoing immune response against normal B cells?FIG1
Immunogenicity of MHC class II binding VH peptides. See the complete figure in the article beginning on page 752.
Immunogenicity of MHC class II binding VH peptides. See the complete figure in the article beginning on page 752.
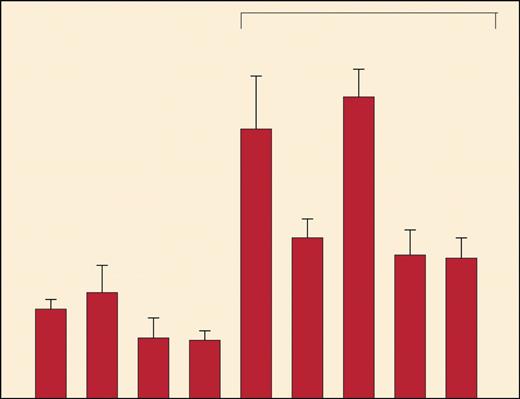
In this issue of Blood, Lou and colleagues have addressed this critical issue. For their model, they used a B-cell hybridoma expressing a conserved germ line VH gene with specificity for dextran, identified peptides that bind to MHC class I and class II molecules, and tested the binding and immunogenicity of these peptides. They demonstrate that these germ line VH peptides are indeed immunogenic in vitro and, as can be shown, induce effective tumor-specific protective immunity (Lou and colleagues [Figure 4]) but that this does not affect the magnitude of normal B-cell responses to dextran. The present study does not address the mechanisms whereby normal B cells escape this immune response, but, as suggested by the authors, those cells' escape may be due to the inability of normal as opposed to malignant B cells to process such peptides. There is a T-cell response against some clones but not others that are responsible for the polyclonal B-cell response against antigen.
Regardless of the mechanism, these elegant studies clearly demonstrate that protective immune responses can be generated against germ line region peptides without impairing the magnitude of normal humoral responses. The major logistical difficulty in Id vaccination is the generation of patient-specific vaccines. The implications of these studies are clear since they demonstrate that it should be possible to develop vaccines using shared peptides from framework regions that are shared among many patients, thereby using autoimmune-type responses to treat cancer.3 Immune responses against these weakly immunogenic peptides are insufficient to induce complete protection, and studies using heteroclitic peptides that are altered to increase their immunogenicity are ongoing.4,5

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal